Amalgamating 4′-substituted 4,2′:6′,4′′-terpyridine ligands with double-helical chains or ladder-like networks†
Guoqi Zhang*a,
Jiawen Tana,
Tonya Phoenixa,
David R. Mankeb,
James A. Golenb and
Arnold L. Rheingoldc
aDepartment of Sciences, John Jay College and The Graduate Center, The City University of New York, New York, NY 10019, USA. E-mail: guzhang@jjay.cuny.edu
bDepartment of Chemistry and Biochemistry, University of Massachusetts Dartmouth, North Dartmouth, MA 02747, USA
cDepartment of Chemistry, University of California San Diego, La Jolla, CA 92093, USA
First published on 15th January 2016
Abstract
The reaction of five 4,2′:6′,4′′-terpyridine ligands containing diverse 4′-substituents with Hg(NCS)2 by layering methods afforded crystals of five novel HgII-based coordination polymers (1–5) that have been structurally characterized by both powder and single-crystal X-ray diffraction analysis. Similar one-dimensional (ID) polymeric chains were observed in the structures of polymers 1–3 where HgII serves as a mononuclear node to link ligands in a linear mode. All 1D chains in these complexes form double-helical structures through significant inter-chain π-stacking interactions, whereas the packing modes were slightly different in the three compounds, resulting from the influence of 4′-substituents. It was interesting to note that the structures of isomorphic 4 and 5 are completely different from those in 1–3. In the former cases, 1D ladder-like architectures that are assembled through the bridging thiocyanate groups were revealed, while remaining the extra 4′-pyridyl units non-coordinated.
Introduction
4,2′:6′,4′′-Terpyridine (4,2′:6′,4′′-tpy) is a divergent ditopic N-donor ligand that has received increasing attention in the area of metallosupramolecular and coordination chemistry during the past fifteen years.1–4 4′-Substituted 4,2′:6′,4′′-tpy derivatives mainly containing aryl groups have been extensively explored, owing to the facile one-pot synthesis that was developed recently and their versatility in coordination chemistry, as well as many intriguing properties.5–12 A number of transition metals including ZnII, CdII, CoII, CuII, NiII, and MnII have been utilized to synthesize diverse metal coordination assemblies including discrete molecules, metallocycles, polycatenated metallocages, and one- to three-dimensional (1D to 3D) coordination polymers/frameworks, with the latter obviously remaining dominated.5–12 The recent reviews by Housecroft documented concisely the recent progress in the coordination assemblies containing ZnII, CuII and CdII metal centers, and discussed the role of both metal salts and the substituents in the 4′-position of 4,2′:6′,4′′-tpy in driving the selective formation of coordination polymers, metallomacrocycles, or cluster-containing polymers.3,4As a heavier element analogue of ZnII and CdII, HgII is, however, not investigated for the coordination chemistry involving 4,2′:6′,4′′-tpy ligands. In contrast to ZnII and CdII, HgII is a much softer metal and tends to form less predictable structures when coordinating to N-donor organic ligands.13–22 The study on mercury(II)-derived coordination polymers is rather limited and known examples concerned mainly over some flexible pyridine-containing ligands,23 although mercury(II) is quite attractive in fabricating hybrid materials that have found widespread applications in many fields such as energy-saving light bulbs, batteries, thermometers, manometers, and paper and paint industries, and so on.24–26
In continuation with our recent efforts in exploring the remarkable substituent effects on the structures of the resulting metal assemblies of various 4,2′:6′,4′′-tpy and its structurally related ligands as well as their catalytic properties,5–12,27 we were interested in studying the HgII-mediated coordination self-assembly and structural diversity of the corresponding metal–organic hybrid materials. It was known that switching from zinc(II) to cadmium(II) significantly changes the supramolecular or network structures of the resulting coordination assemblies with 4,2′:6′,4′′-tpys,3,4 we expect that introducing mercury(II) as new metal nodes would produce interesting structural types distinct with those reported structures. Therefore, we herein report the first HgII coordination polymers based on five previously known 4,2′:6′,4′′-tpy ligands (L1–5, Scheme 1). Structural diversity in five mercury(II) coordination polymers resulting from small differences of the 4′-substituents in 4,2′:6′,4′′-tpy is revealed.
