Etherification over Hβ zeolite modified by lanthanum ion exchange combined with low-temperature steam treatment
Ximing Yan,
Ming Ke*,
Zhaozheng Song,
Qingzhe Jiang and
Pei Yu
State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing, China. E-mail: keming@cup.edu.cn; Tel: +86 10 89733372
First published on 21st January 2016
Abstract
Lanthanum ion exchange, low-temperature steam treatment and La/steam combined modification were used to modify Hβ zeolite, and then comparative studies were performed on the influences of single and compound modified methods on the etherification activity. XRD, XRF, BET, FETEM-EDS, FTIR, OH-FTIR, NH3-TPD and Py-IR analysis methods were used to characterize the catalysts. The evaluation results show that the best modification method is lanthanum ion exchange followed by low-temperature steam treatment; the highest conversion of isoamylene was 67.72% and the yield of TAME was 65.63%. The characterization results implied that large pore sizes, high acidity, moderate acid strength and a suitable Brønsted acid proportion are all favourable for etherification.
1. Introduction
With increasingly stringent environmental requirements, developing low cost and green procedures to improve the quality of gasoline has become an urgent matter. The quality requirements for gasoline include low sulphur content, low olefin content, low aromatics content and high octane number. On this basis, a method called as light gasoline etherification technology has been developed for producing clean gasoline, which not only reduces the olefin content and vapor pressure, but also increases the octane number and oxygen content. This technology converts active olefins in FCC light gasoline into alkyl ether compounds with high octane numbers. Common ethers such as methyl tert-butyl ether (MTBE), methyl tert-amyl ether (TAME), and ethyl tert-butyl ether (ETBE) are good octane enhancers, which improve the anti-knocking performance and solubility of gasoline, as well as reduce the content of CO in the tail gas.1Etherification is an acid catalytic reaction, catalysts generally including macroporous sulfonic acid resins, molecular sieves, and solid heteropolyacids. Molecular sieves are considered to be a potential substitute for acid resins due to significant advantages, such as adjustable pore structure and acidity, excellent thermal stability and easy regeneration after inactivation, although they show poor activity at low temperatures. Several zeolites have been researched with respect to etherification, wherein β zeolite is most frequently researched, due to its higher etherification activity compared to other zeolites.2–8 β-Zeolite was first synthesized by Wadlinger in 1967, which has a unique three-dimensional 12-ring topology and suitable acidity, it is widely applied in hydrocracking, alkylation, isomerization, and esterification.9–12
Molecular sieves are commonly modified by metal ion exchange. Due to their stable electronic structure, high magnetic moments, variable valency state and empty 5d orbital, rare earth metals usually have a high catalytic activity, making them good “electron transfer stations”. Moreover, RE–O–RE bonds present in cavities can bridge with a zeolite tetrahedral framework, stabilizing the structure; this can drastically improve the thermal stability of the catalyst.13,14 Steam treatment is often used in catalyst modification because it can remove framework aluminum and reduce the quantity of acid, especially for strong acid sites, thus improving the activity and stability of the catalyst.15,16
There have been some studies on etherification using a single-modification method, such as metal ion exchange, acid leaching and steam treatment, but there has been no research on etherification using catalysts modified by lanthanum ion exchange combined with low-temperature steam treatment. The purpose of this study is to explore the possible uses of etherification catalysts, and therefore lanthanum ion exchange, low-temperature steam treatment and La/steam combined modification were used to modify Hβ zeolite. Comparative studies were performed on the influences of single and compound modification on the etherification activity, and the most suitable modification method was selected for the preparation of highly active catalysts. In addition, using a variety of characterization methods, relevant studies were performed on the influences of these modified methods upon the catalysts' crystal phase, pore structure, frame structure and acid quality.
2. Experimental
2.1 Materials
Commercial Hβ zeolite and pseudo-boehmite were purchased from the Catalyst Plant of Nankai University and the Shandong Aluminum Inc., respectively. Sesbania powder was provided by the Sinopec Research Institute of Petroleum Processing. Lanthanum nitrate and nitric acid were purchased from Aladdin Industrial Inc., Shanghai, China.2.2 Catalyst preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, then an appropriate amount of sesbania powder (extrusion assistant) and 5 wt% nitric acid solution (peptizer) was added before extruding the product into strips, drying at 110 °C overnight and calcining in air at 550 °C for 4 h. Finally, the catalyst was milled to a size of 0.42–0.84 mm; the geometric mean diameter was 0.63 mm.
