Nitrogen-functionalised carbon nanotubes as a novel adsorbent for the removal of Cu(II) from aqueous solution†
Oluwaseun A. Oyetade,
Vincent O. Nyamori*,
Bice S. Martincigh and
Sreekantha B. Jonnalagadda
School of Chemistry and Physics, University of KwaZulu-Natal, Westville Campus, Private Bag X54001, Durban 4000, South Africa. E-mail: nyamori@ukzn.ac.za; Fax: +27 31 260 3091; Tel: +27 31 260 8256
First published on 23rd December 2015
Abstract
This study investigated the introduction of 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine (HO-Phttpy) onto the surface of multiwalled carbon nanotubes (MWCNTs) to obtain nitrogen-functionalized MWCNTs (MWCNT-ttpy). This novel material was characterised and tested for its possible use in the remediation of wastewater contaminated with heavy metal ions. Its efficacy was compared with that of acid-functionalized MWCNTs (MWCNT-COOH) for the removal of the heavy metal ion Cu2+ through adsorption. HO-Phttpy was first synthesized, followed by the functionalization of MWCNT-COOH to afford MWCNT-ttpy. MWCNT-ttpy showed significant textural enhancement due to an increase in the extent of functionalization. This was demonstrated by an increase in the surface area and pore volume of MWCNT-ttpy, from 126.8 to 189.2 m2 g−1 and 0.692 to 1.252 cm3 g−1, respectively, relative to MWCNT-COOH. Its application for Cu2+ removal showed a marked increase in uptake (qe), i.e. 19.44 to 31.65 mg g−1, compared with MWCNT-COOH. This is attributed to the introduction of more active/chelating sites for adsorption. Adsorption experiments were conducted at pH 5 at which equilibrium was reached after 360 min. The results showed that the adsorption process was best described by the pseudo-second order model. Among the isotherms tested, the Langmuir isotherm provided the best fit for the equilibrium data. Thermodynamic studies revealed that the adsorption process was spontaneous and endothermic. Desorption studies demonstrated a better removal efficiency of Cu2+ from MWCNT-ttpy, indicating its possible regeneration and the recovery of the Cu2+ adsorbate for reuse. Thus, MWCNT-ttpy shows superior properties for wastewater remediation compared to MWCNT-COOH.
1. Introduction
Carbon nanotubes (CNTs) are tubular shaped carbon nanomaterials, possessing excellent electrical, mechanical and optical properties and thermal stability.1,2 They are made up of a hexagonal lattice of carbon atoms, consisting of hollow graphite, rolled at specific angles into cylinders. CNTs are classified into single-walled carbon nanotubes (SWCNTs), double-walled carbon nanotubes (DWCNTs) and multiwalled carbon nanotubes (MWCNTs), depending on the number of concentric sheets contained in the tubes.3 They form large aggregates with low dispersibility in aqueous and organic solutions due to strong van der Waals interactions between them. This requires the development of both covalent and non-covalent strategies for CNT functionalization, in order to introduce functional groups which will enhance their application for different purposes. Covalent functionalization is often carried out through oxidation of CNTs with acids such as HNO3 and H2SO4, to introduce oxygen-containing groups such as –OH and –COOH onto the walls of the tubes.4 This approach improves the wettability of CNTs, thereby increasing their dispersibility in aqueous/organic solutions. New functional groups, such as –NR, –F, –Cl and –SR, can also be incorporated onto the walls/sides of the tubes through solution chemistry.5In recent times, the application of CNTs in various fields such as medicine, engineering, environmental science, energy, catalysis and catalyst supports, amongst many others, has been reported by various authors.4,6,7 This is largely attributed to their inherent properties such as large surface area, pore volume and the ease in introducing new functional groups onto their walls. In spite of these studies, functionalization of CNTs to contain multiple functional groups which will further enhance chemical reactions with other molecules is still under-researched.1
The tridentate chelating ligand, 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine (HO-Phttpy), containing three nitrogen atoms is a good contender for the functionalization of MWCNTs, in order to introduce nitrogen-containing donor atoms to the structure of CNTs. HO-Phttpy has profound flexibility to form bridged metal centres by using two or more moieties, construction of supramolecular structures and formation of macrocyclic ligands. Due to the strong affinity of this ligand for transition metal ions, the chelates produced have been used in luminescence and chemical sensing devices.8 In spite of the vast amount of work on CNTs, to the best of our knowledge, no study has investigated the effect of functionalization of oxidized MWCNTs (MWCNT-COOH) with HO-Phttpy for their increased activity as adsorbents.
One possible area of application of CNTs functionalized with nitrogen-donor ligands is the remediation of wastewater contaminated with heavy metal ions. In this work, the synthesized material was applied for the removal of Cu2+ as an example of a typical heavy metal ion discharged into the environment. The choice of copper was based on the fact that copper and its compounds are regularly used in agriculture for fertilizer production, electronics, textiles, medicine and metal cleaning purposes.9 The discharge of copper-contaminated effluents into aquatic environments introduces humans to necrotic changes in the liver and kidney, gastrointestinal irritations such as diarrhoea and melena, and including a number of capillary diseases; resulting in severe deleterious health effects.10 Although copper is a known essential metal at low concentrations, an excessive amount is considered harmful to both aquatic and human life.11 As a result of the numerous health consequences, a variety of techniques have been used for the removal of Cu2+ from municipal wastewaters and industrial effluents.12,13 Of these methods, adsorption is considered most reliable for its removal from wastewater.13 This approach is simple, efficient, and cost-effective, with a high potential for recycling the metal ion and the adsorbent. Thus, the removal of Cu2+ from aqueous solution by adsorption has been reported through the use of tree fern,14 bagasse,15 peanut hull,16 rice husk,17 magnetite,18 peat19 and activated carbon.20 Reports have shown that these adsorbents possess good potential for Cu2+ removal; however, drawbacks such as slow sorption and regeneration of adsorbents limits their usage.19,21 Pristine and functionalized CNTs have also been applied as adsorbents for Cu2+ removal.10,22,23 However, increased removal efficiency can be achieved by using CNT-based nanocomposites as adsorbents. Composites such as CNT/bagasse,24 CNT/magnetite,25 and CNT/chitosan26 have demonstrated good sorption ability for Cu2+ removal. To further improve the efficiency of CNT-based nanomaterials, nitrogen-containing ligands can be used as modifiers for CNTs, hence, increasing the number of chelating sites available for adsorption on the adsorbent.13
In this communication, we report the synthesis of nitrogen-functionalized MWCNTs (MWCNT-ttpy) through the functionalization of acid-functionalized MWCNTs (MWCNT-COOH) by incorporating 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine (HO-Phttpy) onto their structure. The effectiveness of this material in the adsorption of Cu2+ from aqueous solution was examined through a series of batch adsorption processes and compared with that of MWCNT-COOH. The effects of pH, contact time, adsorbent dose, temperature and initial Cu2+ concentration were investigated to determine the optimum conditions for the effective remediation of Cu2+-polluted wastewater. In addition, desorption studies were undertaken to investigate the potential reuse of the adsorbent and recovery of the adsorbate.