Experimental section
General
Solvents and reagents were purchased from Fisher or Sigma-Aldrich in the US. All reactions were performed under ambient conditions. Solution electronic absorption spectra were recorded on a Shimadzu UV-1800 spectrophotometer, and FT-IR spectra using a Shimadzu 8400S instrument with solid samples using a Golden Gate ATR accessory. Elemental Analyses were performed by Midwest Microlab LLC in Indianapolis. Powder X-ray diffraction (PXRD) was performed with a Bruker D8 Discover microdiffractometer with the General Area Detector Diffraction System (GADDS) equipped with a VÅNTEC-2000 2D detector. The X-ray beam was monochromated with a graphite crystal (λ Cu-Kα = 1.54178 Å) and collimated with a 0.5 mm capillary (MONOCAP). The integrated 1D pattern was analyzed by the software DIFFRACplus EVA.28 The simulated powder pattern was calculated by the software Mercury v. 2.4.29 Ligands L1–L5 were synthesized according to previously published procedures.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) was placed in a long test tube. A mixture of MeOH and CH2Cl2 (5 cm3, 1
4, v/v) was placed in a long test tube. A mixture of MeOH and CH2Cl2 (5 cm3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was layered on the top of this solution, followed by a solution of Hg(NCS)2 (31.8 mg, 0.100 mmol) in MeOH (10 cm3). The tube was sealed and allowed to stand at room temperature for two weeks, during which time X-ray quality colorless crystals of 1 grew on the bottom of the tube. The crystals were collected by decanting the solvent, washed with MeOH and dried in air. Yield: 50.9 mg (81.3%). FT-IR (solid, cm−1) 3057w, 2121s, 1605s, 1595s, 1558m, 1496w, 1437m, 1403m, 1321w, 1217m, 1060s, 1024s, 900w, 841s, 766s, 747m, 730s, 663s, 643s, 615m. Anal. calcd for C23H15HgN5S2: C 44.12, H 2.41, N 11.19%. Found C 44.47, H 2.46, N 11.09%.
1, v/v) was layered on the top of this solution, followed by a solution of Hg(NCS)2 (31.8 mg, 0.100 mmol) in MeOH (10 cm3). The tube was sealed and allowed to stand at room temperature for two weeks, during which time X-ray quality colorless crystals of 1 grew on the bottom of the tube. The crystals were collected by decanting the solvent, washed with MeOH and dried in air. Yield: 50.9 mg (81.3%). FT-IR (solid, cm−1) 3057w, 2121s, 1605s, 1595s, 1558m, 1496w, 1437m, 1403m, 1321w, 1217m, 1060s, 1024s, 900w, 841s, 766s, 747m, 730s, 663s, 643s, 615m. Anal. calcd for C23H15HgN5S2: C 44.12, H 2.41, N 11.19%. Found C 44.47, H 2.46, N 11.09%.Crystal structure determinations
Suitable crystals of 1–5 were mounted on Cryoloops with Paratone-N oil. Data were collected at 100 K with a Bruker APEX II CCD using Mo-Kα radiation and corrected for absorption with SADABS and structures solved by direct methods. For structure 5, large residual electron densities of 3.63 e and 1.90 e noted within 0.91 and 0.94 Å, respectively, of mercury atom Hg1 were attributed in part to the empirical absorption correction used. All non-hydrogen atoms were refined anisotropically by full-matrix least squares on F2. Hydrogen atoms were found from Fourier difference maps and refined isotropically, otherwise they were placed in calculated positions with appropriate riding parameters. Refinement details are summarized in Table 1. CCDC no. 1017551 and 1413156–1413159 contain the supplementary crystallographic data for this paper.| a R1 = (Fo − Fc)/Fo.b wR2 = [w(Fo2 − Fc2)2/w(Fo)2]1/2. | |||||
|---|---|---|---|---|---|
| Compound | 1 | 2 | 3 | 4 | 5 |
| Formula | C23H15HgN5S2 | C22.50H20.50ClHgN6S2 | C24H15HgN5O2S2 | C22H14HgN6S2 | C22H13HgN6S2 |
| Formula weight | 626.11 | 711.14 | 670.12 | 627.10 | 626.09 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | C2/c | C2/c | P2(1)/n | P2(1)/m | P2(1)/m |
| a/Å | 31.4424(10) | 28.2486(17) | 14.4085(4) | 7.195(3) | 7.3024(13) |
| b/Å | 7.9438(3) | 8.1596(3) | 14.5536(5) | 27.428(9) | 26.158(5) |
| c/Å | 20.5260(6) | 22.5028(11) | 22.9421(7) | 10.889(4) | 11.2559(15) |
| α/° | 90 | 90 | 90 | 90 | 90 |
| β/° | 124.2599(5) | 99.253(2) | 99.9220(10) | 107.890(7) | 108.694(10) |
| γ/° | 90 | 90 | 90 | 90 | 90 |
| U/Å3 | 4237.3(2) | 5119.3(4) | 4738.9(3) | 2045.1(12) | 2036.7 (6) |
| Dc/mg m−3 | 1.963 | 1.845 | 1.879 | 2.037 | 2.042 |
| Z | 8 | 4 | 8 | 4 | 4 |
| μ/mm−1 | 15.044 | 6.308 | 13.569 | 7.754 | 7.786 |
| T/K | 200(2) | 100(2) | 200(2) | 100(2) | 100(2) |
| Reflections/unique | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485/4139 485/4139 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670/5784 670/5784 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666/8439 666/8439 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 802/4220 802/4220 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 381/3951 381/3951 |
| Parameters | 280 | 321 | 623 | 292 | 274 |