3, then an appropriate amount of sesbania powder (extrusion assistant) and 5 wt% nitric acid solution (peptizer) was added before extruding the product into strips, drying at 110 °C overnight and calcining in air at 550 °C for 4 h. Finally, the catalyst was milled to a size of 0.42–0.84 mm; the geometric mean diameter was 0.63 mm.2.3 Physical and chemical characterization
Structural characterization was performed by powder XRD, with a Bruker D8 Advance X-ray diffractometer made in Germany, using Cu Kα (λ = 0.15406 nm) radiation with a nickel filter. Samples were scanned at 40 kV and 30 mA in the range of 5–90° at a scanning rate of 4° min−1. The crystalline phases were identified by matching them with the reference patterns in the Joint Committee on Powder Diffraction Standards (JCPDS) database. The textural characterization of solids was performed by N2 adsorption–desorption using a US Micromeritics ASAP2400 static nitrogen adsorption analyser. First, samples were degassed at 300 °C for 8 h. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method in the relative pressure range of 0 < p/p0 < 0.1. The total pore volume was calculated using the absorbed N2 volume at a relative pressure of 0.99, whereas pore size distributions were determined by the Barrett–Joyner–Halenda (BJH) method. Elemental analyses were performed with an Axiosm AX XRF analyser made in the Netherlands. TEM images were obtained using an FEI F20 field emission transmission electron microscope (FETEM) and elemental local, mapping and line-scanning analyses were acquired by energy dispersive spectroscopy (EDS). Fourier transform infrared (FT-IR) absorbance spectra were recorded on a US MAGNA-IR560 spectrometer in the wavenumber range of 400–4000 cm−1; the wafer was prepared by mixing the powdered solids with pressed KBr disks in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and dried overnight. The acid properties of the catalysts were determined by the ammonia temperature programmed adsorption–desorption (NH3-TPD) method, using a US Autosorb-1C-TCD-MS chemisorption analytical instrument. First, the samples were preheated in high purity argon at 550 °C for 1 h and saturated with NH3 at 100 °C for 1 h, and then the physically adsorbed NH3 was removed; finally, the operation was carried out from 100 °C to 600 °C at a heating rate of 10 °C min−1, and the amount of NH3 desorbed was monitored by mass spectrometry (MS). In addition, the acid type was determined by Py-IR on a US MAGNAIR-IR560 IR instrument. The samples were pressed into self-supported wafers and activated at 450 °C for 0.5 h, followed by pyridine adsorption at ambient temperature for 0.5 h; Py-IR spectra were then recorded at temperatures of 200 and 350 °C.
100 and dried overnight. The acid properties of the catalysts were determined by the ammonia temperature programmed adsorption–desorption (NH3-TPD) method, using a US Autosorb-1C-TCD-MS chemisorption analytical instrument. First, the samples were preheated in high purity argon at 550 °C for 1 h and saturated with NH3 at 100 °C for 1 h, and then the physically adsorbed NH3 was removed; finally, the operation was carried out from 100 °C to 600 °C at a heating rate of 10 °C min−1, and the amount of NH3 desorbed was monitored by mass spectrometry (MS). In addition, the acid type was determined by Py-IR on a US MAGNAIR-IR560 IR instrument. The samples were pressed into self-supported wafers and activated at 450 °C for 0.5 h, followed by pyridine adsorption at ambient temperature for 0.5 h; Py-IR spectra were then recorded at temperatures of 200 and 350 °C.
2.4 Etherification activity measurement
Measurements of etherification activity were carried out in a self-assembled fixed-bed down-flow reactor. In each test, 5 mL catalyst was placed in the thermostatic section of the reactor, while the rest was filled with porcelain balls. Typically, a feedstock of hexane, hexene and isopentene (mass ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was fed into the reactor with a double-plunger pump. Through experiments to eliminate internal and external diffusion, a suitable liquid hourly space velocity and a catalyst particle size were determined. The reaction was carried out under the optimum conditions: the methanol/isopentene molar ratio was 1.0, the reaction temperature was 80 °C, pressure was maintained at 1 MPa, and the liquid hourly space velocity was 1 h−1. After reaching a steady state of reaction, the products were collected and analyzed using gas chromatography (SP3420A), with a PONA capillary column and a flame ionization detector, and then identified by GC/MS analysis.
2) was fed into the reactor with a double-plunger pump. Through experiments to eliminate internal and external diffusion, a suitable liquid hourly space velocity and a catalyst particle size were determined. The reaction was carried out under the optimum conditions: the methanol/isopentene molar ratio was 1.0, the reaction temperature was 80 °C, pressure was maintained at 1 MPa, and the liquid hourly space velocity was 1 h−1. After reaching a steady state of reaction, the products were collected and analyzed using gas chromatography (SP3420A), with a PONA capillary column and a flame ionization detector, and then identified by GC/MS analysis.
The isopentene conversion (Cisop) is defined in eqn (1):
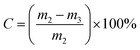 | (1) |
