Structure and electrical properties of lead-free Sr1−x(K,Ce)x/2(Na0.5Bi0.5)Bi4Ti5O18 piezoelectric ceramics
Zhongran Yaoa,
Ruiqing Chu*a,
Zhijun Xua,
Jigong Haoa,
Juan Dua and
Guorong Lib
aCollege of Materials Science and Engineering, Liaocheng University, Liaocheng 252059, People’s Republic of China. E-mail: ruiqingchu@sohu.com; rqchu@lcu.edu.cn; Fax: +86 635 8239106; Tel: +86 635 8239106
bThe State Key Lab of High Performance Ceramics and Superfinemicrostructure, Shanghai Institute of Ceramics, Chinese Academy of Science, Shanghai 200050, People’s Republic of China
First published on 21st January 2016
Abstract
Bismuth layer-structured ferroelectrics with a formula of Sr1−x(K,Ce)x/2(Na0.5Bi0.5)Bi4Ti5O18 (KCSNBT-x, x = 0.0, 0.1, 0.2 and 0.3), were prepared by a conventional solid-state reaction method. The X-ray diffraction analysis suggested that the substitution led to the formation of a layered perovskite structure. Plate-like morphologies of the grains were clearly observed in all samples. The activation energy value of the ionic conductivity suggested that defects were related to oxygen vacancies. Excellent electrical properties (e.g., d33 ∼ 21 pC N−1, 2Pr ∼ 16.4 μC cm−2 and Tc ∼ 567 °C) were simultaneously obtained in the ceramic where x = 0.3. Additionally, thermal annealing studies indicated that the piezoelectric constant (d33) of the KCSNBT-0.1 ceramic remains almost unchanged (22 pC N−1, only decreased by 4%) at temperatures below 400 °C, demonstrating that this ceramic is a promising candidate for high-temperature applications.
1. Introduction
Bismuth layer-structured ferroelectrics (BLSFs), also known as the Aurivillius family of oxides, have been considered as promising lead-free piezoelectric materials in high-temperature applications.1,2 Compared with the traditional perovskite structure, the structure of these ferroelectrics, represented as (Bi2O2)2+(Am−1BmO3m+1)2−, consists of [Bi2O2]2+ layers interleaved with perovskite blocks of [Am−1BmO3m+1]2− units along the c-axes. The A-site in the perovskite blocks can be occupied by 12-coordinated cations such as Bi3+, La3+, Ba2+, Sr2+, Ca2+, etc., while the B-site can be occupied by 6-coordinated cations such as Ti4+, Nb5+, Ta5+, W6+, Mo6+, Co3+, etc. In addition, m, in the range of 1–5, represents the number of perovskite blocks, and the ferroelectric and dielectric properties of BLSFs are strongly dependent on its value.3–7 BLSF ceramics possess some outstanding electrical properties, such as a high Curie temperature, low dielectric loss, excellent fatigue endurance and low aging rate, making BLSFs a promising candidate for ferroelectric non-volatile random access memory (FRAM) storage devices.8SrNa0.5Bi4.5Ti5O18 (abbreviated as SBT), is the m = 5 member of the Aurivillius family, where Bi2O2 layers alternate with (SrNa0.5Bi2.5Ti5O16) perovskite blocks built by five TiO6 octahedral layers and hosting Sr and Bi at the A site. This material has drawn special attention because of its high Curie temperature.9 However, similar to other BLSF compounds, this material exhibits relatively low piezoelectric coefficients (d33) and low remnant polarization (2Pr) because spontaneous polarization is restricted in the a–b plane.9,10 To overcome this shortcoming, many efforts have been made to improve its dielectric and piezoelectric properties by adding some additives in the A-site and/or B-site.11,12 In these studies, A-site substitution is more effective than that of B-site substitution because the cations in the B-site have a similar size and do not make a major contribution to the polarization process in BLSFs. For instance, Ji et al.13 reported that the SrNa0.5Bi4.5Ti5O18 + 1 wt% CeO2 sample shows an excellent Curie temperature (586 °C) and very large piezoelectric coefficient (24 pC N−1). Chen et al.10 investigated the properties of Sr2−x(Na, K)xBi4Ti5O18 composite ceramics and found that the piezoelectric constant d33 and remnant polarization 2Pr were improved, with values of 20 pC N−1 and 20 μC cm−2 respectively, whereas the Curie temperature was only 324 °C. Additionally, it was found that (MCe) (M = Li, Na, K) dopants can efficiently enhance the piezoelectric coefficients for even-layer structured Aurivillius type compounds compared to the pure compositions.14,15
Nevertheless, very little is known about the detailed electrical properties of (KCe) modified Sr(Na0.5Bi0.5)Bi4Ti5O18 (SNBT) ferroelectric ceramics. Therefore, in the present work, A-site (KCe)-doped Sr(Na0.5Bi0.5)Bi4Ti5O18 ceramics were prepared using a conventional solid-state sintering method, and the effect of (KCe) modification in the A-site on their electrical properties was investigated. Additionally, the underlying physical mechanisms for enhanced piezoelectricity and electrical conductivity have been addressed.