| R1a, wR2b [I > 2σ(I)] | 0.0346, 0.0954 | 0.0233, 0.0715 | 0.0554, 0.1270 | 0.0579, 0.1307 | 0.0743, 0.1396 |
| R1a, wR2b (all data) | 0.0351, 0.0960 | 0.0268, 0.0742 | 0.0672, 0.1355 | 0.0755, 0.1391 | 0.1000, 0.1470 |
| GOF | 1.095 | 1.005 | 1.081 | 1.095 | 1.263 |
Results and discussion
Synthesis and characterization
Ligands L1–L5 containing various substituents were prepared according to the literature procedure by one-pot Kröhnke condensations between 4-acetylpyridine and the corresponding aromatic aldehydes.8 Their molecular structures as illustrated in Scheme 1. The solution reaction of ligands with Hg(NCS)2 in CH2Cl2–MeOH gave immediately precipitates that are insoluble in common solvent, preventing from further characterization. Instead, a layering diffusion method was utilized to produce good-quality crystals of compounds 1–5 that were suitable X-ray structural analysis. Specifically, slow diffusion between two layers of solutions containing Hg(NCS)2 and the ligand using a CH2Cl2–MeOH solvent system led to the formation of crystals in one to three weeks. The synthetic details will be described below. It was noticed that the crystals of compounds 2 and 3 appeared to be orange and yellow in colour, respectively, while those of other compounds were colourless. This is probably owing to the existence of strongly electron-donating substituents in the corresponding ligands L2 and L3. The bulk samples of 1–5 are insoluble in both common organic solvents and water. All the compounds were characterized by FT-IR spectroscopy and elemental analysis, and structurally determined by X-ray crystallography. Powder X-ray diffraction (PXRD) data were recorded to confirm the phase purity of the bulk crystalline samples and the results indicated that the PXRD patterns of all samples matched with those simulated from single crystal X-ray diffraction data, indicating that samples 1–5 are all in good purity as a single phase. The crystal refinement results for 1–5 are summarized in Table 1.1-D polymeric chains in 1–3
Layering a solution of Hg(NCS)2 in MeOH onto the top of a solution of L1 in a CH2Cl2–MeOH solution with a Hg : ligand ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
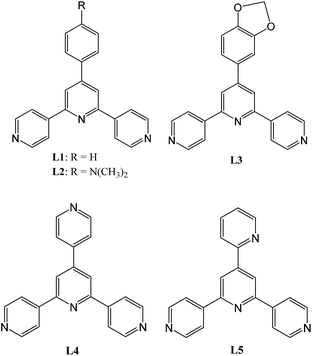
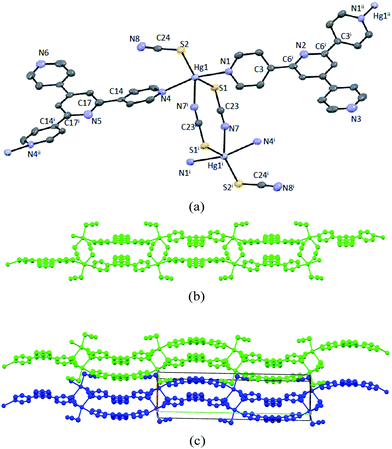
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, separated with a blank solvent layer afforded colourless crystals of 1 after two weeks. Elemental analysis revealed the bulk crystalline sample as a component with the empirical formula of Hg(L1)(NCS)2, in which the existence of NCS− group was evidenced by a strong IR absorption at 2121 cm−1. Single-crystal X-ray structural analysis confirmed unambiguously the formation of polymeric complex [Hg(L1)(NCS)2]n (1). 1 crystallizes in the monoclinic space group C2/c, and the asymmetric unit contains one independent Hg atom and one ligand molecule. An ORTEP representation of the repeating structural unit in 1 is shown in Fig. 1a and relevant bond parameters are given in the caption. Hg atom is four-coordinate with two SSCN atoms and two nitrogen atoms from the outer pyridines of two symmetrically related ligands, adopting a distorted tetrahedral geometry. All bond lengths and angles are unexceptional and close to those reported for similar Hg(NCS)2 complexes of N-ligands.19–23 The tpy domain of L1 in 1 is not well planar and the torsion angles between the least square planes of pairs of adjacent pyridine rings are 18.56 and 2.03°, respectively, yet it is even more distorted between the planes of the phenyl group and central pyridine ring with a dihedral angle of 22.83°. Each ligand binds to two symmetrically related Hg centers with its outer pyridine N atoms, remaining the central pyridine uncoordinated. This is in agreement with all previously reported examples of coordination polymers involving 4,2′:6′,4′′-tpy.1–12
1, separated with a blank solvent layer afforded colourless crystals of 1 after two weeks. Elemental analysis revealed the bulk crystalline sample as a component with the empirical formula of Hg(L1)(NCS)2, in which the existence of NCS− group was evidenced by a strong IR absorption at 2121 cm−1. Single-crystal X-ray structural analysis confirmed unambiguously the formation of polymeric complex [Hg(L1)(NCS)2]n (1). 1 crystallizes in the monoclinic space group C2/c, and the asymmetric unit contains one independent Hg atom and one ligand molecule. An ORTEP representation of the repeating structural unit in 1 is shown in Fig. 1a and relevant bond parameters are given in the caption. Hg atom is four-coordinate with two SSCN atoms and two nitrogen atoms from the outer pyridines of two symmetrically related ligands, adopting a distorted tetrahedral geometry. All bond lengths and angles are unexceptional and close to those reported for similar Hg(NCS)2 complexes of N-ligands.19–23 The tpy domain of L1 in 1 is not well planar and the torsion angles between the least square planes of pairs of adjacent pyridine rings are 18.56 and 2.03°, respectively, yet it is even more distorted between the planes of the phenyl group and central pyridine ring with a dihedral angle of 22.83°. Each ligand binds to two symmetrically related Hg centers with its outer pyridine N atoms, remaining the central pyridine uncoordinated. This is in agreement with all previously reported examples of coordination polymers involving 4,2′:6′,4′′-tpy.1–12