The selectivity to TAME (STAME) is defined in eqn (2):
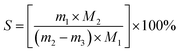 | (2) |
The yield of TAME (YTAME) was calculated using eqn (3):
| Y = C × S | (3) |
3. Results
The etherification performances of xLa/HBC, T–t/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC were tested and the results are shown in Table 1. The activity of HBC was also investigated for comparison. In these experiments, all the samples reached the highest activity at about 80 °C, except for the blank sample, HBC. For HBC, the maximum isopentene conversion was 48.07%, which was achieved at 90 °C, while the maximum Cisop was only 39.25% at a temperature of 80 °C. This shows that the etherification activity of HBC is relatively lower at 80 °C, while it reaches its maximum activity at a higher temperature than the modified samples. As a reversible and exothermic reaction, etherification will be dampened at higher reaction temperatures and side reactions will be intensified, as well as the stability and lifetime of catalysts will be negatively affected. After introducing lanthanum into the HBC, the isopentene conversion increased, while the selectivity decreased slightly. Among all the lanthanum ion-exchange samples, the 0.1La/HBC catalyst exhibits the best etherification performance, with the isopentene conversion above 61%, implying that an over-high concentration of lanthanum nitrate is bad for the reaction. Generally, low-temperature steam treatment can improve the conversion rate and selectivity; however, in this reaction, as the treatment temperature increased, the conversion rate increased slightly, reaching a maximum at 400 °C, and subsequently the activity decreased rapidly as the temperature continued to rise. Steam treatment time also affects the etherification activity; it has been proved that the most suitable steam treatment time is 3 h. However, the two combined methods, which have different sequences of lanthanum exchange and steam treatment, both showed improved effects on the etherification activity, especially 0.1La(400-3)/HBC, which achieved a higher yield of TAME (65.63%) than that of 400-3(0.1La)/HBC under the optimal conditions.| Sample | Cisop (%) | STAME (%) | YTAME (%) |
|---|---|---|---|
| a The optimum temperature was 90 °C, other conditions remain unchanged. When the temperature was 80 °C, Cisop was 39.25%, STAME was 99.31%, and Y was 38.98%. | |||
| HBCa | 48.07 | 98.47 | 47.33 |
| 0.05La/HBC | 50.64 | 98.43 | 49.84 |
| 0.1La/HBC | 61.04 | 97.40 | 59.45 |
| 0.15La/HBC | 59.88 | 97.06 | 58.12 |
| 0.4La/HBC | 48.35 | 98.31 | 47.53 |
| 300-1/HBC | 48.76 | 99.74 | 48.63 |
| 400-1/HBC | 49.86 | 99.69 | 49.71 |
| 500-1/HBC | 42.21 | 99.95 | 42.19 |
| 400-2/HBC | 55.60 | 98.90 | 54.99 |
| 400-3/HBC | 57.05 | 99.71 | 56.88 |
| 400-4/HBC | 49.02 | 98.78 | 48.42 |
| 0.1La(400-3)/HBC | 67.72 | 98.37 | 65.63 |
| 400-3(0.1La)/HBC | 61.92 | 98.90 | 60.25 |
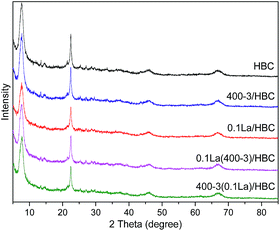
XRD patterns of HBC, 0.1La/HBC, 400-3/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC are shown in Fig. 1. γ-Al2O3 diffraction peaks are at 2θ = 37.63°, 39.52°, 45.82°, and 66.82° (JCPDS PDF-29-0063),17 while peaks at 2θ = 7.70°, 11.68°, 21.46°, and 22.42° belong to Hβ zeolite (JCPDS PDF-48-0074).18,19 As shown in Fig. 1, all the samples exhibit typical peaks of γ-Al2O3 and Hβ zeolite. The phases of La2O3 are not detected in 0.1La/HBC.20 These results suggest that the crystal structures of these catalysts remained intact during the modification process and the lanthanum species were highly dispersed on the catalyst surface, under the monolayer dispersion threshold.21 Fig. 1 also shows the intensities of the diffraction peaks that were reduced after La exchange and steam treatment. The crystallinity of HBC was set as 100%, and the relative crystallinities of other samples are listed in Table 2, in which the weight percentage of lanthanum oxide is also listed. These results demonstrate that the relative crystallinities of all the modified samples decreased, and the influence of steam treatment on the crystallinity was smaller than that of lanthanum exchange. Among the samples, the crystallinity of 400-3(0.1La)/HBC was the lowest, and the crystallinity of 0.1La/HBC was slightly increased after steam treatment, which implies that steam treatment followed by lanthanum exchange will further decrease the crystal stability. It is worth noting that destruction of the crystal structure caused by lanthanum exchange can be repaired by low-temperature steam treatment. On the one hand, due to the higher absorption coefficient of lanthanum compounds for X-rays, La3+ incorporated into Na+ or Al3+ cationic sites probably leads to a decrease in catalyst crystallinity.22 An acid environment induces some Al species to enter from the framework into solution, resulting in a reduction in crystallinity. However, low-temperature steam treatment can remove non-framework aluminum and clear the channels.16 In addition, the content of La2O3 in 400-3(0.1La)/HBC is the same as that in 0.1La/HBC, while the relative crystallinity of 400-3(0.1La)/HBC was further decreased. The weight percentage of lanthanum oxide in 0.1La(400-3)/HBC was decreased, while its crystallinity was improved. This may be due to the loss of a small portion of lanthanum oxide during the hydrothermal process, thus the relative crystallinity can be restored.