2. Experimental details
Sr1−x(K,Ce)x/2(Na0.5Bi0.5)Bi4Ti5O18 (abbreviated as KCSNBT-x, x = 0, 0.1, 0.2 and 0.3) ceramics were prepared by a conventional solid-state reaction method using reagent-grade metal oxides or carbonate powders of K2CO3 (99%, Sinopharm Chemical Reagent Co., Ltd., China), CeO2 (99.95%, Sinopharm Chemical Reagent Co., Ltd., China), Bi2O3 (99.99%, Sinopharm Chemical Reagent Co., Ltd., China), SrCO3 (99%, Sinopharm Chemical Reagent Co., Ltd., China), TiO2 (99.5%, Sinopharm Chemical Reagent Co., Ltd., China) and Na2CO3 (99.49%, Sinopharm Chemical Reagent Co., Ltd., China) as the starting materials. All raw materials were weighed in stoichiometric proportions and then mixed using planetary ball milling in polyethylene with stabilized zirconia balls for 15 h, using ethanol as the solvent. After drying, the mixed powders were calcined at 800 °C for 2 h. After calcination, the mixture was milled again for 12 h, and then dried. The powders were mixed with an appropriate amount of polyvinyl butyral (PVB) binder, and pressed into pellets with a diameter of 12 mm and a thickness of 0.7 mm under a pressure of about 200 MPa. After burning off the PVB at 850 °C, the ceramics were sintered in an alumina crucible at 1180 °C for 3 h. For the electric measurements, disk samples with about 0.3 mm thickness were used.The density of the sintered ceramics was measured by means of the Archimedes method. The crystal structure of the ceramics was determined by X-ray diffraction (XRD) using Cu Kα radiation (λ = 1.54178 Å) (D8 Advance, Bruker Inc., Germany). The surface morphology of the ceramics was observed using a scanning electron microscope (SEM) (JSM-6380, Japan). The ferroelectric hysteresis loops were measured through a standardized ferroelectric test system (TF2000, Germany). The temperature dependence of dielectric properties and impedance spectroscopy for the samples was performed using a Broadband Dielectric Spectrometer (Novocontrol Germany). The samples were polarized in silicon oil in the range of 150–180 °C for 20 min, and piezoelectric measurements were carried out with a quasi-static d33-meterYE2730 (SINOCERA, China).
3. Results and discussion
The X-ray diffraction spectra of the KCSNBT-x ceramics (x = 0.0, 0.1, 0.2, 0.3) in the 2θ range of (a) 20–50° and (b) 28–32° are plotted in Fig. 1. As shown in Fig. 1(a), the Aurivillius structure can be identified by indexing all the diffraction peaks on the basis of an orthorhombic cell (PDF#no. 05-0626),13 indicating that the K+ and Ce3+ ions diffused into the A-site lattice and formed solid solutions as expected. Meanwhile, it is obvious that the highest diffraction peak in KCSNBT-x ceramics corresponds to the (10![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ) orientation, which is consistent with the fact that it is the strongest diffraction peak in BLSFs.16 In addition to the main Aurivillius phase, secondary phase Bi2Ti2O7 (PDF#no. 32-0118, labeled by *) in the KCSNBT-x samples can also be detected regardless of the existence of (KCe), as shown in Fig. 1(b). It seems that this peak could be related to the volatilization of bismuth species at elevated temperatures.17 Similar deficiency behavior of bismuth species was also found during the sintering of another bismuth-layered compound, SrBi4Ti4O15.18,19 In order to further confirm the crystallographic evolution of KCSNBT-x ceramics, the lattice parameters of the samples were calculated and are shown in the inset part of Fig. 1(a). It was found that the lattice parameters a, b, and c varied with increasing x values, demonstrating that the (KCe) substitution reduced the lattice distortion of SNBT-based ceramics. Such a lattice distortion can be attributed to the replacement of the A-site Sr2+ by K+ and Ce3+, and it is supposed to lead to enhanced electrical properties.