Propagation of the structure in Fig. 1a along the crystallographic a-axis leads to a one-dimensional infinite polymeric chain as shown in Fig. 1b. The ligands arrange in an alternate mode around the Hg atoms and the distance between two adjacent Hg centers is 13.105 Å. In addition, the distance of two alternating Hg centers is 26.140 Å. A side-view of the chain shows that the adjacent ligand molecules lie in the same side of Hg centers with a dihedral angle of approximately 60° between central pyridine ring planes of the ligands (Fig. 1c), resulting in a butterfly-like structure in which the aligned ligands look like two wings. Although 1-D polymeric chain containing 4,2′:6′,4′′-tpy linkers and metal nodes has been frequently found in structures of Zn(OAc)2 assemblies of 4′-substituted 4,2′:6′,4′′-tpys including L1 and the zig–zag chain-like arrangement of ligands was most popular,8 the 1-D chain in 1 is essentially different. More interestingly, it was noticed that in the extended molecular packing mode two adjacent 1-D chains are twisted with respect to one another through effective π-stacking interactions between the tpy domains to form a double-helix structure (Fig. 1d). Specifically, the interchain π-stacking results from the head-to-tail arrangement of the closest tpy moieties of two chains and the central pyridine rings of two packing tpy domains are largely displaced. While the centroid…centroid distance of central pyridines is 4.891 Å, the interatomic distance for π⋯π interactions is as short as 3.212 Å, indicating very strong intermolecular forces. It is worth mentioning that such double-stranded helical packing of 1-D polymeric chains was not observed in any known transition metal assemblies of 4,2′:6′,4′′-tpys.3,4
Next, the 4,2′:6′,4′′-tpy with a 4,4′-diaminophenyl substituent (L2) was examined for the reaction with Hg(NCS)2 (metal–ligand ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
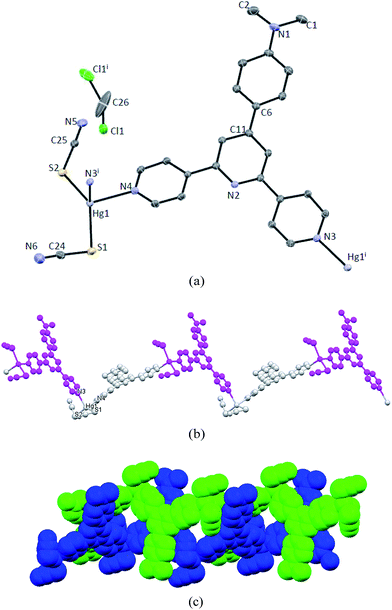
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). By using the layering method block-like organge crystals of 2 was obtained in one week under the same conditions. X-ray structural analysis was then conducted to reveal the molecular structure of 2. Similar to 1, 2 also crystallizes in the monoclinic space group C2/c, and the asymmetric unit contains one independent Hg atom and one ligand molecule. However, one dichloromethane molecule was found to co-crystallize in the asymmetric unit of 2, which tends to depart from the crystal upon exposing the bulk crystals to the air, as supported by the elemental analysis data of air-dried crystals. Three outer aromatic rings of L2 in 2 are slightly distorted out of the plane of the central pyridine ring, with tortion angles being in the range of 6.70–19.10°. The Hg centers in 2 also adopt similar coordination environment as in 1. Each Hg atom coordinates to two Npyridine atoms from two symmetrically related ligands and two SSCN atoms. However, the bond parameters around the Hg center are distinct. Except that one of the Hg–N bonds in 2 is slightly longer than that in 1, the bond angles are remarkably different. In 2 the N–Hg–N angle is much smaller than that in 1 (91.49(7)° vs. 108.25(12)°), and accordingly the S–Hg–S angle is larger (148.23(9)° vs. 126.63(4)°). Consequently, expansion of the repeating units in 2 forms an infinite zig–zag chain that is different from that of 1 (Fig. 2b). Although the distance between two adjacent Hg centers is almost identical to the relevant length in 1 (Hg1⋯Hg1i = 13.321 Å), the distance of two alternating Hg centers is 22.503 Å, significantly longer than that for 1. This should be attributed to more bent arrangement of the ligands around Hg centers. Further inspection of the packing mode in 2 leads to the observation of a similar double-helix structure that was discussed for 1 (Fig. 1d and 2c). Again, the helical structure was formed through significant π-stacking interactions between the aromatic regions of the ligand. Except for the tpy domains, the N,N-dimethylaminophenyl moieties also participate in interchain π⋯π packing event, and the shortest interatomic distance for this contact is 3.331 Å.