Nitrogen adsorption–desorption isotherms for HBC, 0.1La/HBC, 400-3/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC are shown in Fig. 2. The isotherm for HBC is similar to the type-IV isotherm, which is characteristic of mesoporous materials and indicates an average pore diameter of 4.47 nm (see Table 3). An H4-type hysteresis loop appears for the untreated sample; a large hysteresis loop usually indicates that the pore diameter distribution is relatively wide. The shapes of the adsorption–desorption isotherms and the hysteresis loops remain unchanged after modification. Although steam treatment can reduce specific areas, the pore volume and pore size increased, which indicates that the removal of non-framework aluminum by steam treatment will connect some micropores to form secondary pores. However, some of the mesopores collapsed. Although lanthanum incorporation led to a dramatic decrease in specific area, especially the external surface area, the mesopore size significantly increased. This is because lanthanum species covered the external surface and blocked micropores. The BET surface area of 0.1La/HBC was slightly decreased after steam treatment, but the pore volume and pore size increased. The micropore area of 400-3(0.1La)/HBC was decreased, and the pore volume and pore size also decreased significantly, while the external surface area increased. This means that the modification method, irrespective of the order of the two steps, will always further reduce the micropore area and increase the external surface area. On the other hand, lanthanum ion exchange followed by low-temperature steam treatment was more successful in increasing the total pore volume and average pore size; this was due to the dealuminating effect of steam treatment, which enhanced the rearrangement of the surface structure of 0.1La/HBC and the generation of secondary pores.15
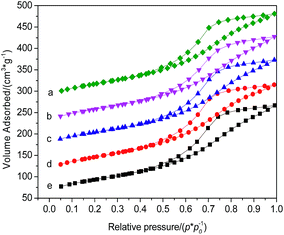 | ||
| Fig. 2 Nitrogen adsorption–desorption isotherms of (a) HBC, (b) 400-3/HBC, (c) 0.1La/HBC, (d) 400-3(0.1La)/HBC and (e) 0.1La(400-3)/HBC. | ||
| Sample | Specific area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | |||||
|---|---|---|---|---|---|---|---|---|
| SBET | Smicro | Sexternal | VTotal | Vmicro | VBJH | Daverage | DBJH | |
| HBC | 381.5 | 155.6 | 225.9 | 0.427 | 0.074 | 0.343 | 4.47 | 6.13 |
| 400-3/HBC | 368.4 | 149.7 | 218.7 | 0.429 | 0.071 | 0.348 | 4.66 | 6.48 |
| 0.1La/HBC | 355.5 | 146.8 | 208.7 | 0.422 | 0.070 | 0.346 | 4.75 | 6.54 |
| 0.1La(400-3)/HBC | 351.7 | 136.7 | 215.0 | 0.428 | 0.065 | 0.300 | 4.87 | 6.50 |
| 400-3(0.1La)/HBC | 363.5 | 132.2 | 231.3 | 0.425 | 0.063 | 0.327 | 4.68 | 6.05 |
The FETEM images of pure γ-Al2O3 and pure Hβ zeolite are shown in Fig. 3a and b. It can be seen that pure γ-Al2O3 shows a typical layered or wrinkled laminated structure,23,24 while Hβ zeolite is made up of relatively larger particles formed from aggregated particles with diameters of 20–50 nm.25 From Fig. 3c–g, it can be seen that the microstructures of all the samples are similar to each other and that the structure of γ-Al2O3 and Hβ zeolite still exist in all the samples. It can be clearly seen that there are wormlike porous channels in a crisscross distribution on γ-Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23,24 and that the Hβ nanoparticles have long-range order, in which intergranular porous channels resulting from aggregation of nanoparticles can be seen.25 In addition, EDS analysis results for 0.1La/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC show that the surfaces of all these samples contain La. Taking 0.1La (400-3)/HBC as an example, the EDS mapping scanning results are shown in Fig. 4. The highlighted part in one of the images is where Si is distributed, which represents Hβ nanoparticles, and it is worth noting that La also aggregated in this part, thus it can be speculated that lanthanum ion exchange mainly occurs on Hβ zeolite and lanthanum is homogeneously distributed on the surface of Hβ zeolite.
23,24 and that the Hβ nanoparticles have long-range order, in which intergranular porous channels resulting from aggregation of nanoparticles can be seen.25 In addition, EDS analysis results for 0.1La/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC show that the surfaces of all these samples contain La. Taking 0.1La (400-3)/HBC as an example, the EDS mapping scanning results are shown in Fig. 4. The highlighted part in one of the images is where Si is distributed, which represents Hβ nanoparticles, and it is worth noting that La also aggregated in this part, thus it can be speculated that lanthanum ion exchange mainly occurs on Hβ zeolite and lanthanum is homogeneously distributed on the surface of Hβ zeolite.
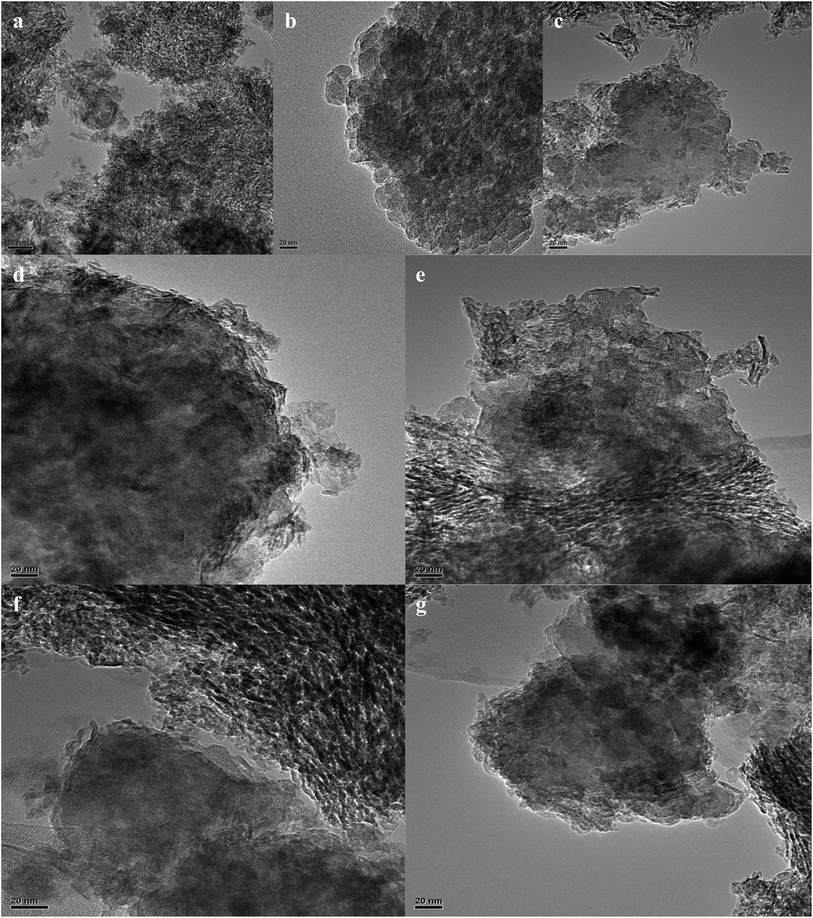 | ||
| Fig. 3 FETEM images of (a) pure γ-Al2O3, (b) pure Hβ, (c) HBC, (d) 0.1La/HBC, (e) 400-3/HBC, (f) 0.1La(400-3)/HBC and (g) 400-3(0.1La)/HBC. | ||
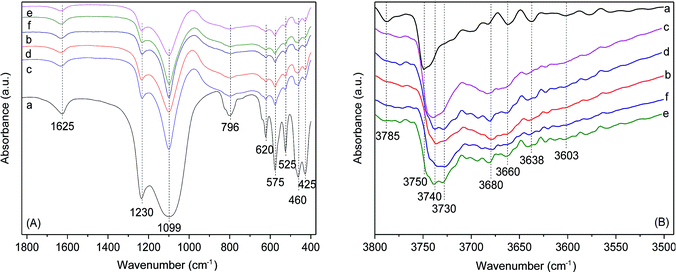