) orientation, which is consistent with the fact that it is the strongest diffraction peak in BLSFs.16 In addition to the main Aurivillius phase, secondary phase Bi2Ti2O7 (PDF#no. 32-0118, labeled by *) in the KCSNBT-x samples can also be detected regardless of the existence of (KCe), as shown in Fig. 1(b). It seems that this peak could be related to the volatilization of bismuth species at elevated temperatures.17 Similar deficiency behavior of bismuth species was also found during the sintering of another bismuth-layered compound, SrBi4Ti4O15.18,19 In order to further confirm the crystallographic evolution of KCSNBT-x ceramics, the lattice parameters of the samples were calculated and are shown in the inset part of Fig. 1(a). It was found that the lattice parameters a, b, and c varied with increasing x values, demonstrating that the (KCe) substitution reduced the lattice distortion of SNBT-based ceramics. Such a lattice distortion can be attributed to the replacement of the A-site Sr2+ by K+ and Ce3+, and it is supposed to lead to enhanced electrical properties.
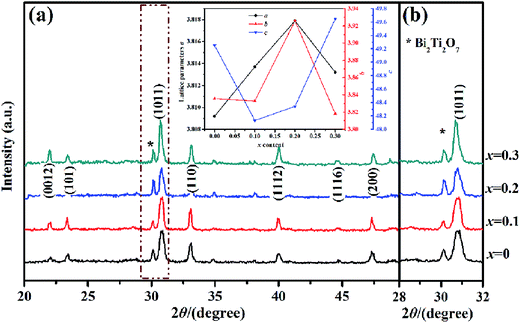 | ||
| Fig. 1 XRD patterns of the KCSNBT-x ceramics sintered at 1180 °C; inset: detailed information on the response of the variation of lattice parameters as a function of x. | ||
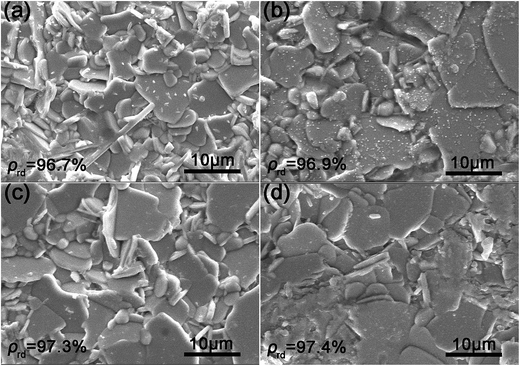
The SEM images of the surface morphologies of the KCSNBT-x ceramics (x = 0.0, 0.1, 0.2, 0.3) are displayed in Fig. 2. It was found that all grains in all of the samples were well-packed, and pore-free microstructures were observed. In addition, (KCe)-substituted SNBT ceramics have uniform grain sizes, indicating that the increased doping with (KCe) does not change the microstructure dramatically. Due to the highly anisotropic grain growth rate in the direction of the a–b plane and the lower surface energies of the (001) planes induced predominantly during sintering, all the specimens are composed of plate-like grains, which is a typical characteristic of Aurivillius ceramics.13 Moreover, all ceramics have a high relative density ρrd (>96%), suggesting that all samples have been well sintered.