1). By using the layering method block-like organge crystals of 2 was obtained in one week under the same conditions. X-ray structural analysis was then conducted to reveal the molecular structure of 2. Similar to 1, 2 also crystallizes in the monoclinic space group C2/c, and the asymmetric unit contains one independent Hg atom and one ligand molecule. However, one dichloromethane molecule was found to co-crystallize in the asymmetric unit of 2, which tends to depart from the crystal upon exposing the bulk crystals to the air, as supported by the elemental analysis data of air-dried crystals. Three outer aromatic rings of L2 in 2 are slightly distorted out of the plane of the central pyridine ring, with tortion angles being in the range of 6.70–19.10°. The Hg centers in 2 also adopt similar coordination environment as in 1. Each Hg atom coordinates to two Npyridine atoms from two symmetrically related ligands and two SSCN atoms. However, the bond parameters around the Hg center are distinct. Except that one of the Hg–N bonds in 2 is slightly longer than that in 1, the bond angles are remarkably different. In 2 the N–Hg–N angle is much smaller than that in 1 (91.49(7)° vs. 108.25(12)°), and accordingly the S–Hg–S angle is larger (148.23(9)° vs. 126.63(4)°). Consequently, expansion of the repeating units in 2 forms an infinite zig–zag chain that is different from that of 1 (Fig. 2b). Although the distance between two adjacent Hg centers is almost identical to the relevant length in 1 (Hg1⋯Hg1i = 13.321 Å), the distance of two alternating Hg centers is 22.503 Å, significantly longer than that for 1. This should be attributed to more bent arrangement of the ligands around Hg centers. Further inspection of the packing mode in 2 leads to the observation of a similar double-helix structure that was discussed for 1 (Fig. 1d and 2c). Again, the helical structure was formed through significant π-stacking interactions between the aromatic regions of the ligand. Except for the tpy domains, the N,N-dimethylaminophenyl moieties also participate in interchain π⋯π packing event, and the shortest interatomic distance for this contact is 3.331 Å.
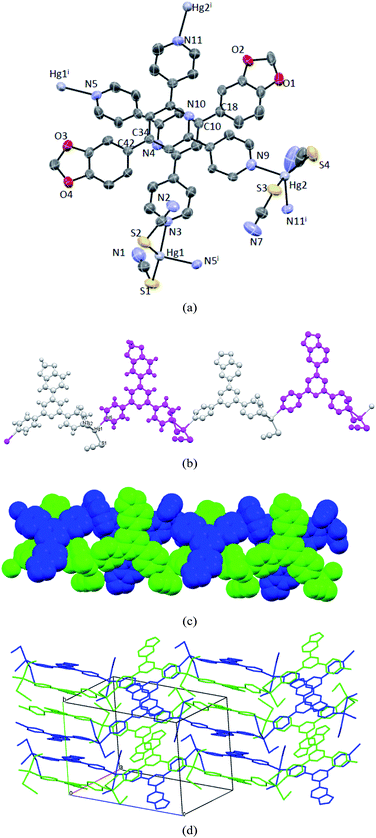
The same procedure was applied to the synthesis of Hg(NCS)2 complex with ligand L3, and yellow crystals of 3 were collected after one week. X-ray studies on one of the good-quality crystals reveal that 3 crystallizes in the monoclinic space group P2(1)/n and is composed of two independent ligands and Hg centers in the asymmetric unit (Fig. 3a). 3 is remarkably distinct from 1 and 2 with regard to the cell parameters, although in 3 each Hg atom coordinates to two ligands and two thiocyanate ions again and adopts a distorted tetradedral geometry. All bond parameters around Hg centers are unexceptional and close to those in 2, and the N–Hg–N angles are 93.9(3)° and 96.3(3)°, respectively. The ligand molecules in 3 are almost co-planar except one outer-pyridine ring (pyridine containing N5 or N11) deviated from the plane by 19.14°. Two independent ligand molecules are aligned in parallel through intermolecular π-stacking between their central pyridine domains and the shortest interatomic distance is 3.396 Å. Like the extended structures in 1 and 2, 3 also features 1-D polymeric structures and two independent chains are developed via the expansion of ligands around Hg1 and Hg2 centers (Fig. 3b), and the chains are interwoven through the interchain π-stacking to form a double-helix chain as shown in Fig. 3c. The separations between adjacent Hg centers for two chain are 12.901 and 12.978 Å, respectively, while the distances of alternating Hg atoms are both 24.900 Å, which falls into approximately the middle of relevant values in 1 and 2. Although the double-helix structure present in 3 involves only π-stacking between central pyridine rings of ligands from independent chains, the 3-D packing in the crystal also shows significant π⋯π interactions between double helixes resulting from the aromatic overlapping between one of outer pyridines and the phenyl ring (Fig. 3d).
The common feature observed in the structures of 1–3 is that each Hg(II) center adopts a four-coordinate environment with a distorted tetrahedral geometry and links two ligand molecules to form infinite zig–zag chains, although different 4′-substituents in the 4,2′:6′,4′′-tpy backbone slightly affect the inter-chain interactions by altering the π-stacking. Previously, a plethora of 1-D Hg(II) coordination polymers have been reported,18–22 where flexible ditopic pyridine-containing were popularly employed. However, the entanglement of 1-D chains into helical networks was not found in these examples. The finding of novel double helical networks in 1–3 is thus remarkable. We attribute this to the existence of both the rigid 4,2′:6′,4′′-tpy backbone and aromatic 4′-substituents in the ligands which resulted in the occurrence of significant π-stacking between infinite zig–zag chains.
Bilayered polymeric chains in 4 and 5
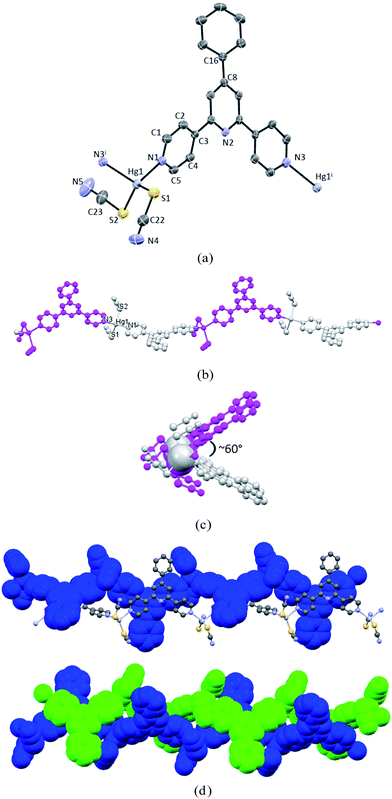
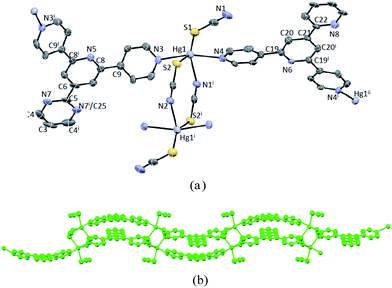
On going from ditopic ligands L1–3 to L4 which contains an extra 4-pyridyl coordination unit in the 4′-position of 4,2′:6′,4′′-tpy, we expected to add an additional dimension to the 1-D chains in 1–3 through a possible divergent tridentate coordination to HgII, as both polycatenated and double-walled cage frameworks have been previously reported in L4/ZnX2 (X = Cl, I) assemblies and CoII or NiII networks involving M7F122+ (M = Co, Ni) cores.7,30Similarly, the layering reaction of solutions of Hg(NCS)2 with L4 in CH2Cl2–MeOH afforded colourless crystals of 4 in three weeks. X-ray structural determination revealed that this is a 1-D bilayered coordination polymer, distinct remarkably from those found in 1–3. 4 crystallizes in the monoclinic space group P2(1)/m. The repeating structural unit is shown in Fig. 4a and relevant bond parameters around the HgII centers listed in the caption. Each Hg center is five-coordinate with two outer pyridine-N atoms of two distinct ligands and the S and N atoms from three thiocyanate groups, with one of which being generated by symmetry operation (code: −x + 1, −y + 1, −z + 2). The Hg–Npyridine distances are 2.569(9) and 2.503(9) Å, respectively, significantly longer than those relevant Hg–N bonds in 1–3, while the Hg–NNCS distance is 2.481(9) Å. Unexpectedly, in 4 each ligand binds to the Hg center with two of its terminal pyridine rings and the extra pyridine ring in the 4′-position is found to be uncoordinated. The observation of the bidentate coordinating mode of L4 here is clearly different from that in most of known metal assemblies with this ligand.7,30 However, this is not unusual, as we have reported a 1-D zig–zag chain in Zn2(L4)(OAc)4, where the 4′-(4-pyridyl) group of 4,2′:6′,4′′-tpy remained non-bound.8 Despite the non-coordinating nature of the 4′-substituent in L4, the 4′-(4-pyridyl) group seems to play an important role in altering the coordination patterns around Hg centers and hence the resulting coordination networks. In 4, the ligands act as bidentate linear linkers to extend the NCS-bridging HgII centres to form a bilayered ladder-like structure with chains running along the crystallographic b-axis (Fig. 4b). The separation between the bridged HgII atoms is 5.603 Å, while the distance between adjacent Hg centers around the same ligand molecule is 14.013 Å. In the ligand, the outer pyridines of tpy region are distorted out of the central pyridine plane by 16.99° and 17.23°, respectively, whereas the 4′-pyridine ring is well co-planar with the center pyridine. Interestingly, ligand molecules besides bridged Hg centers within the ladder-like chain pack closely in a head-to-head fashion via significant π-stacking interactions (the shortest carbon⋯centroid distance between the terminal and central pyridine rings of the two layers is 3.293 Å). Furthermore, packing of the ladder-like chains along the crystallographic c-axis leads to an 2-D arrangement through effective π-stacking between the 4′-(4-pyridyl) and central pyridyl rings (Fig. 4c). Similar layered ladder-like polymeric chains have been observed in coordination assemblies including bilayered, trilayered or even multilayered polymeric chains with several 4′-substituted 4,2′:6′,4′′-tpy ligands as linkers, where a variety of metal cluster nodes such as {Zn2(OAc)4}, {Cd2(OAc)4}, {Mn3(OAc)6}, {Zn5(OAc)10} and {Zn7(μ-OAc)10(μ4-O)2} were found.10–12 However, all these structures are composed of acetate anions as bridging ligands, and ligand L4 were unexplored in those reports. Therefore, the use of {Hg2(NCS)4} cluster as a bridging node in 4 opens a new pathway to assemble novel layered coordination networks with ligands of this type.
To further investigate the role of 4′-(4-pyridyl) group in determining the coordinating mode of the resulting Hg assemblies, we switched the 4′-substituent to a 4′-(2-pyridyl) group and accordingly ligand L5 was utilized for the same reaction. In contrast, L5 is anticipated to behave as a ditopic ligand similar to L1–3, as the 2-pyridyl group in the 4′-position of 4,2′:6′,4′′-tpy is believed to be inert to metal coordination due to large steric hindrance. Thus, the same layering reaction was conducted and X-ray quality crystals of 5 were harvested after two weeks. 5 was found to be isomorphous to 4 and the structural analysis revealed a similar bilayered ladder-like structure (Fig. 5a and b), although some of bond parameters are slightly different from those of 4. In 5, the 4′-(2-pyridyl) group is almost co-planar with the central pyridine ring and not bound to any metal center as expected, yet participate in π-stacking interactions within the ladders. It is interesting to note that although the non-coordinated 4′-(4-pyridyl) groups in L1 and L5 are structurally similar to the phenyl group in L1, the corresponding Hg(NCS)2 assemblies are essentially different. It is very possible that the co-planarity between the 4′-substituent and central pyridine has driven to generate the bilayered structure in 4 and 5 through the formation of interlayer head-to-head π-stacking which is absent in 1–3, although other factors including electronic and steric effects can not be excluded.
Conclusions
In conclusion, five new HgII(NCS)2-based coordination polymers from the known 4,2′:6′,4′′-tpy ligands with various 4′-substituents have been prepared and characterized by X-ray structural analyses. Single-crystal structures of compounds 1–5 revealed the important influence of different 4′-substituents in 4,2′:6′,4′′-tpy on the structures of the resulting HgII assemblies. Whereas similar 1-D polymer chain was observed in the structures of 1–3, slight differences on both the chains and the packing modes between chains led to distinct helical structures, owing to the existence of differing 4′-substituents. However, polymers 4 and 5 assembled from ligands containing additional N-binding sites in the 4′-position of 4,2′:6′,4′′-tpy adopt totally new bilayered ladder-like structures formed through the bridging thiocyanate groups, despite the N-donor remain uncoordinated in the metal assemblies. The results represent the first examples of mercury(II) complexes derived from 4,2′:6′,4′′-tpy ligands, further expanding the transition metal chemistry of 4,2′:6′,4′′-tpys.Acknowledgements
We are grateful to the donors of the American Chemical Society Petroleum Research Fund for partial support of this research (#54247-UNI3) and the Program for Research Initiatives for Science Majors (PRISM) at John Jay College funded by the Title V, HSI-STEM and MSEIP programs within the U.S. Department of Education; the PAESMEM program through the National Science Foundation; and New York State's Graduate Research and Teaching Initiative. Funding for this work was also provided by a Seed grant from the Office for the Advancement of Research at CUNY John Jay College.Notes and references
- M. Barquín, J. Cancela, M. González, J. Garmendia, J. Quintanilla and U. Amador, Polyhedron, 1998, 17, 2373 CrossRef.
- G. W. V. Cave and C. L. Raston, J. Supramol. Chem., 2002, 2, 317 CrossRef CAS.
- C. E. Housecroft, Dalton Trans., 2014, 436, 594 Search PubMed.
- C. E. Housecroft, CrystEngComm, 2015, 17, 7461 RSC.
- E. C. Constable, G. Zhang, C. E. Housecroft, M. Neuburger and J. A. Zampese, CrystEngComm, 2009, 11, 2279 RSC.
- E. C. Constable, G. Zhang, E. Coronado, C. E. Housecroft and M. Neuburger, CrystEngComm, 2010, 12, 2139 RSC.
- E. C. Constable, G. Zhang, C. E. Housecroft and J. A. Zampese, CrystEngComm, 2011, 13, 6864 RSC.
- G. Zhang, Y. Jia, W. Chen, W. F. Lo, N. Brathwaite, J. A. Golen and A. L. Rheingold, RSC Adv., 2015, 5, 15870 RSC.
- Z. Yin, S. Zhang, S. Zheng, J. A. Golen, A. L. Rheingold and G. Zhang, Polyhedron, 2015, 101, 139 CrossRef CAS.
- Y. M. Klein, E. C. Constable, C. E. Housecroft and J. A. Zampese, Polyhedron, 2014, 81, 98 CrossRef.
- F. Yuan, X. Wang, H.-M. Hu, S.-S. Shen, R. An and G.-L. Xue, Inorg. Chem. Commun., 2014, 48, 26 CrossRef CAS.
- E. C. Constable, C. E. Housecroft, S. Vujovic and J. A. Zampese, CrystEngComm, 2014, 16, 328 RSC.
- T. Hajiashrafi, A. Morsali and M. Kubicki, Polyhedron, 2015, 100, 257 CrossRef CAS.
- H. R. Khavasi and B. M. M. Sadegh, Inorg. Chem., 2010, 49, 5356 CrossRef CAS PubMed.
- B. Liu, D.-J. Qian, M. Chen, T. Wakayama, C. Nakamura and J. Miyake, Chem. Commun., 2006, 30, 3175 RSC.
- F. Jin, H. Wang, Y. Zhang, M. Yang, J. Zhang, J. Wu, Y. Tian and H. Zhou, J. Coord. Chem., 2013, 66, 3686 CrossRef CAS.
- A. Morsali and L.-G. Zhu, Helv. Chim. Acta, 2006, 89, 81 CrossRef CAS.
- G. Mahmoudi and A. Morsali, CrystEngComm, 2007, 9, 1062 RSC.
- G. Mahmoudi and A. Morsali, Inorg. Chim. Acta, 2009, 362, 3238 CrossRef CAS.
- G. Mahmoudi, A. Morsali, A. D. Hunter and M. Zeller, CrystEngComm, 2007, 9, 704 RSC.
- G. Mahmoudi, A. Morsali and L.-G. Zhu, Polyhedron, 2007, 26, 2885 CrossRef CAS.
- M. Nolte, I. Pantenburg and G. Meyer, Z. Anorg. Allg. Chem., 2005, 631, 2923 CrossRef CAS.
- A. Morsali and M. Y. Masoomi, Coord. Chem. Rev., 2009, 253, 1882 CrossRef CAS.
- W. Levason and C. A. McAuli, The Chemistry of Mercury, McMillan, London, 1977 Search PubMed.
- Z. H. Chohan and S. K. A. Sheazi, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 1999, 29, 105 CrossRef CAS.
- H. Sahebalzamani, S. Ghammamy, K. Mehrani and F. Salimi, Der Chemica Sinica, 2010, 1, 39 CAS.
- Z. Yin, G. Zhang, T. Phoenix, S. Zheng and J. C. Fettinger, RSC Adv., 2015, 5, 36156 RSC.
- DOFFRACplus EVA (version 15), Software Package for Powder Diffraction, Bruker AXS Inc., Madison, WI, 2009 Search PubMed.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, Mercury: visualization and analysis of crystal structures, J. Appl. Crystallogr., 2006, 39, 453 CrossRef CAS.
- K.-J. Chen, J. J. Perry IV, H. S. Scott, Q.-Y. Yang and M. J. Zaworotko, Chem. Sci., 2015, 6, 4784 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1017551 and 1413156–1413159. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra24044a |
| This journal is © The Royal Society of Chemistry 2016 |