2. Experimental
2.1. Materials
Copper metal powder was obtained from Johnson Matthey Chemicals (Pty) Ltd (Gauteng, South Africa) while sodium hydroxide (NaOH, 98%) and sodium bicarbonate (NaHCO3, 99%) were purchased from Merck Chemicals (Pty) Ltd (Gauteng, South Africa). Sodium carbonate (Na2CO3, 99%) was purchased from Associated Chemical Enterprises (Johannesburg, South Africa). Chemicals such as 4-hydroxybenzaldehyde (99%), 2-acetylpyridine (99%), indium bromide (InBr3, 99%) and solvents such as absolute ethanol, N,N-dimethylformamide (DMF, 99%), dimethyl sulfoxide-d6 (DMSO-d6, 99%) and triethylsilane (Et3SiH, 97%) were purchased from Sigma-Aldrich (St Louis, USA). Tetrahydrofuran (THF, 99%), chloroform (99%) and thionyl chloride (SOCl2, 99%) were purchased from Merck Chemicals (Pty) Ltd (Gauteng, South Africa) while aqueous ammonia (25%) was purchased from Associated Chemical Enterprises (Johannesburg, South Africa). Nitric (55%), sulfuric (98%) and hydrochloric acids (32%) were obtained from C C Imelmann Ltd (Robertsham, South Africa). All materials and chemicals were of analytical grade and used as received from suppliers without further purification. MWCNTs (purity > 95%) (P-MWCNTs), synthesized by chemical vapour deposition (CVD), were obtained from Cheap Tubes Incorporation (Brattleboro, USA).2.2. Characterization
The ligand, 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine, was characterized by using Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR) and mass spectrometry, and melting point measurements. The FTIR spectra were recorded on a Perkin Elmer RX 1 spectrophotometer and the melting point was determined by using a Bibby Stuart Scientific model SMP3 apparatus. 1H and 13C NMR spectra were obtained from a 400 MHz Bruker Avance III spectrometer and mass spectra were obtained on a Waters Synapt G2 mass spectrometer in electrospray positive mode.Structural characterization of the adsorbents was carried out with a transmission electron microscope (TEM) (JEOL, TEM 1010) and a scanning electron microscope (SEM) (JEOL, TSM 6100) to visualize the morphology, structure, shape and size distribution of the nanomaterials. Images were captured by means of a Megaview 3 camera and analysed on iTEM software. The surface area of the adsorbents was determined with nitrogen as the flow gas by means of a Micromeritics Tristar II 3020 surface area and porosity analyser. Data were captured and analysed by using Tristar II 3020 version 2 software. Fourier transform infrared (FTIR) spectra of the synthesized materials were obtained with the samples were embedded into KBr pellets and were recorded on a Perkin Elmer Spectrum RX 1 spectrophotometer, in order to identify the surface functional groups present on the materials. Raman spectroscopy (DeltaNu Advantage 532™) measurements were also performed to provide information on the purity and crystallinity of the adsorbents. Thermogravimetric (TGA) analyses (Q Series™ Thermal Analyzer DSC/TGA Q600) were performed to determine the thermal stability and fraction of volatile components in the samples while the Boehm titration was applied to quantitatively estimate the amount of acidic and basic groups present on the adsorbents.27,28 Elemental analysis (ThermoScientific Flash 2000) of adsorbents was also carried out to investigate the percentage composition of carbon, hydrogen, oxygen and nitrogen present in the samples. The point of zero charge (pHPZC) was also performed as described by Oyetade et al.4 and Khan et al.29 to estimate the electrical charge density of the adsorbents. Details of the Boehm titration and point of zero charge determination are provided in the ESI.†
2.3. Procedure for the synthesis of 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine (HO-Phttpy)
The ligand was synthesized as reported by Patel et al.,30,31 with some modifications. 2-Acetylpyridine (2.423 g, 20.0 mmol) was added to 15 cm3 of a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of ethanol and water containing 4-hydroxybenzaldehyde (1.221 g, 10.0 mmol). To the solution, NaOH pellets (1.458 g, 26.0 mmol) and 30 cm3 aqueous NH3 were added and stirred continuously at room temperature for 8 h to yield a cream-coloured precipitate. The resulting mixture was filtered, the solid obtained was washed with deionised water (5 × 10 cm3), followed by absolute ethanol (3 × 5 cm3) to obtain the crude white product (508.8 mg, 42%). m.p. 199–201 °C; IR (ATR, cm−1) 3375, 1614, 1588, 1565; 1H NMR (400 MHz, DMSO-d6) δ: 6.92 (d, 2H, J = 8.6 Hz), 7.49–7.52 (m, 2H), 7.75 (d, 2H J = 8.68 Hz), 7.99–8.04 (m, 2H), 8.67–8.74 (m, 6H); 13C NMR (400 MHz, DMSO-d6) δ: 160.2, 155.4, 155.1, 149.4, 149.2, 137.3, 128.0, 126.8, 124.3, 120.8, 116.8, 116.4; HR-MS [C21H15N3O] ES: [M + H+] m/z calcd 326.1215, found 326.1293. Additional spectral information is shown in ESI Fig. S1–S3.†
1 (v/v) mixture of ethanol and water containing 4-hydroxybenzaldehyde (1.221 g, 10.0 mmol). To the solution, NaOH pellets (1.458 g, 26.0 mmol) and 30 cm3 aqueous NH3 were added and stirred continuously at room temperature for 8 h to yield a cream-coloured precipitate. The resulting mixture was filtered, the solid obtained was washed with deionised water (5 × 10 cm3), followed by absolute ethanol (3 × 5 cm3) to obtain the crude white product (508.8 mg, 42%). m.p. 199–201 °C; IR (ATR, cm−1) 3375, 1614, 1588, 1565; 1H NMR (400 MHz, DMSO-d6) δ: 6.92 (d, 2H, J = 8.6 Hz), 7.49–7.52 (m, 2H), 7.75 (d, 2H J = 8.68 Hz), 7.99–8.04 (m, 2H), 8.67–8.74 (m, 6H); 13C NMR (400 MHz, DMSO-d6) δ: 160.2, 155.4, 155.1, 149.4, 149.2, 137.3, 128.0, 126.8, 124.3, 120.8, 116.8, 116.4; HR-MS [C21H15N3O] ES: [M + H+] m/z calcd 326.1215, found 326.1293. Additional spectral information is shown in ESI Fig. S1–S3.†
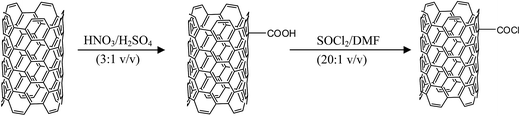
2.4. Preparation of MWCNT-COOH
Oxidation of MWCNTs was carried out as reported by Oyetade et al.4 and Santangelo et al.32 In brief, pristine MWCNTs (1.5 g) were placed in a round-bottomed flask containing 100 cm3 of concentrated hydrochloric acid, and stirred for 4 h to remove residual metal impurities from the tubes. The resulting mixture was filtered, and the solid was washed with deionised water until a neutral pH was obtained. The sample obtained was dried in a vacuum oven at 80 °C overnight and stored in a desiccator for future analysis. The purified MWCNTs were then oxidized by using a mixture of sulfuric and nitric acids in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and refluxed at 80 °C for 12 h. The resulting mixture was diluted with deionised water, filtered, and the residue obtained was washed continuously with deionised water until a neutral pH was obtained.
3, and refluxed at 80 °C for 12 h. The resulting mixture was diluted with deionised water, filtered, and the residue obtained was washed continuously with deionised water until a neutral pH was obtained.
2.5. Preparation of MWCNT-COCl
MWCNT-COOH (150 mg) were placed in a solution containing 30 cm3 of a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture of SOCl2 and dry DMF, and then refluxed at 70 °C for 24 h. The resulting mixture was filtered and the solid washed with anhydrous THF (5 × 5 cm3).33 The solid was dried in a vacuum oven at 80 °C, and stored under an inert atmosphere of argon for further analysis.
1 (v/v) mixture of SOCl2 and dry DMF, and then refluxed at 70 °C for 24 h. The resulting mixture was filtered and the solid washed with anhydrous THF (5 × 5 cm3).33 The solid was dried in a vacuum oven at 80 °C, and stored under an inert atmosphere of argon for further analysis.
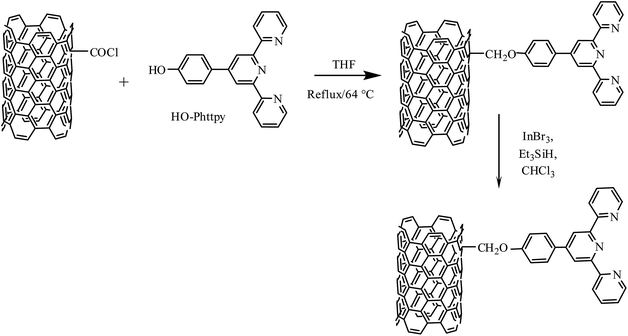
2.6. Preparation of MWCNT-COOttpy
MWCNT-COCl (100 mg) were added to HO-Phttpy (100 mg) in a round-bottomed flask containing 20 cm3 of dry THF and 2–3 drops of glacial acetic acid. The mixture was refluxed at 64 °C for 24 h under an inert atmosphere of argon. After cooling to room temperature, the resulting suspension was filtered and the product obtained was washed with THF and dried in a vacuum oven at 80 °C overnight.2.7. Preparation of MWCNT-ttpy
The reduction of MWCNT-COOttpy to obtain an ether was carried out as reported by Sakai et al.34 MWCNT-COOttpy (100 mg), InBr3 (10.6 mg, 0.03 mmol) and Et3SiH (380 μL, 2.4 mmol) were added to a 30 cm3 volume of freshly distilled chloroform, and refluxed at 60 °C for 1 h under an inert atmosphere of argon. The suspension was filtered and the solid obtained was washed with chloroform, followed by water until a neutral pH was obtained. The resulting solid was dried in a vacuum oven at 80 °C overnight and stored in a desiccator for future analysis.2.8. Adsorbate preparation
A standard stock solution of Cu2+ was prepared by dissolving approximately 1.0 g of pure copper metal into 50 cm3 of 5 mol dm−3 nitric acid. The solution was made up to 1000 dm3 with deionized water. From this solution, working solutions were prepared by diluting the stock solution in accurate proportions to obtain the required concentrations.2.9. Sorption experiments
The adsorption of Cu2+ on prepared adsorbents was investigated by using batch adsorption experiments. All adsorption experiments were conducted in duplicate by using 50 cm3 polypropylene plastic vials. Freshly prepared working solutions of 100 mg dm−3 Cu2+ were prepared from the stock solution daily. Adsorption experiments were performed by agitating 20 cm3 of a known Cu2+ concentration (100 mg dm−3) in a thermostated water bath at a fixed temperature (20 °C) for 24 h with an adsorbent dose of 50 mg. The pH of the solution was adjusted by adding appropriate amounts of 0.1 mol dm−3 NaOH or HNO3 to obtain the desired pH. After the required time interval, the mixtures were filtered and the final concentration of Cu2+ in the filtrate was determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Perkin Elmer Optima 5300 DV). The effect of pH, amount of adsorbent, initial Cu2+ concentration, contact time and temperature was studied in order to determine the optimum conditions necessary for Cu2+ removal from aqueous solution. The adsorption efficiency (% adsorbed) and adsorption capacity (qe) were calculated by using eqn (1) and (2), respectively.