FT-IR spectra of Hβ, HBC, 0.1La/HBC, 400-3/HBC and La/steam-modified samples are shown in Fig. 5A. As can be seen from the spectra of pure Hβ, the bands below 700 cm−1 suggest the characteristic framework vibrations. The band at 425 cm−1 can be attributed to the bending vibration of the T–O framework, and the band at 460 cm−1 is a characteristic peak of an Hβ zeolite pore structure. The bands at 525 cm−1 and 575 cm−1 are assigned to six-membered and four-membered-ring vibrations, respectively. The band present at 620 cm−1 corresponds to the characteristic vibration of a double ring. The band at around 796 cm−1 can be attributed to the symmetric stretching vibration of T–O–T and the band at around 1100 cm−1 can be attributed to asymmetric vibrations, which are highly sensitive to the content of framework silicon and aluminum. The band at 1230 cm−1 is associated with the asymmetric external tetrahedron vibrations of TO4. The hydroxyl bending vibration of physisorbed water is observed at 1625 cm−1.18 Fig. 5A shows that the characteristic peak position of HBC is basically consistent with that of pure Hβ, while the peak intensity is obviously decreased. The spectra of all the modified samples exhibit similar structural characteristics to the basal catalyst, which indicates that the addition of binder (alumina) has no obvious effect on the framework structure of Hβ zeolite. The two absorption peaks at about 782 cm−1 and 617 cm−1 are characteristic vibration peaks of Al–O in γ-Al2O3, which overlap with the T–O–T vibration of β zeolite.26,27 In addition, lanthanum ion exchange and low-temperature steam treatment cause no damage to the framework structure of Hβ zeolite, and no vibrational peaks of La–O in bulk La2O3 were observed.
Fig. 5B shows the FT-IR spectra of hydroxyl groups of Hβ, HBC, 0.1La/HBC, 400-3/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC in the 3800–3500 cm−1 range. The band at 3785 cm−1 is attributed to OH in Al–OH groups, which was found to have some acidic characteristics.28 The main peak at 3750 cm−1 is assigned to the OH stretching vibration of isolated silanol groups, which are located both on the external surface of the zeolite microcrystals and at defect positions in the zeolite framework.29 The band present at 3660 cm−1 is assigned to the stretching of isolated OH groups, which are attached to extra-framework Al3+ species. The band at 3603 cm−1 is attributed to the Si(OH)Al bridging hydroxyl groups, which were found to be associated with the Brønsted acid center.30 Fig. 5B shows that the main peak of Hβ (3750 cm−1) red shifted to 3740 cm−1 after being kneaded with the alumina, which may be due to the formation of more stable hydrogen bonds between the silanol groups and hydroxyl aluminum. In addition, the peaks at 3680 cm−1 and 3730 cm−1, which are derived from the weak acid sites in the alumina, show significantly increased intensities.24 On the other hand, single lanthanum ion exchange and steam treatment (especially low-temperature steam treatment) can increase the peak intensity at 3740 cm−1, and both single-modification methods are beneficial with respect to the formation of hydrogen bonds. Moreover, after the combination of both modification methods, the peak intensity at 3740 cm−1 remains unchanged, while the band at 3680 cm−1 was obviously weakened, which means that combined modification can cover the weak acid sites on alumina.
Temperature-programmed desorption of ammonia (NH3-TPD) was carried out to determine the strength and quantity of different acid sites, wherein the acidic strength can be determined by the desorption temperature, and the quantity of different acid sites can be calculated according to the amount of desorbed ammonia. The NH3-TPD profiles of HBC, 400-3/HBC, 0.1La/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC are presented in Fig. 6. All the samples show two obvious desorption peaks, among which the peak at the lower temperature can be attributed to physisorbed NH3 or NH4+ species, while the peak at the higher temperature is assigned to NH3 that was strongly adsorbed at the strong Brønsted acid sites.31
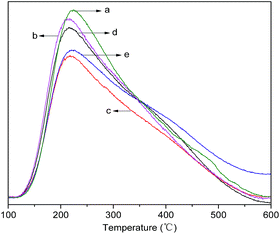 | ||
| Fig. 6 NH3-TPD profiles of (a) HBC, (b) 400-3/HBC, (c) 0.1La/HBC, (d) 0.1La(400-3)/HBC and (e) 400-3(0.1La)/HBC. | ||
The curve area integral can characterize the acid content in different catalysts, and the integral results showed that the acid contents in the catalysts were in the sequence HBC > 0.1La(400-3)/HBC > 400-3/HBC > 400-3(0.1La)/HBC > 0.1La/HBC; moreover, the acid content in all the modified catalysts were decreased by different degrees. Among them, the lanthanum exchanged sample has the minimum quantity of acid, while 0.1La(400-3)/HBC has the maximum acidity, the ratio of strong acid to weak acid being relatively low. However, 400-3(0.1La)/HBC has the largest acid ratio, while 400-3/HBC has the lowest ratio. In addition, from Fig. 6, it can be seen that the peak temperatures of weak acids and strong acids for the modified catalysts are moving toward the low-temperature zone, which means that the acid strengths of both weak and strong acids were reduced.