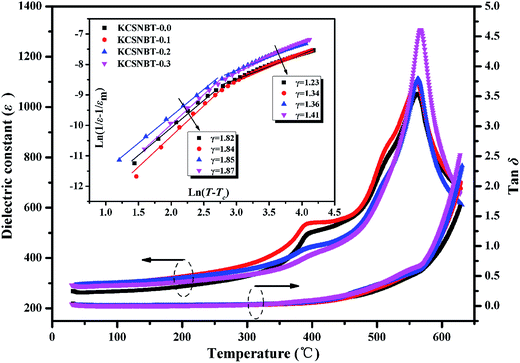
The temperature dependence of the dielectric constant (ε) and loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of the KCSNBT-x ceramics (x = 0.0, 0.1, 0.2, 0.3) measured at 10 kHz is depicted in Fig. 3. Two phase transitions were observed at ∼400 °C and ∼550 °C, corresponding to the ferroelectric–ferroelectric and ferroelectric–paraelectric phase transitions.20 Such a phenomenon was also reported in Ca-doped SrNa0.5Bi4.5Ti5O18 ceramics.13 In addition, Fig. 3 clearly shows that the value of the Curie temperature (Tc) corresponding to the ferroelectric–paraelectric phase transition of ferroelectrics was slightly increased from 562 °C to 570 °C, which is higher than the reported results for other Aurivillius material systems (Table 1). Moreover, as shown Fig. 3, the dielectric loss values were found to be very low (less than 7%) and very stable when the measurement temperature is below 400 °C for all compositions. These results show that the KCSNBT-x ceramics possess high stability in their dielectric behavior, which is of great importance for high-temperature device applications. When the temperature was above 500 °C, the tan
δ) of the KCSNBT-x ceramics (x = 0.0, 0.1, 0.2, 0.3) measured at 10 kHz is depicted in Fig. 3. Two phase transitions were observed at ∼400 °C and ∼550 °C, corresponding to the ferroelectric–ferroelectric and ferroelectric–paraelectric phase transitions.20 Such a phenomenon was also reported in Ca-doped SrNa0.5Bi4.5Ti5O18 ceramics.13 In addition, Fig. 3 clearly shows that the value of the Curie temperature (Tc) corresponding to the ferroelectric–paraelectric phase transition of ferroelectrics was slightly increased from 562 °C to 570 °C, which is higher than the reported results for other Aurivillius material systems (Table 1). Moreover, as shown Fig. 3, the dielectric loss values were found to be very low (less than 7%) and very stable when the measurement temperature is below 400 °C for all compositions. These results show that the KCSNBT-x ceramics possess high stability in their dielectric behavior, which is of great importance for high-temperature device applications. When the temperature was above 500 °C, the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ value significantly increased, arising from the space charge carriers induced by the increase of electrical conductivity.21
δ value significantly increased, arising from the space charge carriers induced by the increase of electrical conductivity.21
It is well known that the nature of the phase transition is determined by calculating the degree of diffusion (γ) in the measured dielectric constant by modifying Curie–Weiss law:5
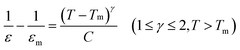 | (1) |
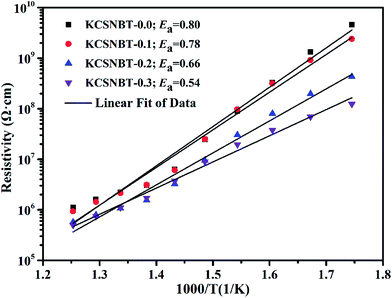
Fig. 4 shows the DC resistivity of the KCSNBT-x ceramics as a function of reciprocal temperature. The resistivity of all compositions is higher than 105 Ω cm at 525 °C. This is important for high temperature piezoelectric sensor applications. Furthermore, the behavior of the temperature dependent resistivity follows the Arrhenius relationship:
ρ = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/kBT) exp(−Ea/kBT)
| (2) |
| Samples (x) | ε | tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (%) δ (%) |
Tc (°C) | Ea (eV) | ρrd (%) | Ec (kV cm−1) | 2Pr (μC cm−2) | d33 (pC N−1) |
|---|---|---|---|---|---|---|---|---|
| 0.0 | 267 | 3.4 | 562 | 0.80 | 96.7 | 34 | 8 | 20 |
| 0.1 | 291 | 1.2 | 563 | 0.78 | 96.9 | 36 | 11 | 23 |
| 0.2 | 293 | 1.4 | 564 | 0.66 | 97.3 | 50 | 12.8 | 21 |
| 0.3 | 291 | 3.0 | 567 | 0.54 | 97.4 | 59 | 16.4 | 21 |
Fig. 5(a) shows P–E hysteresis of the KCSNBT-x ceramics measured at a frequency of 10 Hz under a maximum electric field of 140 kV cm−1 and at a temperature of 180 °C. The measured remnant polarization (2Pr) and coercive field (Ec) values with varying (KCe) content (x) in SNBT ceramics are listed in Table 2. It is evident that the remnant polarization (2Pr) and coercive field (Ec) both increase gradually with an increase of the (KCe) doping content. When the doping content is 0.3, 2Pr and Ec for KCSNBT-x simultaneously exhibit maximum values of 16.4 μC cm−2 and 59 kV cm−1, respectively. 2Pr is much higher than that of the pure SNBT ceramic, indicating that the ferroelectric properties of the SNBT ceramic have been enhanced by (KCe)-doping. The remarkable enhancement in ferroelectric polarization could be mainly attributed to the crystal lattice distortion originating from the replacement of the A-site Sr2+ by K+ and Ce3+, which is also substantiated by the XRD results, in which the lattice parameters a, b and c varied with increasing x values. Moreover, the ferroelectricity in BLSFs is related to the tilting of oxygen in octahedral BO6 from the c axis and its rotation in the a–b plane.30,31 In view of the difference of the mean ionic radius caused by the introduction of (KCe) into the SNBT ceramics, the tilting and rotation of oxygen in octahedral TiO6 have been increased, which should be responsible for the enhanced ferroelectric properties.32 Meanwhile, the increased Ec is consistent with the assumption (Fig. 4) that oxygen vacancies are formed. Under high electric fields, mobile oxygen vacancies can assemble at the low energy domain walls, and thereby hinder domain switching due to domain pinning.33 In order to further realize the polarization state of the KCSNBT-x ceramics, the polarization current curves of the ceramics were collected, as shown in Fig. 5(b). One sharp polarization current peak could be observed when the applied electric field reached Ec, indicating that the ferroelectric domain could be easier to switch when the driving electric field increases to Ec.34
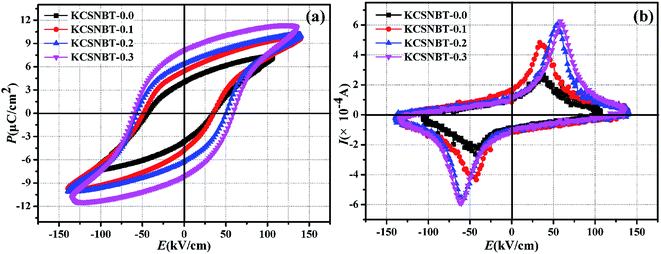 | ||
| Fig. 5 (a) P–E hysteresis loops of the KCSNBT-x ceramics with different values of x at 180 °C; (b) I–E loops of the KCSNBT-x ceramics at 180 °C. | ||
Fig. 6 gives the thermal annealing behavior of the piezoelectric constant (d33) of the KCSNBT-x ceramics depolarized at different temperatures held for 10 min. An excellent piezoelectric coefficient of 23 pC N−1 is found when x is 0.1 as shown in Table 2, which is higher than the reported results in other Aurivillius material systems (Table 1).22–24 In addition, the values of d33 were not zero when the depolarization temperature was higher than the first dielectric anomalies (∼400 °C). However, when the depolarization temperature was higher than the second dielectric anomalies, d33 was zero. Therefore, in the x range of 0–0.3, the KCSNBT-x piezoelectric materials underwent a ferroelectric–ferroelectric transition at the temperature of the first dielectric anomalies and a ferroelectric–paraelectric transition at the temperature of the second dielectric anomalies, as shown in Fig. 3. Moreover, the inset of Fig. 6 clearly shows that the piezoelectric constant (d33) of the KCSNBT-0.1 ceramic remains almost unchanged (22 pC N−1, a decrease of only 4%) at temperatures below 400 °C, indicating that the ceramic has excellent temperature stability, so that it is very tolerant to thermal annealing and might be an appropriate candidate for high temperature applications.
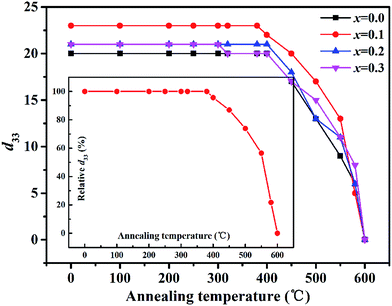 | ||
| Fig. 6 Annealing temperature dependence of the piezoelectric coefficient (d33) for the KCSNBT-x specimens. The inset shows the relative d33 (%) values of the KCSNBT-0.1 sample. | ||
4. Conclusion
KCSNBT-x ceramics were prepared by a conventional solid-state sintering method, and their structure and electrical properties were studied. The KCSNBT-x ceramics presented a typical layered perovskite structure. The morphologies of these Aurivillius ceramics show the grains in all the samples are all well-defined and the plate-like morphologies of the samples can be observed. The Tc increases slightly from 562 °C to 567 °C. The activation energy (Ea) values of the ionic conductivity suggested that defects were related to oxygen vacancies. The 2Pr increased up to a value of 16.4 μC cm−2 with (KCe)-modifications. Meanwhile, the ceramics showed excellent thermal stability when the annealing temperature was below 400 °C. As a result, a high Tc and d33, and good thermal stability have been attained in the KCSNBT-x ceramics. Therefore, such a material system is a potential candidate for high-temperature piezoelectric applications.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51372110, 51402144, 51302124, 51302025), National High Technology Research and Development Program of China (No. 2013AA030801), Science and Technology Planning Project of Guangdong Province, China (No. 2013B091000001), Independent innovation and achievement transformation in Shandong Province special, China (No. 2014CGZH0904), The Project of Shandong Province Higher Educational Science and Technology Program (No. J14LA11, No. J14LA10) and Research Foundation of Liaocheng University (No. 318011301, No.318011306).References
- B. H. Park, B. S. Kang, S. D. Bu, T. W. Noh and J. Lee, Nature, 1999, 401, 682–684 CrossRef CAS.
- P. Xiao, Y. Guo, M. Tian, Q. Zheng, N. Jiang, X. Wu, Z. Xia and D. Lin, Dalton Trans., 2015, 44, 17366–17380 RSC.
- J. S. Kim, M. S. Jang, I. W. Kim and K. S. Lee, J. Electroceram., 2006, 17, 129–133 CrossRef CAS.
- J. Xiao, H. Zhang, Y. Xue, Z. Lu, X. Chen, P. Su, F. Yang and X. Zeng, Ceram. Int., 2015, 41, 1087–1092 CrossRef CAS.
- S. Ye, J. Fuh, L. Lu, Y.-l. Chang and J.-R. Yang, RSC Adv., 2013, 3, 20693–20698 RSC.
- J. Wu, Y. Wang and H. Wang, RSC Adv., 2014, 4, 64835–64842 RSC.
- A. Kaushal, S. M. Olhero, B. Singh, R. Zamiri, V. Saravanan and J. M. F. Ferreira, RSC Adv., 2014, 4, 26993–27002 RSC.
- Y. Zhong-Ran, C. Rui-Qing, X. Zhi-Jun, H. Ji-Gong and L. Guo-Rong, J. Inorg. Mater., 2015, 30, 989–994 CrossRef.
- L. Fei, Z. Zhou, S. Hui and X. Dong, Ceram. Int., 2015, 41, 9729–9733 CrossRef CAS.
- Q. Chen, Z. Xu, R. Chu, J. Hao, Y. Zhang, G. Li and Q. Yin, Phys. B, 2010, 405, 2781–2784 CrossRef CAS.
- J. D. Bobić, M. M. Vijatović Petrović, J. Banys and B. D. Stojanović, Ceram. Int., 2013, 39, 8049–8057 CrossRef.
- D. Peng, H. Zou, C. Xu, X. Wang and X. Yao, J. Alloys Compd., 2013, 552, 463–468 CrossRef CAS.
- W. Ji, R. Chu, Z. Xu, J. Hao, R. Cheng, X. Chen and Y. Xu, J. Mater. Sci.: Mater. Electron., 2015, 26, 5686–5689 CrossRef CAS.
- L. Sun, Q. Chen, D. Wu, J. Wu, Z. Tan, D. Xiao and J. Zhu, J. Alloys Compd., 2015, 625, 113–117 CrossRef CAS.
- L. Sun, Q. Chen, J. Wu, Z. Peng, Z. Tan, D. Xiao and J. Zhu, Ceram. Int., 2014, 40, 14159–14163 CrossRef CAS.
- Z. Yao, R. Chu, Z. Xu, J. Hao, D. Wei and G. Li, J. Mater. Sci.: Mater. Electron., 2015, 26, 8740–8746 CrossRef CAS.
- L. Nibou, A. Aftati, M. El Farissi and J.-P. Mercurio, J. Eur. Ceram. Soc., 1999, 19, 1383–1386 CrossRef CAS.
- C.-H. Lu and C.-H. Wu, J. Eur. Ceram. Soc., 2002, 22, 707–714 CrossRef CAS.
- E. Khomyakova, J. Pavlic, M. Makarovic, H. Ursic, J. Walker, T. Rojac, B. Malic and A. Bencan, J. Eur. Ceram. Soc., 2015, 35, 4163–4171 CrossRef CAS.
- Z. Xu, R. Chu, J. Hao, Y. Zhang, Q. Chen, L. Zhao, G. Li and Q. Yin, J. Alloys Compd., 2009, 487, 585–590 CrossRef CAS.
- Z. Peng, Q. Chen, D. Liu, Y. Wang, D. Xiao and J. Zhu, Curr. Appl. Phys., 2013, 13, 1183–1187 CrossRef.
- P. Nayak, S. R. Mohapatra, P. Kumar and S. Panigrahi, Ceram. Int., 2015, 41, 9361–9372 CrossRef CAS.
- X. Meng, W. Ma, T. Chen, M. Wang and Y. Guo, Electron. Mater. Lett., 2015, 11, 902–905 CrossRef CAS.
- R. Singh, V. Luthra, R. S. Rawat and R. P. Tandon, Ceram. Int., 2015, 41, 4468–4478 CrossRef CAS.
- C. Diao, H. Li, Z. Chen and H. Zheng, Ceram. Int., 2016, 42, 621–626 CrossRef CAS.
- V. Senthil, T. Badapanda, A. Chandrabose and S. Panigrahi, Mater. Lett., 2015, 159, 138–141 CrossRef CAS.
- A. Peláiz-Barranco and Y. González-Abreu, Solid State Commun., 2009, 149, 2082–2084 CrossRef.
- A. Palanduz and D. Smyth, J. Electroceram., 2003, 11, 191–206 CrossRef CAS.
- S. Bharadwaja and S. Krupanidhi, J. Appl. Phys., 1999, 86, 5862–5869 CrossRef CAS.
- J. Rödel, W. Jo, K. T. Seifert, E. M. Anton, T. Granzow and D. Damjanovic, J. Am. Ceram. Soc., 2009, 92, 1153–1177 CrossRef.
- Z. Yao, H. Liu, Y. Liu, Z. Wu, M. Cao and H. Hao, Appl. Phys. Lett., 2008, 92, 2905 Search PubMed.
- T.-L. Zhao, C.-M. Wang, C.-L. Wang, Y.-M. Wang and S. Dong, Mater. Sci. Eng., B, 2015, 201, 51–56 CrossRef CAS.
- A. Khokhar, P. K. Goyal, O. P. Thakur, A. K. Shukla and K. Sreenivas, Mater. Chem. Phys., 2015, 152, 13–25 CrossRef CAS.
- R. Cheng, C. Wang, Z. Xu, R. Chu, J. Hao, H. Li, W. Li, J. Du and G. Li, RSC Adv., 2015, 5, 90508–90514 RSC.
| This journal is © The Royal Society of Chemistry 2016 |