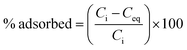 | (1) |
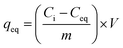 | (2) |
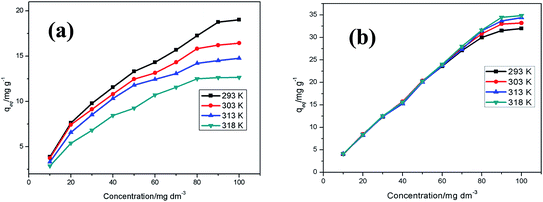
Adsorption isotherms were investigated by using varying Cu2+ concentrations, ranging from 10–100 mg dm−3, at a constant pH of 5. Aliquots of 20 cm3 were mixed with 50 mg of the adsorbents and agitated on a thermostated shaking water bath under varying temperatures of 293, 303, 313 and 318 K for 24 h. The solutions were filtered by gravity and the concentrations of Cu2+ in the filtrates determined by ICP-OES. The experimental adsorption equilibrium data were analysed by eight adsorption isotherm models consisting of both two- (Langmuir,39 Freundlich,40 Temkin41 and Dubinin–Radushkevich42 models) and three-parameter (Sips,43 Toth,44 Redlich–Peterson45 and Khan46) models. The equations of the models are given in ESI Table S2.† Thermodynamic parameters such as change in Gibbs energy (ΔG°), change in enthalpy (ΔH°), and change in entropy (ΔS°) were also calculated over the studied temperature range.
2.10. Analysis of real samples
Three water samples were collected from three sites on the Umgeni river: (i) the tributary on the confluence of the Umgeni and Msunduzi rivers, (ii) the outflow from the EThekwini wastewater treatment plant and (iii) the Blue Lagoon (mouth of Umgeni rivers). The initial concentrations of Pb2+, Zn2+ and Cu2+ were determined by using ICP-OES. An aliquot of 25 cm3 of the water samples conditioned to pH 5, was measured into 50 cm3 polypropylene bottles with an addition of 50 mg of MWCNT-ttpy. The suspensions were agitated in a thermostated water bath at 20 °C for 1 h. After agitation, the suspensions were filtered and their supernatants analysed for the final concentrations of metal ions in solution. The removal efficiency and adsorption capacity for each metal ion were evaluated according to eqn (1) and (2), respectively.2.11. Data analysis
The data obtained were fitted to the isotherm and kinetics models by means of the nls nonlinear regression routine in the R statistical computing environment.47 The R statistical software takes into account the minimization of the sum of squared residuals (SSR) and the residual square errors (RSE). A comparison of all SSR and RSE values was done in order to assess the adequacy of the models. The model chosen was that with the lowest SSR.3. Results and discussion
The ligand and nanomaterials synthesised were characterized by a series of techniques to ascertain the authenticity and properties of the adsorbents for Cu2+ removal. Subsequently, the efficiency of the novel material to adsorb Cu2+ was assessed and compared with that of acid-functionalized MWCNTs by means of batch adsorption processes.3.1. Synthesis of 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine (HO-Phttpy)
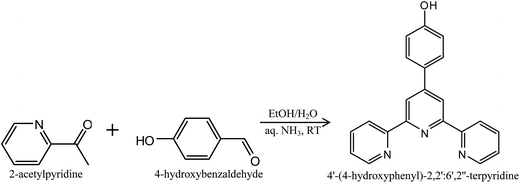
The synthesis of 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine was carried out by using a “greener” synthetic approach as illustrated in Fig. 1. The use of green solvents such as ethanol and water at room temperature affords an opportunity of obtaining products in moderate yields. Upon reacting 4-hydroxybenzaldehyde and 2-acetylpyridine in an ethanolic solution, a cream-coloured precipitate was formed. The completion of the reaction was monitored by thin layer chromatography (TLC) and FTIR and NMR spectroscopy. The formation of the product was confirmed by the disappearance of the carbonyl absorption band at ≈1700 cm−1 and the appearance of sharp peaks at 1634 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 1250–1335 cm−1 (C–N str), 712 cm−1 and 634 cm−1 (indicating γ and δ Py-ring in-plane and out-of-plane deformation vibrations respectively) in the FTIR spectra (Fig. 4c).48–50 1H NMR spectrometry further confirmed the formation of the product by the disappearance of the methyl proton resonance peak (≈2.10 ppm) and the appearance of aromatic proton resonance peaks (≈7.5–8.0 ppm) (see ESI Fig. S1†). Further verification of the product was done by 13C NMR and mass spectroscopy (see ESI Fig. S2 and S3†).
N str), 1250–1335 cm−1 (C–N str), 712 cm−1 and 634 cm−1 (indicating γ and δ Py-ring in-plane and out-of-plane deformation vibrations respectively) in the FTIR spectra (Fig. 4c).48–50 1H NMR spectrometry further confirmed the formation of the product by the disappearance of the methyl proton resonance peak (≈2.10 ppm) and the appearance of aromatic proton resonance peaks (≈7.5–8.0 ppm) (see ESI Fig. S1†). Further verification of the product was done by 13C NMR and mass spectroscopy (see ESI Fig. S2 and S3†).
3.2. Synthesis of MWCNT-ttpy
The synthesis of the novel nanomaterial was carried out first by the acylation of MWCNT-COOH with a mixture of SOCl2 and DMF in a volume ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as illustrated in Fig. 2.
1 as illustrated in Fig. 2.
Acylated nanotubes (MWCNT-COCl) were further reacted in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with HO-Phttpy to afford esterified MWCNTs (MWCNT-COOttpy). Reduction of MWCNT-COOttpy was carried out in distilled CHCl3, by using Et3SiH as a reducing agent and InBr3 as a catalyst, to afford ether-functionalized CNTs (MWCNT-ttpy). The route to the synthesis of MWCNT-ttpy is shown in Fig. 3.
1 with HO-Phttpy to afford esterified MWCNTs (MWCNT-COOttpy). Reduction of MWCNT-COOttpy was carried out in distilled CHCl3, by using Et3SiH as a reducing agent and InBr3 as a catalyst, to afford ether-functionalized CNTs (MWCNT-ttpy). The route to the synthesis of MWCNT-ttpy is shown in Fig. 3.
3.3. Characterization of adsorbents
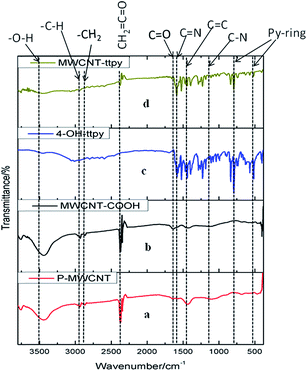
The synthesized products were characterized by FTIR spectroscopy. Fig. 4 shows the FTIR spectra of (a) P-MWCNT, (b) MWCNT-COOH, (c) HO-Phttpy and (d) MWCNT-ttpy. For pristine MWCNTs (P-MWCNTs), the peaks observed at ≈1498 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations (Fig. 4a) which are representative of graphitic structures characteristic of CNTs.1 The peaks observed around ≈3500 and ≈2700 cm−1 are attributed to the presence of water molecules and carbon dioxide, respectively. Acidic functional groups such as carboxylic groups were introduced onto the walls of tubes after oxidation, hence a new peak at ≈1750 cm−1, assigned to C
C stretching vibrations (Fig. 4a) which are representative of graphitic structures characteristic of CNTs.1 The peaks observed around ≈3500 and ≈2700 cm−1 are attributed to the presence of water molecules and carbon dioxide, respectively. Acidic functional groups such as carboxylic groups were introduced onto the walls of tubes after oxidation, hence a new peak at ≈1750 cm−1, assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, was noticeable (Fig. 4b), indicating the presence of new groups after oxidation was carried out.23 For HO-Phttpy (Fig. 4c), peaks at 3050 cm−1 (aromatic C–H str), 1475–1585 cm−1 (aromatic C
O stretching, was noticeable (Fig. 4b), indicating the presence of new groups after oxidation was carried out.23 For HO-Phttpy (Fig. 4c), peaks at 3050 cm−1 (aromatic C–H str), 1475–1585 cm−1 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C str), 1634 cm−1 (C
C str), 1634 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N str), 2890–2970 cm−1 (CH2 and CH groups), 3500 cm−1 (O–H str), 1250–1335 cm−1 (C–N str), 712 cm−1 and 634 cm−1 (indicating γ and δ Py-ring in-plane and out-of-plane deformation vibrations respectively) were obtained.48–50 Of importance to note is that MWCNT-ttpy (Fig. 4d) showed a similar profile to that of the ligand (Fig. 4c). This therefore confirms that the successful functionalization of MWCNT-COOH with HO-Phttpy was achieved, since functional groups characteristic to the ligand were present for MWCNT-ttpy.
N str), 2890–2970 cm−1 (CH2 and CH groups), 3500 cm−1 (O–H str), 1250–1335 cm−1 (C–N str), 712 cm−1 and 634 cm−1 (indicating γ and δ Py-ring in-plane and out-of-plane deformation vibrations respectively) were obtained.48–50 Of importance to note is that MWCNT-ttpy (Fig. 4d) showed a similar profile to that of the ligand (Fig. 4c). This therefore confirms that the successful functionalization of MWCNT-COOH with HO-Phttpy was achieved, since functional groups characteristic to the ligand were present for MWCNT-ttpy.
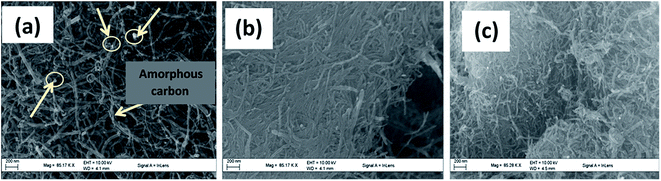
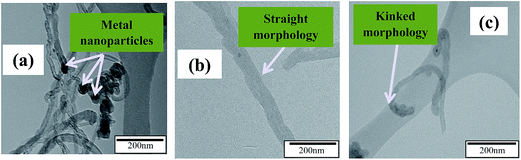
To confirm the shape and structure, and determine the morphology of the synthesized adsorbents, images were collected from transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The micrographs shown in Fig. 5 represent the SEM images of (a) P-MWCNT, (b) MWCNT-COOH and (c) MWCNT-ttpy while Fig. 6 shows the TEM images of (a) P-MWCNT, (b) MWCNT-COOH and (c) MWCNT-ttpy. As shown in Fig. 5a and 6a, a large entanglement of tubes was observed for P-MWCNT demonstrating a high presence of metal catalyst (shown with arrows) and amorphous carbon on the surface of the tubes. More alignment and less agglomeration was observed for MWCNT-COOH (i.e. tubes purified and functionalized with acids) (Fig. 5b). This is in agreement with the study of Rosca et al.51 Oxidation of CNTs accounts for the shortening of tubes, thereby introducing new functional groups, such as carboxylate groups, onto open ends of CNTs. Purification of CNTs removes the metal catalyst and amorphous carbon present on the surface of the tubes.1 Also, Fig. 6c further demonstrates that the tubular structure characteristic of MWCNTs was preserved after functionalization with the nitrogen ligand was carried out. A higher interspaced region was also noticed between tubes when compared with MWCNT-COOH (Fig. 5b vs. c). The images shown in Fig. 6a and b demonstrate the straight morphology for P-MWCNTs and MWCNT-COOH, while a curved/bent morphology was obtained for MWCNT-ttpy (Fig. 6c) due to the introduction of the ligand. Evidence of functionalization was also noted as long strands of CNTs were cut to obtain short and open-ended tubes.
In order to establish the thermal stability, purity and amount of volatile components in the synthesized adsorbents, thermogravimetric analysis (TGA) was carried out. Fig. S4† represents the (a) thermograms (TGA) and (b) derivative thermograms (DTG) of P-MWCNT, MWCNT-COOH and MWCNT-ttpy. As observed from Fig. S4a,† both P-MWCNTs and MWCNT-COOH were more thermally stable than MWCNT-ttpy. A lower decomposition temperature was obtained for MWCNT-ttpy than for P-MWCNTs and MWCNT-COOH (Fig. S4a†). This could be attributed to the increase in defects induced by the functionalization of MWCNT-COOH. This result is consistent with data obtained from Raman spectroscopy (Table 1), which demonstrates that MWCNT-ttpy had a higher ID/IG ratio than either P-MWCNTs or MWCNT-COOH. A higher ratio was also obtained for MWCNT-COOH when compared with P-MWCNTs. Hence, decreasing crystallinity in MWCNT-COOH and MWCNT-ttpy was associated with increasing functionalization, which thereby induces an increase in defects on P-MWCNTs, resulting in a larger ID/IG ratio. An increase in defective sites also accounts for a decrease in the decomposition temperature of MWCNT-COOH and MWCNT-ttpy obtained from TGA. The thermal stability of MWCNTs therefore increased in the order MWCNT-ttpy < MWCNT-COOH < P-MWCNTs. These results are consistent with recently published studies by Zhao et al.,1 Ombaka et al.,7 and Chizari et al.,52 indicating that increasing functionalization results in a reduced decomposition temperature and larger ID/IG ratio. Purification of P-MWCNTs also resulted in the decrease of metal catalyst present in MWCNT-COOH (Fig. S4a†) as indicated by the residual mass remaining after 620 °C. Three thermal decomposition stages were obtained for MWCNT-ttpy. The first stage (in circle) showed a mass loss (Δm = 9.88%) in the interval of 282–412 °C, suggesting the release of CH4O (9.85%, calcd). The second decomposition step (shown with arrow) demonstrates a mass loss (Δm = 35.84%) in the temperature range of 434–555 °C, suggesting the release of C9H6 (35.08%, calcd). The third decomposition step was observed for all three types of CNTs (i.e. P-MWCNTs, MWCNT-COOH and MWCNT-ttpy), representing the total decomposition of the graphitic structure of CNTs.
| Entry | Adsorbents | Surface area/m2 g−1 | Pore volume/cm3 g−1 | Pore diameter/nm | ID/IG |
|---|---|---|---|---|---|
| 1 | P-MWCNT | 108.8 | 0.494 | 18.44 | 1.17 |
| 2 | MWCNT-COOH | 126.8 | 0.692 | 22.95 | 1.19 |
| 3 | MWCNT-ttpy | 189.2 | 1.252 | 27.26 | 1.31 |
The surface area of the adsorbents was noticed to increase in the order P-MWCNTs < MWCNT-COOH < MWCNT-ttpy (Table 1). The results obtained indicated an increase in surface area and pore volume of MWCNT-COOH when compared with P-MWCNT (Table 1, Entry 2 vs. 1). This is attributed to the treatment of P-MWCNT, which results in cutting of large entanglements of tubes (Fig. 6a), thereby causing an increase in surface area and pore volume as observed in MWCNT-COOH.7 Furthermore, an increase in surface area and pore volume of MWCNT-ttpy (Table 1, Entry 3 vs. 2) was obtained, due to increasing functionalization of MWCNT-COOH. This result confirms that surface area/pore volume of nanomaterials can be increased based on the extent of functionalization. All adsorbents were mesoporous in nature since the pore diameters were less than 50 nm. Hence, MWCNT-COOH and MWCNT-ttpy possess moderately large surface areas and increased pore volume, which might enhance faster and better sorption ability of the adsorbents for the removal of Cu2+ from aqueous solutions.
Elemental analysis of the adsorbents confirmed the presence of nitrogen atoms attached to the ligand on the MWCNT-ttpy. However, as expected, nitrogen atoms were absent in P-MWCNTs and MWCNT-COOH (Table S3†). Thus, the presence of nitrogen atoms in MWCNT-ttpy confirms the successful functionalization of MWCNT-COOH with HO-Phttpy. The elemental analysis also showed an increase in the amount of oxygen in the order P-MWCNTs < MWCNT-COOH < MWCNT-ttpy. Acidic functionalization of P-MWCNTs accounts for the increase in the oxygen content of MWCNT-COOH due to the incorporation of oxygen-containing functional groups onto the walls of tubes.4 An increase in the oxygen content of MWCNT-ttpy is accounted for by the functionalization of MWCNT-COOH, which introduces nitrogen and more oxygen atoms onto the tubes. This result is consistent with data obtained from the Boehm titration (Table 2) which demonstrates an increase in the surface acidic groups of the MWCNTs as the extent of functionalization increases.
| Adsorbents | Carboxyl/mmol g−1 | Lactonic/mmol g−1 | Phenolic/mmol g−1 | Total acidic groups/mmol g−1 | Total basic groups/mmol g−1 | pHPZC |
|---|---|---|---|---|---|---|
| P-MWCNTs | 0.136 | 0.014 | 0.114 | 0.264 | 0.145 | 5.04 |
| MWCNT-COOH | 0.719 | 0.104 | 0.401 | 1.224 | 0.226 | 4.02 |
| MWCNT-ttpy | 0.613 | 0.165 | 0.544 | 1.322 | 0.752 | 4.48 |
The nature of the acidic and basic functional groups on the adsorbents was determined by the Boehm titration method.28,53 Functionalization of P-MWCNT with acids markedly increased the concentration of carboxyl, phenolic and lactonic groups on MWCNT-COOH (Table 2). This was consistent with the FTIR spectra (Fig. 4b) obtained, which revealed the appearance of a new peak at ≈1750 cm−1 corresponding to the formation of carboxylic groups. This result conforms with data reported by Biniak et al.27 wherein an increase in acidic properties was attributed to oxidation of adsorbents with acids. However, further functionalization with the nitrogen ligand decreased the concentration of the carboxyl groups but increased the concentrations of phenolic and lactonic groups. It is also worthy of note that an increase in the total number of basic groups of the adsorbents was observed as the extent of functionalization increased. MWCNT-ttpy contained the largest amount of basic groups justifying the presence of nitrogen-containing groups on the adsorbent.
3.4. Batch adsorption processes
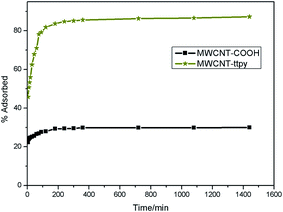
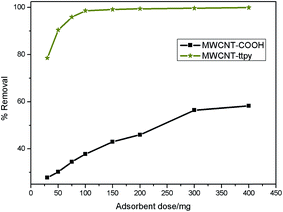
Sorption processes were carried out to examine the effectiveness and efficiency of MWCNT-COOH and MWCNT-ttpy for the removal of Cu2+ from a simulated wastewater. The role of various parameters that influence adsorption such as pH, contact time, adsorbent dose, temperature and initial adsorbate concentration were investigated to ascertain the ideal conditions suited for Cu2+ removal. Kinetics, isotherm and thermodynamic studies were also carried out by using the data obtained.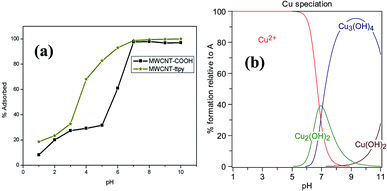 | ||
Fig. 7 (a) Effect of pH on sorption of Cu2+ onto MWCNT-COOH and MWCNT-ttpy, [conditions: 20 cm3 of 100 mg dm−3 Cu2+, 24 h equilibration time, 50 mg adsorbent dose, agitation speed 150 rpm, temperature 20 °C] and (b) speciation of Cu2+ as a function of pH in aqueous solution. Numerical values of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β for the metal hydroxides used in the calculation of the speciation curves were obtained from critical stability constants compiled by Smith and Martell,56 and plots obtained with the aid of HySS software.57 β for the metal hydroxides used in the calculation of the speciation curves were obtained from critical stability constants compiled by Smith and Martell,56 and plots obtained with the aid of HySS software.57 | ||
A significant increase is noticed for the removal of Cu2+ from aqueous solution as the pH of the solution is increased (Fig. 7a). The surface charge of the adsorbents is positive at pH values less than their pHPZC (Table 2), resulting in a low removal of the adsorbate due to strong competitive activity between Cu2+ and H+ ions for active sites on the adsorbent. Increasing the pH of the solution led to a decrease in H+, which enabled electrostatic/coordination attraction of Cu2+ onto the active sites of the adsorbents. Cationic adsorption is favourable at pH values higher than pHPZC;55 hence, sorption of Cu2+ was propitious at pH conditions greater than 4.5. Higher removal efficiencies of Cu2+ were noticeable from pH 3 to 7 when MWCNT-ttpy was used as adsorbent. This is attributed to an increase in the number of chelating sites on the adsorbent, which permits strong binding between the nitrogen-donor atoms in the adsorbent and Cu2+. Modification of MWCNT-COOH with HO-Phttpy significantly improved the textural properties of MWCNT-COOH, resulting in an increase in surface area, pore volume (Table 1) and active sites on MWCNT-ttpy (Table 2), hence, enabling better sorption ability for Cu2+ removal. Although the data suggest that pH 7 is optimal for the removal of Cu2+, further adsorption studies were carried out at a pH value of 5, in order to limit the effect of precipitation on Cu2+ removal.
| Model | Parameter | MWCNT-COOH | MWCNT-ttpy |
|---|---|---|---|
| a RSE – residual square error.b SSR – sum of squared residuals. | |||
| Pseudo-first order | k1/min−1 | 0.259 | 0.073 |
| qeq/mg g−1 | 10.06 | 33.30 | |
| RSEa | 0.741 | 3.350 | |
| SSRb | 8.242 | 168.3 | |
| Pseudo-second order | k2/g mg−1 min−1 | 0.044 | 0.003 |
| qeq/mg g−1 | 10.44 | 35.25 | |
| RSE | 0.447 | 1.858 | |
| SSR | 2.994 | 51.77 | |
| Intraparticle diffusion | kid/mg g−1 min−0.5 | 0.491 | 1.573 |
| l/mg g−1 | 7.764 | 14.66 | |
| RSE | 5.639 | 15.64 | |
| SSR | 508.8 | 3916 | |
| Elovich | α/mg g−1 min−1 | 5.237 | 192.7 |
| β/g mg−1 | 1.864 | 0.287 | |
| RSE | 0.217 | 2.082 | |
| SSR | 0.705 | 65.00 | |
Adsorption proceeds via one/more of these four stages: (i) the transfer of solute from the solution to the surface of the adsorbent, (ii) solute transfer from the bulk solution to the boundary film which surrounds the adsorbent surface (film diffusion), (iii) solute transfer through the internal pores of the adsorbent (intraparticle diffusion), and (iv) interaction between adsorbate molecules with the active sites on the external surface of the adsorbent. One of these processes usually determines the rate at which the adsorbate is removed from aqueous solutions. To further elucidate the diffusion mechanism involved in adsorption, experimental data were modelled with the intraparticle diffusion model. A plot of qt against was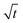 plotted to obtain a straight line which did not pass through the origin. This indicates that adsorption proceeded through the intraparticle diffusion of Cu2+ to the pores of the adsorbent; however, it was not the only rate-controlling step.63–65 Adsorption therefore occurred via a multi-step process involving an initial rapid stage, followed by the intraparticle diffusion of adsorbates to the pores of adsorbents and then to a slower phase which proceeds towards saturation due to low adsorbate concentration.58 Higher kid and l values were obtained for MWCNT-ttpy than MWCNT-COOH, showing that adsorption was boundary-controlled and indicates better Cu2+ removal for MWCNT-ttpy.
plotted to obtain a straight line which did not pass through the origin. This indicates that adsorption proceeded through the intraparticle diffusion of Cu2+ to the pores of the adsorbent; however, it was not the only rate-controlling step.63–65 Adsorption therefore occurred via a multi-step process involving an initial rapid stage, followed by the intraparticle diffusion of adsorbates to the pores of adsorbents and then to a slower phase which proceeds towards saturation due to low adsorbate concentration.58 Higher kid and l values were obtained for MWCNT-ttpy than MWCNT-COOH, showing that adsorption was boundary-controlled and indicates better Cu2+ removal for MWCNT-ttpy.