An etherification catalyst cannot perform well without having suitable acid sites, acid type, acid strength and acid distribution. A pyridine molecule coordinated on a Brønsted acid site can be simply distinguished from that coordinated on a Lewis acid site by IR. Fig. 7 presents the IR spectra of pyridine adsorbed on HBC, 0.1La/HBC, 40-3/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC in the 1650 to 1400 cm−1 region. The strong band at 1454 cm−1 was caused by the adsorption of pyridine molecules on Lewis acid sites, and the band at 1545 cm−1 was due to protonation of the pyridine molecule on Brønsted acid sites; the bands at around 1490 cm−1 and 1620 cm−1 can be assigned to pyridine coordinated to Brønsted and Lewis acid sites, while the bands at 1440 cm−1, 1600 cm−1 and 1638 cm−1 were assigned to pyridine adsorbed on weak Lewis acid sites.32–34
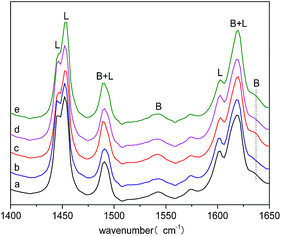 | ||
| Fig. 7 Py-IR profiles of (a) HBC, (b) 400-3/HBC, (c) 0.1La/HBC, (d) 0.1La(400-3)/HBC and (e) 400-3(0.1La)/HBC. | ||
Table 4 lists the Brønsted acidity, Lewis acidity, acid distribution and B/L ratios determined by Py-IR characterization. These results are consistent with the results from NH3-TPD. It can been seen that the blank sample has the maximum total acidity, while 0.1La/HBC has the minimum acidity. The Lewis acidity on weak and strong acid sites of 0.1La/HBC decreased, while the Brønsted acidity significantly increased on the strong acid site. Moreover, the Brønsted/Lewis ratio and the proportion of strong acid increased. All variations imply that La modification can reduce the total acid quantity while increasing the proportion of strong acid, especially on Brønsted acid sites. La atoms may replace aluminum atoms on the bridged hydroxyl groups and destroy the original acid sites; however, this will lead to the formation of new strong acid sites in the meantime. A new peak can be seen in Fig. 7c near 1630 cm−1, which belongs to a Brønsted acid site. According to another study, this new peak might be the result of coupled water cation ionization, which caused by the strong electrostatic potential of rare earth hydrated cation.35 Low-temperature steam treatment alone has little impact on the acid quantity and acid distribution; the proportion of weak acid increased slightly, while the Brønsted/Lewis ratio of weak acid sites was greatly reduced, which may be due to the separation of framework aluminate or active hydroxyl from the molecular sieve framework in the process of steam treatment. The characterization results for 0.1La(400-3)/HBC show that remodification by lanthanum after steam treatment can improve the proportion of weak acid, wherein the acid strength was slightly reduced but the Brønsted/Lewis ratio of weak acid sites and the Brønsted acidity were increased. However, the proportion of strong acid and the Brønsted acidity significantly increased for 400-3(0.1La)/HBC and the Lewis acidity reduced, eventually resulting in a significant increase in the Brønsted/Lewis ratio. In addition, the acid strength obviously improved, especially the Brønsted acid strength. All these vibrations were caused by the catalyst, which is more conducive after steam dealumination and dehydroxylation for the formation of new Brønsted acid sites during La modification.
| Sample | Acidity (mmol g−1) | Acid distribution | Brønsted/Lewis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WL | WB | SL | SB | TL | TB | W% | S% | W | S | |
| HBC | 1.495 | 0.189 | 3.159 | 0.359 | 4.654 | 0.548 | 32.38 | 67.62 | 0.13 | 0.11 |
| 400-3/HBC | 1.495 | 0.155 | 3.017 | 0.367 | 4.512 | 0.521 | 32.77 | 67.23 | 0.10 | 0.12 |
| 0.1La/HBC | 1.285 | 0.183 | 2.787 | 0.483 | 4.072 | 0.666 | 30.98 | 69.02 | 0.14 | 0.17 |
| 0.1La(400-3)/HBC | 1.530 | 0.212 | 3.081 | 0.357 | 4.611 | 0.569 | 33.63 | 66.37 | 0.14 | 0.12 |
| 400-3(0.1La)/HBC | 1.216 | 0.210 | 3.119 | 0.483 | 4.335 | 0.693 | 28.36 | 71.64 | 0.17 | 0.16 |
4. Discussion
A comparative study was performed on the influence of different modification methods (lanthanum exchange, low-temperature steam treatment and La/steam combined modification) on the etherification activity. Using a variety of characterization methods, this study investigated the effect of the abovementioned modifications on the crystal phase, pore structure, framework structure and acidic properties.The evaluation results for etherification activity show that the activities of modified samples were improved. It can be seen that the lanthanum modification alone is more effective than low-temperature steam treatment alone and the compound-modification method is superior to both of these methods. In addition, the activity of 0.1La(400-3)/HBC is greater than that of 400-3(0.1La)/HBC. The most suitable modification method is combined modification involving lanthanum ion exchange followed by steam treatment.