| Isotherm | Parameter | MWCNT-COOH | MWCNT-ttpy | |||||
|---|---|---|---|---|---|---|---|---|
| 293 K | 303 K | 313 K | 318 K | 303 K | 313 K | 318 K | ||
| a RSE – residual square error.b SSR – sum of squared residuals. | ||||||||
| Langmuir | qm/mg g−1 | 19.44 | 16.87 | 16.35 | 15.59 | 33.04 | 34.13 | 33.97 |
| b/dm3 mg−1 | 0.169 | 0.184 | 0.123 | 0.068 | 5.494 | 9.065 | 17.25 | |
| RSEa | 1.887 | 1.386 | 0.510 | 0.478 | 3.302 | 3.176 | 5.670 | |
| SSRb | 28.48 | 15.37 | 2.077 | 1.828 | 87.25 | 80.71 | 257.2 | |
| Freundlich | KF | 5.244 | 4.756 | 3.876 | 2.533 | 23.88 | 26.05 | 28.54 |
| n | 3.056 | 3.188 | 2.964 | 2.507 | 6.641 | 7.006 | 8.991 | |
| RSE | 0.604 | 0.575 | 0.701 | 0.585 | 4.295 | 4.589 | 5.951 | |
| SSR | 2.922 | 2.644 | 3.926 | 2.740 | 147.6 | 168.5 | 283.3 | |
Table 4 demonstrates that the Freundlich isotherm best describes the experimental data obtained for MWCNT-COOH, while the Langmuir isotherm best described the data obtained for MWCNT-ttpy. The curves for the best-fit isotherms for MWCNT-COOH and MWCNT-ttpy are shown in ESI Fig. S6 and S7,† respectively. The Langmuir maximum adsorption capacity (qm) varied from 15.50 to 19.44 mg g−1 and 31.65 to 34.13 mg g−1 for MWCNT-COOH and MWCNT-ttpy, respectively over the studied temperature range. Table 4 further indicates a decrease in qm as the temperature of the adsorbate solution was increased for MWCNT-COOH, whereas an increase in qm was obtained with increasing temperature for MWCNT-ttpy. The Langmuir adsorption constant, b (Table 4), indicating the high adsorptive binding power obtained for MWCNT-ttpy, is indicative of good adsorptive forces resulting in the enhancement of the binding strength as the temperature is increased.
The amount of area on the adsorbent covered by Cu2+ can be established by dividing the theoretical specific surface area (S) into the BET surface area. The theoretical surface area was calculated by using eqn (3) as described by Ho et al.68
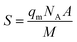 | (3) |
Also, as suggested by Hamza et al.69 and Soon-An et al.,70 the nature of the adsorption process can be estimated depending on the values of the separation factor (RL) as expressed in eqn (4). Adsorption is assumed to be favourable if 0 < RL < 1, unfavourable if RL > 1, irreversible if RL = 0 and linear if RL = 1.69–71
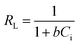 | (4) |
The Langmuir isotherm assumes monolayer coverage of adsorbates onto the surface of adsorbent, while the Freundlich isotherm assumes that the surface of adsorbents is heterogeneous in nature.4 Since MWCNT-ttpy fit the Langmuir isotherm, it can be inferred that the active sites on this adsorbent are equivalent with a uniform surface, hence, the adsorbed Cu2+ ions on this adsorbent do not interact with each other, achieving monolayer coverage of the adsorbate. In the case of MWCNT-COOH, the values of the Freundlich constant n indicate a favourable adsorption process since values were ≤10 (see Table 4).54
Table 5 shows the results of some previously published studies investigating the sorption process of Cu2+ from aqueous solutions onto carbon-structured materials. These results were compared with those obtained in this study. A significant increase in sorption uptake of Cu2+ was obtained by using MWCNT-ttpy in comparison with 8-hydroxyquinoline-functionalized MWCNTs (8-HQ-MWCNT), also functionalized with a N-donor ligand but of lower denticity. Table 5 further demonstrates better uptake of Cu2+ on MWCNT-ttpy in comparison with composite adsorbents such as MWCNT/bagasse and MWCNT/Fe2O4. Thus, the results reported in this study compare very favourably with data obtained from other studies.
| Adsorbents | Conditions | qm/mg g−1 | References |
|---|---|---|---|
| P-MWCNTs | pH 7.0, Ci 1.0 mg dm−3, 250 mg dose, 120 min, 298 K | 0.080 | 72 |
| P-MWCNTs | pH 7.0, Ci 0.5 mg dm−3, 125 mg dose, 120 min, 298 K | 0.398 | 22 |
| MWCNT-COOH | pH 7.0, Ci 20 mg dm−3, 30 mg dose, 120 min, 293 K | 12.34 | 10 |
| 8-HQ-MWCNT | pH 7.0, Ci 1.0 mg dm−3, 250 mg dose, 120 min, 298 K | 0.080 | 72 |
| MWCNT/bagasse | pH 5.5, 100 mg dm−3, 50 mg dose, 360 min, 301 K | 15.60 | 24 |
| MWCNT/Fe2O4 | pH 5.50, 10 mg dm−3, 40 h, 353 K | 8.920 | 25 |
| MWCNT-COOH | pH 5.0, Ci 100 mg dm−3, 50 mg dose, 24 h, 293 K | 19.44 | This study |
| MWCNT-ttpy | pH 5.0, Ci 100 mg dm−3, 50 mg dose, 24 h, 293 K | 31.65 | This study |
The change in Gibbs energy of adsorption (ΔG°) is calculated thus (eqn (5)):14
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K
| (5) |
A plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K against 1/T was found to be linear, and the values of ΔH° and ΔS° were obtained from the slope and intercept of the plot, respectively, according to the van't Hoff equation (eqn (6)):
K against 1/T was found to be linear, and the values of ΔH° and ΔS° were obtained from the slope and intercept of the plot, respectively, according to the van't Hoff equation (eqn (6)):
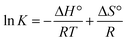 | (6) |
Table 6 presents the thermodynamic parameters for the adsorption of Cu2+ onto MWCNT-COOH and MWCNT-ttpy. Negative values were obtained for ΔG°, indicating that the process was spontaneous and feasible for both adsorbents. An increase in negative values for ΔG° was obtained as the temperature of the adsorbate solution was increased for MWCNT-ttpy. This confirms that increasing the temperature of the adsorbate solution resulted in better sorption for the adsorbent. In contrast, a decrease in the negative values of ΔG° was obtained as temperature is increased when MWCNT-COOH was used as adsorbent. An exothermic process of adsorption was obtained with MWCNT-COOH, indicated by the negative ΔH° value obtained. On the other hand, the application of MWCNT-ttpy as adsorbent yielded an endothermic process, which is confirmed by the positive ΔH° value obtained.
| Adsorbent | T/K | ΔG°/kJ mol−1 | ΔH°/kJ mol−1 | ΔS°/J K−1 mol−1 |
|---|---|---|---|---|
| MWCNT-COOH | 293 | −19.72 | ||
| 303 | −20.26 | |||
| 313 | −19.79 | −32.36 | −99.19 | |
| 318 | −18.42 | |||
| MWCNT-ttpy | 293 | −28.11 | ||
| 303 | −30.50 | |||
| 313 | −32.90 | 50.97 | 211.9 | |
| 318 | −35.11 |
Table 6 further shows a positive ΔS° value for MWCNT-ttpy, which indicates an increase in the disorderliness at the solid/solution interface resulting in a favourable adsorption process. However, a negative ΔS° was obtained for MWCNT-COOH indicating a decrease in disorderliness as the adsorbent interacts with the adsorbate. Hence, the adsorption process for MWCNT-ttpy is entropy-driven, while the process is enthalpy-driven for MWCNT-COOH. The values of ΔH° obtained show that the heat evolved/absorbed was greater than for physisorption (2.1–20.9 kJ mol−1), but lower than for chemisorption processes (80–200 kJ mol−1).4,75 This indicates that the process for the removal of Cu2+ from aqueous solutions by using MWCNT-COOH and MWCNT-ttpy was a physico-chemical process,75 indicating that adsorption was facilitated by both processes.
Thermodynamic parameters therefore suggest that the application of MWCNT-ttpy will be effective for remediating metal-polluted effluents discharged directly from industries.
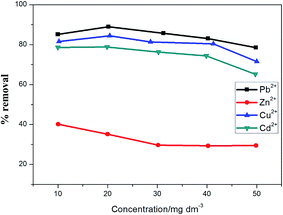
As proposed by Chen et al.,76 the removal of divalent metal ions is inversely proportional to the hydrated radii of the metal species in solution. The ionic radius of Pb2+, Cu2+, Cd2+ and Zn2+ are 1.33 Å, 0.71 Å, 0.95 Å and 0.83 Å respectively. The smaller the ionic radii of the metal ion species, the greater its tendency to hydrolyse, resulting in decreased sorption.24,76,77 Hence, the hydrated radii of the studied metal ions are in the order of Pb2+ (4.01 Å) < Cu2+ (4.19 Å) < Cd2+ (4.29 Å) < Zn2+ (4.30 Å).24,76,77 Metal ions with smaller hydrated radii therefore have easy access to the adsorbent sites, hence accounting for increased sorption in the sequence Pb > Cu > Cd > Zn (Fig. 11). Therefore, this principle could be said to be obeyed, since their removal was in the same order.
The selectivity of metal ions obtained in this study was mostly in agreement with previously reported studies. Srivastava et al.78 and Li et al.79 reported the same sequence order of Pb > Cu > Cd from a multicomponent system onto MWCNT-COOH. Sitko et al.80 and Chen et al.76 also reported similar trends in their studies by using graphene oxide and nano-hydroxyapatite, respectively. However, a sequence order of Pb > Cu > Zn > Cd was reported by Srivastava et al.81 and Sdiri et al.82 from a multicomponent system onto kaolinite and clay, respectively. This order was slightly different from that obtained in this study, hence, the removal of metal ion species is principally dependent on the textural characteristics of the sorbent used.
3.5. Analysis of real samples
In order to affirm the selectivity of MWCNT-ttpy and test its efficacy for metal ion removal in a real life scenario, water samples were collected from three different rivers in Durban, South Africa. The initial metal ion concentrations found in the water samples is given in Table S4.† Interestingly, a removal efficiency greater than 95% was obtained for all three metal ions in the three water samples (Table S4†). Although the initial concentrations of Pb2+, Cu2+ and Zn2+ in samples were in measurable amounts (≥10 mg dm−3), MWCNT-ttpy successfully sequestered these toxic heavy metal ions from the environmental samples. This further authenticates that MWCNT-ttpy and the method of adsorption presented are effective and efficient when applied to real samples. Hence, the application of MWCNT-ttpy should be further explored for the remediation of wastewater or effluents discharged from industries.3.6. Desorption studies
The process of desorption regenerates an adsorbent for reuse, thereby reducing the cost and availability of sorbents for adsorption. This process also limits the introduction of spent adsorbents into the environment, thereby reducing the disposal of secondary pollutants. Similarly, the isolation of copper is possible, and may encourage its reutilization for other valuable purposes.Prior to the desorption of Cu2+ from loaded adsorbents, they were characterized by means of TEM and BET surface area measurements. These were performed in order to confirm the presence of adsorbate on the adsorbents. The data obtained exhibited lower surface areas and pore volumes for both adsorbents after adsorption as compared with their corresponding values before adsorption (Tables 7 vs. 1). This trend can be explained by the increase in agglomeration of nanotubes observed after adsorption as shown in Fig. S8.† The increase in CNT agglomeration after the deposition of Cu2+ onto their surface (Fig. S8†), resulted in a decrease in the surface areas exhibited by both adsorbents (Table 7). Further, the hydrated radius of Cu2+ (0.419 nm)24,77 is low compared with the pore volumes of the synthesized adsorbents (Table 1). Hence, copper species might fill the pore volumes of the adsorbent, thereby resulting in a reduction of the pore volumes of the adsorbents after adsorption (Table 7).
| Adsorbents | Surface area/m2 g−1 | Pore volume/cm3 g−1 | Pore diameter/nm |
|---|---|---|---|
| MWCNT-COOH | 104.22 | 0.314 | 15.18 |
| MWCNT-ttpy | 149.1 | 0.527 | 16.85 |
Desorption experiments were carried out by agitating a Cu2+-loaded adsorbent with 10 cm3 of 0.1 mol dm−3 HCl in a thermostated water bath at 20 °C for 30 min. The mixture was filtered and the final concentration of Cu2+ determined. Results obtained showed good desorption efficiencies of 62% and 73% for MWCNT-COOH and MWCNT-ttpy, respectively. These values signify that the adsorbents can be regenerated for reuse and the adsorbate solution can be isolated as chloride salt and reused for other industrial applications such as electroplating. Again, in this respect MWCNT-ttpy performed better, showing better desorption of Cu2+ than MWCNT-COOH.
4. Conclusions
A novel nanomaterial, synthesized by the use of 4′-(4-hydroxyphenyl)-2,2′:6′,2′′-terpyridine as a modifier for acid-functionalized multiwalled carbon nanotubes (MWCNT-COOH), to produce nitrogen-functionalized MWCNTs (MWCNT-ttpy), was successfully synthesized. The adsorbent proved effective and efficient for the removal of Cu2+ from aqueous solutions, obtaining a higher Langmuir monolayer adsorption capacity (qm) of 31.65 mg g−1 than the 19.44 mg g−1 obtained for MWCNT-COOH. Also, a higher sorption uptake of Cu2+ was exhibited by MWCNT-ttpy when compared with other MWCNT-containing sorbents from other studies.This study revealed that MWCNT-ttpy can be used as an alternative adsorbent for the removal of heavy metal ions from municipal wastewater and industrial effluents, due to the high efficiency exhibited towards Cu2+ removal from aqueous solution. The regeneration of this adsorbent was successful, suggesting that the spent adsorbent and Cu2+ can be recovered and made available for reuse, and thereby reducing secondary pollutants.
Acknowledgements
The authors wish to thank the University of KwaZulu-Natal, Durban, for research facilities and the National Research Foundation (NRF) for provision of funds for the completion of this work.References
- Z. Zhao, Z. Yang, Y. Huc, J. Li and X. Fan, Appl. Surf. Sci., 2013, 276, 476–481 CrossRef CAS.
- N. Arora and N. N. Sharma, Diamond Relat. Mater., 2014, 50, 135–150 CrossRef CAS.
- L. M. Ombaka, P. Ndungu and V. O. Nyamori, Catal. Today, 2013, 217, 65–75 CrossRef CAS.
- O. A. Oyetade, V. O. Nyamori, B. S. Martincigh and S. B. Jonnalagadda, RSC Adv., 2015, 5, 22724–22739 RSC.
- B. Pan and B. Xing, Environ. Sci. Technol., 2008, 42, 9005–9013 CrossRef CAS PubMed.
- G. Keru, P. G. Ndungu and V. O. Nyamori, Int. J. Energy Res., 2014, 38, 1635–1653 CrossRef CAS.
- L. M. Ombaka, P. G. Ndungu and V. O. Nyamori, RSC Adv., 2014, 5, 109–122 RSC.
- L. Hou and D. Li, Inorg. Chem. Commun., 2005, 8, 190–193 CrossRef CAS.
- M. Chua and C.-K. Chui, J. Mech. Behav. Biomed. Mater., 2015, 44, 164–172 CrossRef CAS PubMed.
- I. Mobasherpour, E. Salahi and M. Ebrahimi, J. Saudi Chem. Soc., 2014, 18, 792–801 CrossRef.
- C. M. A. Iwegbue, Vet. Arh., 2008, 78, 401–410 CAS.
- I. Giannopoulou and D. Panias, Hydrometallurgy, 2008, 90, 137–146 CrossRef CAS.
- M. Abdel Salam, G. Al-Zhrani and S. A. Kosa, J. Ind. Eng. Chem., 2014, 20, 572–580 CrossRef CAS.
- Y.-S. Ho, Water Res., 2003, 37, 2323–2330 CrossRef CAS PubMed.
- P. L. Homagai, K. N. Ghimire and K. Inoue, Bioresour. Technol., 2010, 101, 2067–2069 CrossRef CAS.
- H. D. Özsoy, H. Kumbur and Z. Özer, Int. J. Environ. Pollut., 2007, 31, 125–134 CrossRef.
- M. G. A. Vieira, A. F. de Almeida Neto, M. G. C. Da Silva, C. N. Carneiro and A. A. Melo Filho, Braz. J. Chem. Eng., 2014, 31, 519–529 CrossRef.
- S. Sai Bhargav and I. Prabha, Int. J. Chem. Biol. Eng., 2013, 3, 107–112 Search PubMed.
- Y. S. Ho and G. McKay, Water, Air, Soil Pollut., 2004, 158, 77–97 CrossRef CAS.
- H. Liu, S. Feng, N. Zhang, S. Du and Y. Liu, Front. Environ. Sci. Eng., 2014, 8, 329–336 CrossRef CAS.
- Y. Jiang, H. Pang and B. Liao, J. Hazard. Mater., 2009, 164, 1–9 CrossRef CAS PubMed.
- M. Abdel Salam, Int. J. Environ. Sci. Technol., 2013, 10, 677–688 CrossRef CAS.
- M. S. Tehrani, P. A. Azar, P. Ehsani Namin and S. M. Dehaghi, J. Appl. Environ. Biol. Sci., 2014, 4, 316–326 Search PubMed.
- I. A. A. Hamza, PhD Thesis, University of KwaZulu-Natal, Durban, South Africa, 2013.
- J. Hu, D. Zhao and X. Wang, Water Sci. Technol., 2011, 63, 917–923 CAS.
- S. R. Popuri, R. Frederick, C.-Y. Chang, S.-S. Fang, C.-C. Wang and L.-C. Lee, Desalin. Water Treat., 2013, 52, 691–701 CrossRef.
- S. Biniak, G. Szymanski, J. Siedlewski and A. Swiatkowski, Carbon, 1997, 35, 1799–1810 CrossRef CAS.
- H. P. Boehm, Carbon, 2002, 40, 145–149 CrossRef CAS.
- T. A. Khan, M. Nazir and E. A. Khan, Toxicol. Environ. Chem., 2013, 95, 919–931 CrossRef CAS.
- M. N. Patel, P. A. Dosi and B. S. Bhatt, Med. Chem. Res., 2012, 21, 2723–2733 CrossRef CAS.
- M. N. Patel, H. N. Joshi and C. R. I. Patel, Polyhedron, 2012, 40, 159–167 CrossRef CAS.
- S. Santangelo, G. Messina, G. Faggio, S. H. Abdul Rahim and C. Milone, J. Raman Spectrosc., 2012, 43, 1432–1442 CrossRef CAS.
- J. Shen, W. Huang, L. Wu, Y. Hu and M. Ye, Mater. Sci. Eng., A, 2007, 464, 151–156 CrossRef.
- N. Sakai, T. Moriya and T. Konakahara, J. Org. Chem., 2007, 72, 5920–5922 CrossRef CAS PubMed.
- Y. S. Ho, Water Res., 2004, 38, 2962–2964 CrossRef CAS PubMed.
- Y.-S. Ho, J. Hazard. Mater., 2006, 136, 681–689 CrossRef CAS PubMed.
- S. H. Chien and W. R. Clayton, Soil Sci. Soc. Am. J., 1980, 44, 265–268 CrossRef CAS.
- E. Demirbas, M. Kobya, E. Senturk and T. Ozkan, Water SA, 2004, 30, 533–539 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1402 CrossRef CAS.
- H. Freundlich, Z. Phys. Chem., 1906, 57, 385–470 CAS.
- M. I. Temkin and V. Pyzhev, Acta Phys. Chim., 1940, 12, 327–356 CAS.
- M. M. Dubinin and L. V. Radushkevich, Proc. Acad. Sci. USSR, Phys. Chem. Sect., 1947, 55, 327–329 CAS.
- R. Sips, J. Chem. Phys., 1948, 16, 490–495 CrossRef CAS.
- J. Toth, Acta Chim. Acad. Sci. Hung., 1971, 69, 311–328 CAS.
- O. Redlich and D. L. Peterson, J. Phys. Chem., 1959, 63, 1024 CrossRef CAS.
- A. R. Khan, I. R. Al-Waheab and A. Al-Haddad, Environ. Technol., 1996, 17, 13–23 CrossRef CAS.
- The R Development Core Team, The R foundation for statistical Computing, R version 3.0.2, 2013 Search PubMed.
- S. Narimany and S. Ghammamy, Global J. Pharmacol., 2013, 7, 187–191 CAS.
- T. L. Yang, W. W. Qin, Z. F. Xiao and W. S. Liu, Chem. Pap., 2005, 59, 17–20 CAS.
- W. Huang, C. Li, J. Wang and L. Zhu, Spectrosc. Lett., 1998, 31, 1793–1809 CrossRef CAS.
- I. D. Rosca, F. Watari, M. Uo and T. Akasaka, Carbon, 2005, 43, 3124–3131 CrossRef CAS.
- K. Chizari, A. Vena, L. Laurentius and U. Sundararaj, Carbon, 2014, 68, 369–379 CrossRef CAS.
- Z. Wang, M. D. Shirley, S. T. Meikle, R. L. D. Whitby and S. V. Mikhalovsky, Carbon, 2009, 47, 73–79 CrossRef CAS.
- K. S. Tong, M. J. Kassim and A. Azraa, Chem. Eng. J., 2011, 170, 145–153 CrossRef CAS.
- M. A. Salleh, D. K. Mahmoud, W. A. Abdul Karim and A. Idris, Desalination, 2011, 280, 1–13 CrossRef CAS.
- R. M. Smith and A. E. Martell, Critical Stability Constants, Plenum Press, New York, 1976, p. 6 Search PubMed.
- P. Gans, HySS, version 4.0.31, 2009 Search PubMed.
- K. V. Kumar, J. Hazard. Mater., 2006, 137, 1538–1544 CrossRef CAS PubMed.
- J. Lin and L. Wang, Front. Environ. Sci. Eng., 2009, 3, 320–324 CrossRef CAS.
- Y. S. Ho and C. C. Wang, Process Biochem., 2004, 39, 759–763 CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- B. Yu, Y. Zhang, A. Shukla, S. S. Shukla and K. L. Dorris, J. Hazard. Mater., 2000, B80, 33–42 CrossRef.
- S. G. Muntean, M. E. Radulescu-Grad and P. Sfarloaga, RSC Adv., 2014, 4, 27354–27362 RSC.
- C. H. Wu, J. Hazard. Mater., 2007, 144, 93–100 CrossRef CAS PubMed.
- Y. Yao, F. Xu, M. Chen, Z. Xu and Z. Zhu, Bioresour. Technol., 2010, 101, 3040–3046 CrossRef CAS PubMed.
- F. Ekmekyapar, A. Aslan, Y. K. Bayhan and A. Cakici, J. Hazard. Mater., 2006, 137, 293–298 CrossRef CAS PubMed.
- T. A. Khan, S. Dahiya and I. Ali, Appl. Clay Sci., 2012, 69, 58–66 CrossRef CAS.
- Y.-S. Ho, Scientometrics, 2004, 59, 171–177 CrossRef CAS.
- I. A. A. Hamza, B. S. Martincigh, J. C. Ngila and V. O. Nyamori, Phys. Chem. Earth, 2013, 66, 157–166 CrossRef.
- O. Soon-An, S. Chye-Eng and L. Poh-Eng, EJEAFChe, Electron. J. Environ., Agric. Food Chem., 2007, 6, 764–1774 Search PubMed.
- T. K. Sen and D. Gomez, Desalination, 2011, 267, 286–294 CrossRef CAS.
- S. A. Kosa, G. Al-Zhrani and M. Abdel Salam, Chem. Eng. J., 2012, 181–182, 159–168 CrossRef CAS.
- R. Djeribi and Q. Hamdaoui, Desalination, 2008, 225, 95–112 CrossRef CAS.
- S. K. Milonjić, J. Serb. Chem. Soc., 2007, 72, 1363–1367 CrossRef.
- Y. Liu and Y.-J. Liu, Sep. Purif. Technol., 2008, 61, 229–242 CrossRef CAS.
- S. B. Chen, Y. B. Ma, L. Chen and K. Xian, Geochem. J., 2010, 44, 233–239 CrossRef CAS.
- L. Lv, G. Tsoi and X. Zhao, Ind. Eng. Chem. Res., 2004, 43, 7900–7906 CrossRef.
- S. Srivastava, Adv. Mater. Lett., 2013, 4, 2–8 Search PubMed.
- Y.-H. Li, J. Ding, Z. Luan, Z. Di, Y. Zhu, C. Xu, D. Wu and B. Wei, Carbon, 2003, 41, 2787–2792 CrossRef CAS.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Dalton Trans., 2013, 42, 5682–5689 RSC.
- P. Srivastava, B. Singh and M. Angove, J. Colloid Interface Sci., 2005, 290, 28–38 CrossRef CAS PubMed.
- A. Sdiri, T. Higashi, R. Chaabouni and F. Jamoussi, Water, Air, Soil Pollut., 2011, 223, 1191–1204 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23900a |
| This journal is © The Royal Society of Chemistry 2016 |