XRD results show that all the modification methods cause no damage to the crystal phase. Lanthanum oxide is uniformly dispersed on the catalyst surface. Despite the relative crystallinity of samples being slightly decreased, all these samples maintained good crystal stability; in particular, the stability of 0.1La(400-3)/HBC was improved. BET results show that the specific surface area of the modified samples was decreased, which had little effect on the pore volume but played a significant role in enlarging pores. Among all the samples, 0.1La(400-3)/HBC has the largest average pore size. FETEM images showed that the microstructures of all the samples were similar; the typical structure of γ-Al2O3 and Hβ zeolite existed in all the samples. The EDS analysis results for 0.1La/HBC, 0.1La(400-3)/HBC and 400-3(0.1La)/HBC indicated that the surfaces of these samples contained La. In addition, EDS mapping scanning results implied that lanthanum ion exchange mainly occurred on Hβ zeolite, and lanthanum was homogeneously distributed on Hβ zeolite. FT-IR results indicate that neither the γ-Al2O3 (binder) nor the modification methods damage the framework structure of Hβ zeolite and there is no characteristic La–O peak. OH-FTIR results showed a red shift in the main peak of Hβ when it was kneaded with alumina, thus the weak acidity increased. In addition, single-modification methods such as lanthanum exchange and steam treatment can promote the formation of hydrogen bonds, while the combined-modification method can cover the weak acid sites on alumina. Characterization of acid properties attests that the total acidity of modified catalysts are decreased by varying degrees. The influence of low-temperature steam treatment on acidity is relatively small, but the introduction of lanthanum significantly decreased the acidity and also facilitated the generation of new Brønsted acid sites. Among all the samples, 400-3(0.1La)/HBC has the largest proportion of strong acid but a relatively lower acid content, while 0.1La(400-3)/HBC has the highest acidity but a moderate proportion of strong acid.
Combining the evaluation results with the characterization results, it can be seen that the crystal phase, pore structure, framework structure and acid properties impact the etherification activity, and that the pore structure and acid properties exert relatively larger effects. A rich pore structure and a large pore size can be beneficial with respect to the diffusion of reactants and products in the pores, as well for decreasing the deactivation rate and improving catalyst stability. In addition, the acid environment for etherification is very moderate. Acid content, acid distribution and acid strength together exhibit a synergistic effect. Both Brønsted acid and Lewis acid sites contribute to etherification. A high acid content, an appropriate acid strength, a large number of acid sites and a suitable Brønsted/Lewis ratio are favorable conditions for etherification.
With a greater influence on the pore features compared to catalyst acidity, low-temperature steam treatment can remove some of the non-framework aluminum species and effectively clear the channels, and therefore the activity of 400-3/HBC can be improved.16 In addition, La exchange plays an effective role in enlarging the pore size. Although the acidity is significantly reduced, the new Brønsted acid sites generated are favorable for etherification.9 Steam treatment followed by lanthanum ion exchange exerted less effect on pore size than on acid properties; pore-size enlargement was not significant. Moreover, 400-3(0.1La)/HBC has an over-strong acid strength and a too-large Brønsted/Lewis ratio. In contrast, 0.1La(400-3)/HBC has higher etherification activity due to its large pore size, high acidity and suitable acid strength.
5. Conclusions
In summary, both lanthanum ion exchange and low-temperature steam treatment can improve etherification activity, while lanthanum exchange has a greater effect than steam treatment. However, combined-modification methods, which consist of the abovementioned two methods in different orders, can further improve catalyst activity, wherein 0.1La(400-3)/HBC has the highest etherification activity.The characterization results show that the crystal phase and framework structure were not destroyed during any of the modification processes, while the stability of 0.1La(400-3)/HBC was improved. In addition, these modification processes play an effective role in enlarging pore size; in particular, 0.1La(400-3)/HBC has the largest average pore size. All the samples exhibit the typical microstructure of γ-Al2O3 and Hβ zeolite, and EDS mapping scanning results suggest that lanthanum ion exchange mainly occurs on Hβ zeolite. Although the total acid quantity of modified samples was decreased by different degrees, the introduction of lanthanum cations helped to generate new Brønsted acid sites. 0.1La(400-3)/HBC has the highest acidity and a moderate acid strength.
Pore structure and acid properties are the major factors that impact the etherification activity. A large pore size, high acidity, moderate acid strength and a suitable proportion of Brønsted acid sites are all favorable for etherification.
Acknowledgements
The authors are grateful for assistance with catalyst characterization in this study by the New Materials Laboratory of Chemical Engineering College at China University of Petroleum.References
- R. Soto, C. Fité, E. Ramírez, R. Bringué and F. Cunill, Fuel Process. Technol., 2016, 142, 201–211 CrossRef CAS.
- T. F. Hu, J. P. Chen, H. Y. Wang, J. Ma and M. Wei, Microporous Mesoporous Mater., 2006, 94, 283–287 CrossRef CAS.
- W. Q. Zhao, C. H. Yi, B. L. Yang, J. Y. Hu and X. M. Huang, Fuel Process. Technol., 2013, 112, 70–75 CrossRef CAS.
- M. D. González, Y. Cesteros and P. Salagre, Appl. Catal., A, 2013, 450, 178–188 CrossRef.
- F. Collignon and G. Poncelet, J. Catal., 2001, 202, 68–77 CrossRef CAS.
- F. Collignon, R. Loenders, J. A. Martens, P. A. Jacobs and G. Poncelet, J. Catal., 1999, 182, 302–312 CrossRef CAS.
- S. K. Saxena, A. A. H. Al-Muhtaseb and N. Viswanadham, Fuel, 2015, 159, 837–844 CrossRef CAS.
- J. W. Kim, D. J. Kim, J. U. Han, M. Kang, J. M. Kim and J. E. Yie, Catal. Today, 2003, 87, 195–203 CrossRef CAS.
- F. Collignon, M. Mariani, S. Moreno, M. Remy and G. Poncelet, J. Catal., 1997, 166, 53–66 CrossRef CAS.
- P. Liu, Y. Yao, X. G. Zhang and J. Wang, Chin. J. Chem. Eng., 2011, 19, 278–284 CrossRef CAS.
- J. C. Chavarría, J. Ramírez, H. González and M. A. Baltanas, Catal. Today, 2004, 98, 235–242 CrossRef.
- Y. Wang, Y. Y. Sun, C. Lancelot, C. Lamonier, J. C. Morin, B. Revel, L. Delevoye and A. Rives, Microporous Mesoporous Mater., 2015, 206, 42–51 CrossRef CAS.
- B. Thomas, B. B. Das and S. Sugunan, Microporous Mesoporous Mater., 2006, 95, 329–338 CrossRef CAS.
- F. E. Trigueiro, D. F. J. Monteiro, F. M. Z. Zotin and E. Falabella Sousa-Aguiar, J. Alloys Compd., 2002, 344, 337–341 CrossRef CAS.
- X. Y. Lin, Y. Fan, Z. H. Liu, G. Shi, H. Y. Liu and X. J. Bao, Catal. Today, 2007, 125, 185–191 CrossRef CAS.
- R. Otomo, T. Yokoi, J. N. Kondo and T. Tatsumi, Appl. Catal., A, 2014, 470, 318–326 CrossRef CAS.
- A. Cabanas, J. A. Darr, E. Lester and M. Poliakoff, Chem. Commun., 2000, 144, 901–902 RSC.
- M. Mahalakshmi, S. Vishnu Priya, B. Arabindoo, M. Palanichamy and V. Murugesan, J. Hazard. Mater., 2009, 161, 336–343 CrossRef CAS PubMed.
- M. V. Shankar, K. K. Cheralathan, B. Arabindoo, M. Palanichamy and V. Murugesan, J. Mol. Catal. A: Chem., 2004, 223, 195–200 CrossRef CAS.
- J. Gao, Z. Y. Hou, J. Z. Guo, Y. H. Zhu and X. M. Zheng, Catal. Today, 2008, 131, 278–284 CrossRef CAS.
- X. Liu, Y. Liu, X. H. Li, S. Xiang, Y. Zhang, P. L. Ying, Z. B. Wei and C. Li, Appl. Catal., A, 2003, 239, 279–286 CrossRef CAS.
- J. H. Fei, Z. Y. Hou, B. Zhu, H. Lou and X. M. Zheng, Appl. Catal., A, 2006, 304, 49–54 CrossRef CAS.
- J. Lee, H. Jeon, D. G. Oh, J. Szanyi and J. H. Kwak, Appl. Catal., A, 2015, 500, 58–68 CrossRef CAS.
- E. Sayah, D. Brouri and P. Massiani, Catal. Today, 2013, 218–219, 10–17 CrossRef CAS.
- N. D. Hould and R. F. Lobo, Chem. Mater., 2008, 20, 5807–5815 CrossRef CAS.
- X. C. Duan, T. Kim, D. Li, J. M. Ma and W. J. Zheng, Chem.–Eur. J., 2013, 19, 5924–5937 CrossRef CAS PubMed.
- M. Y. Li, Z. C. Si, X. D. Wu, D. Weng and F. Y. Kang, J. Colloid Interface Sci., 2014, 417, 369–378 CrossRef CAS PubMed.
- P. J. Kunkeler, B. J. Zuurdeeg, J. C. van der Waal, J. A. van Bokhoven, D. C. Koningsberger and H. van Bekkum, J. Catal., 1998, 180, 234–244 CrossRef CAS.
- A. Vimont, F. Thibault-Starzyk and J. C. Lavalley, J. Phys. Chem. B, 2000, 104, 286–291 CrossRef CAS.
- A. Zecchina, L. Marchese, S. Bordiga, C. Pazè and E. Gianotti, J. Phys. Chem. B, 1997, 101, 10128–10135 CrossRef CAS.
- R. Q. Long and R. T. Yang, J. Catal., 2001, 198, 20–28 CrossRef CAS.
- Z. K. Xie, J. Q. Bao, Y. Q. Yang, Q. L. Chen and C. F. Zhang, J. Catal., 2002, 205, 58–66 CrossRef CAS.
- Y. Zheng, X. X. Wang, Z. H. Li, X. Z. Fu and K. M. Wei, J. Organomet. Chem., 2005, 690, 3187–3192 CrossRef CAS.
- H. Matsuura, N. Katada and M. Niwa, Microporous Mesoporous Mater., 2003, 66, 283–296 CrossRef CAS.
- V. N. Sheemol, B. Tyagi and R. V. Jasra, J. Mol. Catal. A: Chem., 2004, 215, 201–208 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |