Highly efficient L-lactic acid production from xylose in cell recycle continuous fermentation using Enterococcus mundtii QU 25
Mohamed Ali Abdel-Rahmanab,
Yukihiro Tashirocd,
Takeshi Zendoa,
Kenji Sakaicd and
Kenji Sonomoto*ae
aLaboratory of Microbial Technology, Division of Systems Bioengineering, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan. E-mail: sonomoto@agr.kyushu-u.ac.jp; Fax: +81-92-642-3019; Tel: +81-92-642-3019
bBotany and Microbiology Department, Faculty of Science for Boys, Al-Azhar University, Nasr City, PN 11884, Cairo, Egypt
cLaboratory of Soil and Environmental Microbiology, Division of Systems Bioengineering, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan
dLaboratory of Microbial Environmental Protection, Tropical Microbiology Unit, Center for International Education and Research of Agriculture, Faculty of Agriculture, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan
eLaboratory of Functional Food Design, Department of Functional Metabolic Design, Bio-Architecture Center, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581, Japan
First published on 25th January 2016
Abstract
A few strains of lactic acid bacteria metabolise xylose into optically pure L-lactic acid (LA). This study achieved an effective homofermentative cell recycle continuous fermentation of xylose to L-LA with high concentration, productivity, and yield using Enterococcus mundtii QU 25. In conventional continuous fermentation, the optimal xylose concentration in the feeding solution is 50 g L−1, and the optimal dilution rate is 0.15 h−1. Continuous fermentation with cell recycling using a microfiltration membrane module produced an L-LA concentration of 32.3 g L−1 with a yield of 0.789 g g−1 and a productivity of 5.33 g L−1 h−1. Controlling pH and optimising the feeding medium were important for achieving a high L-LA yield with strain QU 25. Using corn steep liquor-containing medium at pH 6.2, a maximum L-LA concentration, yield and productivity were achieved at 41.0 g L−1, 1.01 g g−1, and 6.15 g L−1 h−1, respectively. This study is the first to report on continuous fermentation with cell recycling for lactic acid production from xylose.
1. Introduction
Lactic acid (LA) demand has been estimated to be growing at 5–8% annually due to its wide applications in the food, and non-food (pharmaceutical, textile, leather) industries.1 Optically pure L- or D-LA has great potential for use in the production of thermoplastics, a biodegradable and biocompatible polylactic acid (PLA), an alternative to traditional non-biodegradable polymers. L- or D-LA can only be produced by microbial fermentation, because chemically synthesised LA from fossil resources is racemic.The high cost of LA manufacturing limits the wide commercial availability of PLA. Therefore, LA production should be improved in terms of (i) utilisation of available substrates and cheap nitrogen sources required for microbial fermentation, (ii) increase of LA concentration, yield and productivity, and (iii) efficient downstreaming processes (LA recovery and purification).1 For that, the use of inedible, abundant, and renewable lignocellulosic substrates composed of cellulose, hemicellulose and lignin as carbon source has attracted great attention in recent years.2,3 Extensive work has been done on the utilisation of the cellulosic fraction, because it consists only of glucose which is available for most microbial fermentations. On the other hand, few lactic acid bacteria (LAB) strains can ferment xylose which is the second abundant sugar in lignocelluloses and the main sugar of hemicellulose fraction. Furthermore, most xylose-fermenting LAB perform hetero-LA fermentation via the phosphoketolase pathway (PK-pathway), which yields equimolar amounts of by-products (acetic acid, ethanol, etc.) to LA.
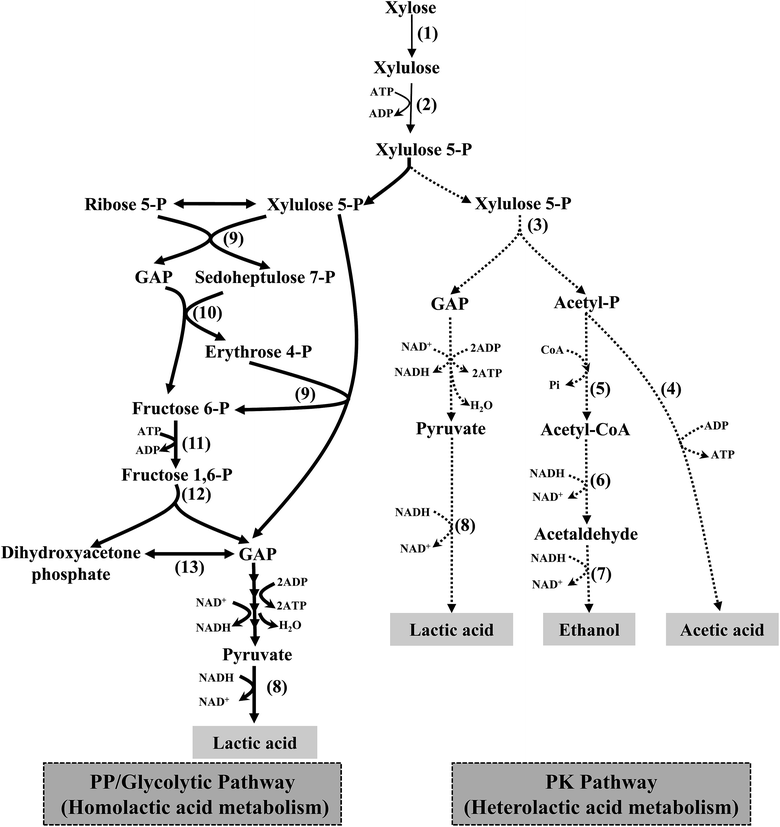
Recently, a potential LAB strain, Enterococcus mundtii QU 25, which is able to produce high yields of optically pure L-LA (>99.9%) via homofermentation from several lignocellulose-derived sugars, including glucose4 and cellobiose,5 was described. In addition, it can also produce LA with minimal amounts of by-products from xylose at 43 °C when the pH is maintained at 7.0. It has been reported that, under high xylose concentrations, only the pentose phosphate pathway (PP-pathway) is used for xylose consumption, thus resulting in high LA yields of up to 0.904 g g−1;6 however, low xylose concentrations lead to low LA yields because, under these conditions, strain QU 25 utilises the PK-pathway as well as the PP-pathway. The hypothetical metabolic pathways of xylose metabolism for LA production are shown in Fig. 1. Furthermore, the low LA productivity of this strain (≤2.08 g L−1 h−1) is an obvious disadvantage for batch fermentation.6
Continuous fermentation is an attractive approach for enhancing LA productivity by reducing end-product inhibition.7,8 To date, there is only one report on conventional continuous LA fermentation with xylose, using Lactococcus lactis IO-1.9 However this strain exhibits heterofermentation when undergoing continuous LA fermentation and thus has low values for yield (0.728 g g−1) and productivity (1.03 g L−1 h−1) as compared with those obtained via batch fermentation by strain QU 25 (0.904 g g−1 and 2.08 g L−1 h−1, respectively).6 Conventional continuous LA fermentation is characterised by low cell and LA concentrations,1 however continuous fermentation with cell recycling achieved high cell density and improved LA concentration and productivity from glucose.10,11 To the authors' knowledge, there have been no reports on continuous fermentation with cell recycling for LA production from xylose. Therefore, the present study aims to investigate the conventional continuous fermentation and cell recycling continuous fermentation with or without cell concentration for homofermentative production of L-LA with high LA concentration, yield, and productivity from xylose by using strain QU 25.
2. Materials and methods
2.1. Microorganism and growth medium
E. mundtii QU 25 NITE BP-965 (ref. 5) was used in this study and stocked at −80 °C in 15% (v/v) glycerol. It was grown and precultured in modified de Man, Rogosa, and Sharpe (mMRS) medium which consisted of 10 g L−1 peptone (Difco™; Becton Dickinson, Franklin Lakes, NJ, USA), 8 g L−1 beef extract (Nacalai Tesque, Kyoto, Japan), 4 g L−1 yeast extract (Nacalai Tesque), 5 g L−1 sodium acetate trihydrate (Nacalai Tesque), 2 g L−1 dipotassium hydrogen phosphate (Sigma, Tokyo, Japan), 2 g L−1 triammonium citrate (Nacalai Tesque), 0.2 g L−1 magnesium sulphate heptahydrate (Nacalai Tesque), 0.05 g L−1 manganese sulphate tetrahydrate (Nacalai Tesque), and 1 mL Tween 80 (Nacalai Tesque) and was supplemented with xylose at various concentrations as described below. Simplified de Man, Rogosa, and Sharpe (sMRS) medium contained 5 g L−1 sodium acetate trihydrate, 2 g L−1 dipotassium hydrogen phosphate, 2 g L−1 triammonium citrate, 0.2 g L−1 magnesium sulphate heptahydrate, 0.05 g L−1 manganese sulphate tetrahydrate, 12.5 g L−1 yeast extract, 12.5 g L−1 corn steep liquor (Sigma), and 1 mL Tween 80. The pH of the media were adjusted to 7.0 using 10 M NaOH or 10 M HCl. The media and xylose solution were sterilised at 121 °C for 15 min separately to prevent heat degradation.2.2. Hollow-fibre microfiltration module
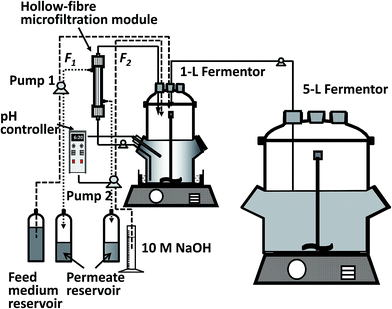
A hollow-fibre microfiltration module (MICROZA PSP 102, Asahi Kasei, Tokyo, Japan) was used to recycle cells back to the fermentor for continuous fermentation with cell recycling. The filtration area of the module was 0.17 m2, and each fibre had a diameter of 0.7 mm and a pore diameter of 0.25 μm. Before use, the module was sterilised with 70% ethanol for >24 h and then washed with sterilised deionised water. After the experiment, the module was washed with sterilised deionised water and sterilised with 1 M NaOH.2.3. Fermentation processes
| D = (F1 + F2)/V, |
To investigate the effect of xylose concentration in the feeding medium, after batch fermentation as described above, continuous fermentation was initiated by feeding with mMRS medium containing 25, 50, 70, or 100 g L−1 xylose at a dilution rate of ca. 0.15 h−1. For each xylose concentration, the feeding medium was changed when biomass and LA production were stable and/or varied less than 10% after at least three retention times (after steady state). Five-millilitre samples of the broth from the fermentor at steady state were withdrawn at least five times for each xylose concentration and analysed for biomass, sugar, and fermentation products.
To investigate the effect of dilution rates, mMRS medium containing ca. 50 g L−1 xylose was used as the feeding medium. Dilution rates were set at 0.08 h−1, 0.12 h−1, 0.15 h−1, and 0.16 h−1. Dilution rates were changed after steady state had been achieved. Samples from the fermentor at steady state were withdrawn at least five times at each dilution rate.
To study the effect of dilution rates on L-LA production, dilution rates were set at 0.093 h−1, 0.113 h−1, and 0.165 h−1. To investigate the effect of pH on LA production and by-product formation, the pH values were set at 5.6, 5.9, 6.2, and 7.0, at a dilution rate of 0.156 ± 0.013 h−1.
2.4. Analytical methods and calculations
Biomass (dry cell weight) was measured by spectrophotometry (UV-1600, Shimadzu, Kyoto, Japan) at a wavelength of 562 nm using a calibration curve. Biomass was calculated as described previously, with an OD of 1.0 corresponding to 0.218 g L−1 dry cell weight of strain QU 25.5 Xylose and fermentation products concentrations were analysed using HPLC (US HPLC-1210, Jasco, Tokyo, Japan) equipped with a SUGAR SH-1011 column (Shodex, Tokyo, Japan). Samples from fermentation broth were centrifuged at 2000 × g for 10 min at 4 °C, the supernatant was filtered using a bacterial filter (Dismic-13HP, Advantec, Tokyo, Japan), and injected into the HPLC system under the following conditions: column temperature, 50 °C; mobile phase, 3 mM HClO4; flow rate, 1.0 mL min−1; and injection volume, 20 μL.The fermentation parameters evaluated in this study were as follows: yield of L-LA to xylose was calculated using the equation YL-LA = CL-LA/CXyl, where YL-LA is the yield of L-LA to xylose (g g−1), CL-LA is the concentration of produced L-LA (g L−1), and CXyl is the xylose consumed (g L−1). The L-LA productivity was calculated using the equation PL-LA = CL-LA × D, where PL-LA is the L-LA productivity (g L−1 h−1), CL-LA is the concentration of produced L-LA (g L−1), and D is the dilution rate (h−1).
3. Results and discussion
3.1. Effect of xylose concentration in the feeding medium on L-LA production in conventional continuous fermentation
To determine suitable xylose concentrations for the feeding medium in continuous fermentation, different concentrations ranging from 25 g L−1 to 100 g L−1 were investigated at a dilution rate of ca. 0.15 h−1 (Table 1). Biomass was ranged from 1.05 g L−1 to 1.61 g L−1, and xylose consumption was ranged from 18.4 g L−1 to 25.4 g L−1. However, the residual xylose increased from 7.43 g L−1 to 80.3 g L−1 as the xylose concentrations in the feeding medium increased from 25.8 g L−1 to 100 g L−1. The highest LA concentration of 21.7 g L−1 was achieved using 50.2 g L−1 xylose in the feeding medium; this concentration also provided the greatest amount of LA productivity (3.14 g L−1 h−1). In contrast, the concentrations of by-products such as acetic acid, formic acid, and ethanol decreased with increasing xylose concentrations in the feeding medium. In particular, continuous fermentation using 25.8 g L−1 xylose in the feeding medium produced the highest by-product concentrations (1.86 g L−1 acetic acid, 3.16 g L−1 formic acid, and 2.58 g L−1 ethanol) and thus resulted in a low LA yield (0.663 g g−1). However, much higher LA yields (≥0.855 g g−1) were obtained with xylose concentrations of 50.2 g L−1 and over, due to the resulting reduction in by-product concentration. These results suggest that the metabolic shift from heterofermentation to homofermentation by strain QU 25 during continuous fermentation is dependent on the xylose concentration in the feeding medium. Consequently, ca. 50 g L−1 was determined to be the optimal concentration of xylose in the feeding medium and was used for further study, even though the level of residual xylose in the fermentation broth was high (24.7 g L−1).| Xylose conc. in feeding media (g L−1) | CXylb (g L−1) | Residual xylose (g L−1) | CL-LAc (g L−1) | Cacetated (g L−1) | Cformatee (g L−1) | Cethanolf (g L−1) | Biomassg (g L−1) | PL-LAh [g L−1 h−1] | YL-LAi (g g−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Conventional continuous fermentation was conducted in a 1 L jar fermentor of 0.4 L working volume at 43 °C and pH controlled at 7.0 with 10 M NaOH, feeding with mMRS medium containing 25.8, 50.2, 71.6, or 100 g L−1 xylose. The dilution rate was set at ca. 0.15 h−1. Averages with standard deviations are based on the averages of measurements for samples withdrawn at least five times in the steady state.b Xylose consumed.c L-Lactic acid produced.d Acetic acid produced.e Formic acid produced.f Ethanol produced.g Dry cell weight.h Productivity of L-lactic acid.i Yield of L-lactic acid to consumed xylose. | |||||||||
| 25.8 | 18.4 ± 0.8 | 7.43 ± 0.84 | 12.2 ± 0.4 | 1.86 ± 0.12 | 3.16 ± 0.21 | 2.58 ± 0.15 | 1.61 ± 0.15 | 1.76 ± 0.06 | 0.663 ± 0.009 |
| 50.2 | 25.4 ± 2.2 | 24.7 ± 2.2 | 21.7 ± 1.2 | 0.621 ± 0.129 | 1.24 ± 0.23 | 1.22 ± 0.23 | 1.35 ± 0.07 | 3.14 ± 0.17 | 0.855 ± 0.027 |
| 71.6 | 18.5 ± 0.9 | 53.1 ± 0.9 | 17.3 ± 1.2 | 0.424 ± 0.086 | 0 | 0 | 1.05 ± 0.18 | 2.39 ± 0.09 | 0.937 ± 0.029 |
| 100 | 20.3 ± 2.7 | 80.3 ± 2.7 | 18.4 ± 1.2 | 0.339 ± 0.065 | 0 | 0 | 1.42 ± 0.08 | 2.47 ± 0.17 | 0.913 ± 0.064 |
Compared with previous work on batch fermentation by strain QU 25 using different initial xylose concentrations, continuous fermentation using similar initial xylose concentrations in the feeding medium resulted in LA productivities that were higher than those obtained with batch fermentation: using initial xylose concentrations of ca. 25, 50, 70, and 100 g L−1, LA productivities for continuous fermentation were 1.76, 3.14, 2.39, and 2.47 g L−1 h−1, respectively, whereas LA productivities for batch fermentation were 1.12, 2.08, 1.25, and 0.904 g L−1 h−1, respectively (Table 1 (ref. 6)). Surprisingly, metabolic shifts from heterofermentation to homofermentation have been observed in both batch and continuous fermentations when the xylose concentration in the feeding medium increased from ca. 25 g L−1 to ca. 50 g L−1, resulting in similar LA yields for batch and continuous fermentations: using initial xylose concentrations of ca. 25, 50, 70, and 100 g L−1, LA yields for continuous fermentation were 0.663, 0.855, 0.937, 0.913 g g−1, respectively, and for batch fermentation were 0.670, 0.904, 0.880, and 0.844 g g−1, respectively. However, the LA produced (21.7 g L−1) was much lower and the residual xylose (24.7 g L−1) was much higher in continuous fermentation than in batch fermentation (44.1 g L−1 and 0.166 g L−1, respectively) when ca. 50 g L−1 xylose was used in the medium; this result may be due to the drastic difference in the resulting biomass (from 5.24 g L−1 to 1.35 g L−1; Table 1 (ref. 6)), and thus may indicate that increasing biomass would improve LA concentration, LA productivity, and residual xylose concentration.
To date, there is only one report on conventional continuous LA fermentation using various xylose concentrations in feeding medium (5.5–69.9 g L−1) by Lc. lactis IO-1.9 Our results for LA concentration, LA yield and LA productivity, and total by-product concentration when 50.2 g L−1 xylose was used in the feeding medium are significantly better than those achieved in that study, which used continuous fermentation with 46.2 g L−1 xylose (13.9 g L−1, 0.596 g g−1, 0.697 g L−1 h−1 and 6.73 g L−1, respectively) (Table 1 (ref. 9)). Note that the high level of LA and low level of by-products achieved with the method described in this study should lead to lower purification costs. Furthermore, changes in LA yield and by-product concentration in response to changes in the xylose concentration in the feeding medium were also observed with Lc. lactis IO-1.9 These data suggest that strain QU 25 may behave in the same manner in continuous fermentation as other LAB and that the xylose concentration in the feeding medium is an important parameter for LA production with continuous fermentation.
Previous studies have revealed that the activities of metabolic enzymes in the PK- and PP-pathways contribute to hetero- and homofermentation in LAB that use xylose as a substrate. Tanaka et al. reported increases in the activity of key enzymes in the PP-pathway (transketolase and transaldolase) and a decrease in the activity of an enzyme in the PK-pathway (phosphoketolase) with increasing xylose concentrations in the feeding medium when using Lc. lactis IO-1 in continuous fermentation. In addition, the activity of pyruvate formate lyase is stimulated at low xylose concentration leading to formic acid formation.9 In batch fermentation using strain QU 25, high transketolase and transaldolase activities and an absence of phosphoketolase activity were detected at a high initial xylose concentration (ca. 100 g L−1), whereas moderate phosphoketolase activity was observed at a low initial xylose concentration (ca. 25 g L−1 (ref. 6)). Gene cluster of PP/glycolytic pathway (including transketolase and transaldolase) for xylose metabolism in QU 25 chromosome was located in a 22 kb region (positions 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904
904![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 895–2
895–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926
926![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 bp), however the genes involved in the phosphoketolase pathway (including phosphoketolase, acetate kinase, phosphotransacetylase, acetaldehyde dehydrogenase, and alcohol dehydrogenase) were dispersed throughout the chromosome.12 These results suggest that changes in the activities of these enzymes may underlie the metabolic shifts in responses to the changes in xylose concentrations of the feeding medium for continuous fermentation with strain QU 25.
710 bp), however the genes involved in the phosphoketolase pathway (including phosphoketolase, acetate kinase, phosphotransacetylase, acetaldehyde dehydrogenase, and alcohol dehydrogenase) were dispersed throughout the chromosome.12 These results suggest that changes in the activities of these enzymes may underlie the metabolic shifts in responses to the changes in xylose concentrations of the feeding medium for continuous fermentation with strain QU 25.
3.2. Effect of dilution rates on L-LA production in conventional continuous fermentation
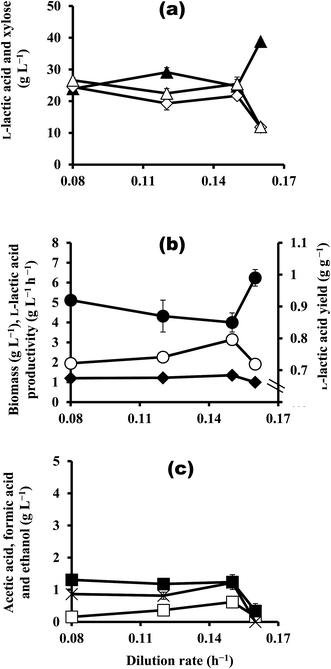
To investigate the effect of dilution rate on continuous LA production, continuous fermentation was conducted at dilution rates of 0.08 h−1, 0.12 h−1, 0.15 h−1, and 0.16 h−1 using feeding medium with 50 g L−1 xylose (Fig. 3a–c). The biomass was almost comparable at dilution rates of 0.08–0.15 h−1, ranging from 1.20 g L−1 to 1.35 g L−1, but biomass fell to 0.997 g L−1 when the dilution rate was increased to 0.16 h−1 (Fig. 3b). At dilution rates of up to 0.15 h−1, the results obtained with the different rates were similar for LA produced (19.3–24.5 g L−1), total by-products (2.33–3.08 g L−1), and residual xylose concentration (23.9–29.1 g L−1); however, when the dilution rate was increased to 0.16 h−1, the results drastically changed, with an LA produced of 11.8 g L−1, a total by-product of 0.510 g g−1, and a residual xylose of 38.3 g L−1 (Fig. 3a and c). These changes may be due to the effects of the dilution rate on biomass. However, high LA yields (0.850–0.991 g g−1) were obtained, indicating that homofermentation occurs at these dilution rates. Furthermore, LA productivity increased from 1.95 g L−1 h−1 to 3.14 g L−1 h−1 as the dilution rate increased from 0.08 h−1 to 0.15 h−1, and then significantly decreased to 1.91 g L−1 h−1 when the dilution rate increased to 0.16 h−1 (Fig. 3b). These results indicate that dilution rate affects LA productivity that increased with an increase of dilution rate, even though similar LA produced (19.3–24.5 g L−1) was obtained with the different dilution rates at 0.08–0.15 h−1. The highest LA productivity, 3.14 g L−1 h−1, was obtained at the dilution rate of 0.15 h−1, together with an LA produced of 21.7 g L−1, an LA yield of 0.855 g g−1, and 1.35 g L−1 of biomass.The dilution rate has been reported to be a critical factor in conventional continuous fermentation and affects various fermentation parameters such as biomass, residual sugars, LA production, and so on.13,14 In particular, a dilution rate that is higher than the threshold value leads to low biomass, which results in high residual sugar concentration and low LA productivity, because a high dilution rate allows insufficient time for the producer strain to ferment sugars.7,15 The results obtained in this study indicated that a dilution rate of 0.15 h−1 is the threshold value for conventional continuous fermentation using strain QU 25 by xylose, because biomass and LA productivity were decreased at dilution rate of 0.16 h−1. Among the reports of LA productivity in conventional continuous fermentation using various sugars, the highest LA productivity (3.14 g L−1 h−1) reported in this study is higher than that by Enterococcus faecium No. 78 using sago starch (1.56 g L−1 h−1)14 and comparable to that by Lactobacillus sp. RKY2 using lignocellulose hydrolysate containing 50 g L−1 glucose (3.1 g L−1 h−1),7 and that by Lactobacillus pentosus CECT-4023 T using trimming vine shoots hydrolysate (3.1 g L−1 h−1).15 LA yields obtained in this study oscillated between 0.850 and 0.991 g g−1 regardless of the dilution rate used. This result indicates that yield is not related to the dilution rate but to the xylose concentration of the feeding media. Several authors have reported that there is no direct relation between dilution rate and LA yield in conventional continuous fermentation.7,15 Because LA is a primary metabolite and its production is associated with cell growth,16 an increase in biomass would improve not only sugar consumption but also LA concentration and productivity.
3.3. Continuous L-LA production with cell recycling
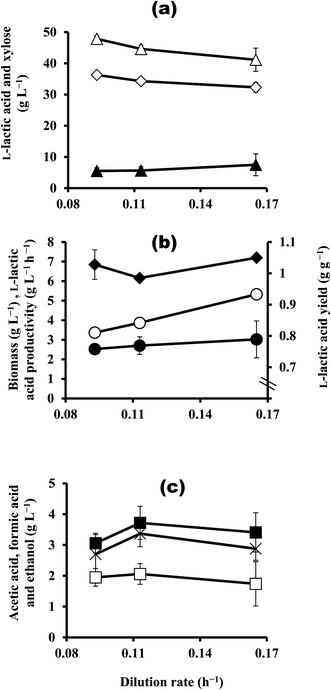
In this study, a hollow-fibre module was integrated into the continuous fermentation system in order to increase the biomass in the fermentor. Continuous fermentation with cell recycling was performed at different dilution rates of 0.093, 0.113, and 0.165 h−1 using feeding medium containing ca. 50 g L−1 xylose at 43 °C and pH 7.0 (Fig. 4a–c). As expected, biomass increased drastically to 6.16–7.20 g L−1 at all the dilution rates (Fig. 4b), compared with 0.997–1.35 g L−1 without cell recycling (Fig. 3b). At the dilution rate of ca. 0.16 h−1 in particular, continuous fermentation with cell recycling exhibited 7.2-times more biomass (7.20 g L−1) than continuous fermentation without cell recycling (0.997 g L−1). This resulted in an increase in xylose consumption (41.2–47.9 g L−1) and a decrease in residual xylose (5.51–7.49 g L−1) as compared to continuous fermentation without cell recycling (11.9–26.6 g L−1 and 23.9–38.8 g L−1, respectively) (Fig. 3a and 4a). As a result, a significant increase in LA produced was observed, with values ranging from 32.3 g L−1 to 36.3 g L−1 (Fig. 4a). With cell recycling, the highest LA productivity, 5.33 g L−1 h−1, was achieved at a dilution rate of 0.165 h−1 (Fig. 4b), as compared to 3.14 g L−1 h−1, which was the highest productivity achieved without cell recycling, at a dilution rate of 0.15 h−1 (Fig. 3b). However, by-product concentrations were higher with cell recycling than without: with cell recycling, the concentration of acetic acid ranged from 1.74 g L−1 to 2.06 g L−1, the concentration of formic acid ranged from 3.06 g L−1 to 3.72 g L−1, and the ethanol concentration ranged from 2.70 g L−1 to 3.37 g L−1 (Fig. 4c); these concentrations were ca. 3–11 fold, 2.8–9 fold, and 2.5–2.7 fold higher, respectively, than those achieved without cell recycling. As a result, the LA yield with cell recycling (0.758–0.789 g g−1; Fig. 4b) was significantly less than that obtained without cell recycling (0.85–0.99 g g−1; Fig. 3b).Enhanced LA productivity via an increase in biomass with cell recycling using microfiltration membranes has been reported for various substrates, including cheese whey permeate,17 sago starch,14 glucose,11 and wood hydrolysate containing mainly glucose.7 The results from this study showed that the use of cell recycling with strain QU 25 increased biomass, LA concentration, and productivity when xylose was used as the substrate, but it also led to high by-product formation. With cell recycling, the residual xylose concentration was quite low (around 5.51–7.49 g L−1) (Fig. 4a); this result is similar to that obtained from continuous fermentation without cell recycling and using 25.8 g L−1 xylose in the feeding medium (7.43 g L−1) (Table 1). These results suggest that the decrease in LA yield may result for the same reasons as described in Section 3.1. In conclusion, although cell recycling continuous fermentation improved cell growth, xylose utilisation, LA concentration, and LA productivity from xylose, the decrease in LA yield will need to be overcome in future studies.
3.4. Effect of pH on L-LA and by-product formation in continuous fermentation with cell recycling
Previously, it was reported that when batch fermentation with strain QU 25 using xylose as a substrate was performed at low pH (≤6.5), not only biomass and LA concentration but also by-product concentration were lower than those obtained when the pH was maintained at 7.0.6 To investigate the effect of pH on continuous fermentation with cell recycling, fermentation was carried out at a dilution rate of 0.156 h−1 at pH values of 5.6, 5.9, 6.2, or 7.0 (Table 2). When continuous fermentation with cell recycling was performed at pH 5.6 or 5.9, the values for not only biomass (3.57 g L−1 and 4.97 g L−1, respectively), xylose consumption (14.0 g L−1 and 26.2 g L−1, respectively), LA produced (13.6 g L−1 and 23.5 g L−1, respectively), and LA productivity (1.94 g L−1 h−1 and 3.71 g L−1 h−1, respectively) but also total by-products (0.313 g L−1 and 1.76 g L−1, respectively) were much lower than those obtained at pH 7.0. In contrast, the results obtained for biomass (8.02 g L−1), xylose consumption (33.0 g L−1), LA produced (30.4 g L−1), and LA productivity (5.13 g L−1 h−1) at pH 6.2 were similar to those obtained at pH 7.0, although total by-products decreased drastically from 8.03 g L−1 to 3.41 g L−1. As expected, maintaining the LA yields obtained at pH 5.6 (0.968 g g−1), 5.9 (0.905 g g−1), and 6.2 (0.915 g g−1) were significantly higher than that obtained at pH 7.0 (0.789 g g−1).| pH | CXylb (g L−1) | Residual xylose (g L−1) | CL-LAc (g L−1) | Cacetated (g L−1) | Cformatee (g L−1) | Cethanolf (g L−1) | Biomassg (g L−1) | PL-LAh [g L−1 h−1] | YL-LAi (g g−1) |
|---|---|---|---|---|---|---|---|---|---|
| a Continuous fermentation with cell recycling was conducted in a 1 L jar fermentor of 0.4 L working volume at 43 °C and pH controlled at different values 5.6, 5.9, or 6.2 with 10 M NaOH, feeding with mMRS medium containing 50 g L−1 xylose. The dilution rate was set at 0.156 ± 0.013 h−1. Averages with standard deviations are based on the averages of measurements for samples withdrawn at least five times in the steady state.b Xylose consumed.c L-Lactic acid produced.d Acetic acid produced.e Formic acid produced.f Ethanol produced.g Dry cell weight.h Productivity of L-lactic acid.i Yield of L-lactic acid to consumed xylose. | |||||||||
| 5.6 | 14.0 ± 1.2 | 35.1 ± 1.2 | 13.6 ± 1.2 | 0.023 ± 0.025 | 0.013 ± 0.043 | 0.277 ± 0.389 | 3.57 ± 0.37 | 1.94 ± 0.17 | 0.968 ± 0.064 |
| 5.9 | 26.2 ± 1.1 | 24.9 ± 1.5 | 23.5 ± 1.4 | 0.140 ± 0.059 | 0.471 ± 0.221 | 1.15 ± 0.35 | 4.97 ± 1.25 | 3.71 ± 0.17 | 0.905 ± 0.024 |
| 6.2 | 33.0 ± 1.7 | 15.4 ± 1.7 | 30.4 ± 0.8 | 0.470 ± 0.138 | 1.16 ± 0.91 | 1.78 ± 0.15 | 8.02 ± 0.39 | 5.13 ± 0.13 | 0.915 ± 0.003 |
| 7.0 | 41.2 ± 3.7 | 7.49 ± 3.50 | 32.3 ± 1.6 | 1.74 ± 0.72 | 3.41 ± 0.64 | 2.88 ± 0.38 | 7.20 ± 0.15 | 5.33 ± 0.26 | 0.789 ± 0.059 |
Although controlling pH during LA fermentation has been shown to be an important factor in batch LA fermentations,6,11,18 to the best of the authors' knowledge, there is no report on the effect of controlling pH values on LA production in cell recycle continuous fermentation using all sugars, including not only glucose but also xylose. This study was the first to achieve homo-LA production with a drastic improvement in LA yield from 0.789 g g−1 to 0.915 g g−1 by shifting the pH from 7.0 to 6.2, even in continuous fermentation with cell recycling (Table 2). Nevertheless, the biomass of 8.02 g L−1 at pH 6.2 is still lower than that obtained when glucose is used in continuous fermentation with cell recycling (86.0 g L−1);19 thus, further studies are needed to enhance overall fermentation by using more biomass.
3.5. Improved L-LA production in continuous fermentation with cell concentration and cell recycling
Previously it was suggested that cell concentration may be an efficient technique for rapidly obtaining high cell density and thus improving LA productivity in continuous fermentation with cell recycling from glucose11 and starch.14 Therefore, after cells were concentrated by reducing 4 L of fermentation broth to 0.4 L, continuous fermentation with cell recycling and high cell density was performed in mMRS medium containing 50 g L−1 xylose at pH 6.2 and with a dilution rate of 0.155 h−1 (Table 3). Approximately three fold higher biomass (26.6 g L−1) and two fold lower residual xylose concentration (7.92 g L−1) in the fermentation broth were achieved in continuous fermentation with cell concentration than without cell concentration (8.02 g L−1 and 15.4 g L−1, respectively) (Tables 2 and 3). In addition, continuous fermentation with cell concentration produced a high LA concentration of 36.0 g L−1 and productivity of 5.80 g L−1 h−1 with a low LA yield (0.856 g g−1) as compared to the values obtained without cell concentration (30.4 g L−1, 5.13 g L−1 h−1, and 0.915 g g−1, respectively) (Tables 2 and 3). Further increasing the dilution rate to 0.205 h−1 resulted in an increased productivity of 6.59 g L−1 h−1 but a reduced LA concentration of 32.1 g L−1 and an increase in the residual xylose concentration to 11.6 g L−1 (Table 3). Thus, continuous fermentation with cell concentration and cell recycling with a dilution rate of 0.155 h−1 improved LA concentration, LA productivity, and residual xylose concentration while maintaining high LA yield.| Feeding media | Db (h−1) | CXylc (g L−1) | Residual xylose (g L−1) | CL-LAd (g L−1) | Cacetatee (g L−1) | Cformatef (g L−1) | Cethanolg (g L−1) | Biomassh (g L−1) | PL-LAi [g L−1 h−1] | YL-LAj (g g−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| a Continuous fermentation with cell concentration and cell recycling was conducted in a 1 L jar fermentor of 0.4 L working volume at 43 °C and pH controlled at 6.2 with 10 M NaOH, feeding with mMRS and sMRS media containing 50 g L−1 xylose. sMRS medium contains the minerals with mMRS medium, 12.5 g L−1 yeast extract, 12.5 g L−1 corn steep liquor. Averages with standard deviations are based on the averages of measurements for samples withdrawn at least five times in the steady state.b Dilution rate.c Xylose consumed.d L-Lactic acid produced.e Acetic acid produced.f Formic acid produced.g Ethanol produced.h Dry cell weight.i Productivity of L-lactic acid.j Yield of L-lactic acid to consumed xylose. | ||||||||||
| mMRS | 0.155 | 42.1 ± 0.9 | 7.92 ± 0.89 | 36.0 ± 1.2 | 0.564 ± 0.281 | 1.57 ± 0.46 | 1.57 ± 0.17 | 26.6 ± 0.1 | 5.80 ± 0.19 | 0.856 ± 0.019 |
| 0.205 | 38.4 ± 2.0 | 11.6 ± 2.0 | 32.1 ± 2.0 | 0.368 ± 0.252 | 1.51 ± 0.36 | 1.60 ± 0.26 | 32.9 ± 2.4 | 6.59 ± 0.40 | 0.838 ± 0.054 | |
| sMRS | 0.155 | 41.5 ± 1.5 | 11.1 ± 1.5 | 41.0 ± 0.9 | 0.586 ± 0.037 | 1.15 ± 0.04 | 1.62 ± 0.08 | 26.1 ± 0.4 | 6.15 ± 0.13 | 1.01 ± 0.02 |
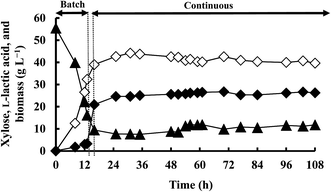
Corn steep liquor, a by-product of the corn wet-milling industry, is considered a good and cheap nitrogen source for most fermentative microorganisms. Therefore, continuous fermentation with sMRS medium, which contains the same minerals as mMRS medium but is supplemented with 12.5 g L−1 yeast extract and 12.5 g L−1 corn steep liquor, was performed using 50 g L−1 xylose and a dilution rate of 0.155 h−1 with cell concentration and cell recycling (Fig. 5 and Table 3). Interestingly, the LA concentration (41.0 g L−1) and productivity (6.15 g L−1 h−1) achieved with sMRS were greater than those obtained using mMRS medium (36.0 g L−1 and 5.80 g L−1 h−1, respectively) (Table 3). In addition, the LA yield significantly increased to 1.01 g g−1. The enhanced LA production might be due to the corn steep liquor, which contains considerable amounts of nutrients that can support growth and fermentation activity such as water soluble vitamins, amino acids, minerals, and growth factors/stimulants (purine and pyrimidine derivatives).20 Consequently, in this study, highly productive and efficient continuous L-LA fermentation was achieved using strain QU 25 with cell concentration and cell recycling at pH of 6.2 and with a dilution rate of 0.155 h−1; under these conditions, the LA concentration was comparable to that obtained with batch fermentation but the productivity was 3-fold higher.6 Furthermore, continuous LA production is enhanced by using a medium containing corn steep liquor as a competitive nitrogen source.
Table 4 compares the results from this study and from the literature on continuous LA fermentation using various LAB with xylose and other substrates. To date, there has been only one other report on continuous LA fermentation from xylose. The present study reports successful homo-LA production from xylose by using strain QU 25, with a 2.0-fold higher LA concentration (41.0 g L−1), 1.4-fold higher LA yield (1.01 g g−1), and 6-fold higher LA productivity (6.15 g L−1 h−1) than that obtained using Lc. lactis IO-1 in the conventional mode (20.6 g L−1, 0.72 g g−1, and 1.03 g L−1 h−1, respectively).9 Many researchers have reported on continuous LA fermentation using glucose, but there are few studies on continuous fermentation using other substrates. Continuous fermentation using glucose exhibits quite high LA productivities; for example, 18.0 g L−1 h−1 was achieved using commercial glucose,11 and 33.1 g L−1 h−1 was achieved using hydrolysed glucose derived from sago starch.21 In contrast, the continuous fermentation method described in this study resulted in comparable or higher LA productivity (6.15 g L−1 h−1) and the highest LA yield (1.01 g g−1) for other substrates and systems published so far (Table 4). Therefore, in this study, a highly productive fermentation system with a high LA yield from xylose was established. These results show that E. mundtii QU 25 can be used to develop a continuous process using xylose for the industrial production of L-LA. Future research efforts will focus on developing a continuous fermentation process using glucose and xylose mixtures derived from lignocellulose hydrolysates.
| Substrates: concentration in feeding medium (derivation) | System | Strain | Lactic acid production | Dd (h−1) | References | ||
|---|---|---|---|---|---|---|---|
| CLAa (g L−1) | YLAb (g g−1) | PLAc [g L−1 h−1] | |||||
| a Lactic acid produced.b Yield of lactic acid to consumed sugars.c Productivity of lactic acid.d Dilution rate.e Commercial sugar.f Not determined. Lb., Lactobacillus; Lc., Lactococcus; E., Enterococcus; Sp., Sporolactobacillus. | |||||||
| Glucose: 20 g L−1 (CSe) | Cell concentration and cell recycling | Lb. delbrueckii subsp. lactis QU 41 | 20.7 | 1.03 | 18.0 | 0.87 | 11 |
| Glucose: 30 g L−1 (sago starch) | Cell recycling | Lc. lactis IO-1 | 30.1 | 0.910 | 33.1 | 1.1 | 21 |
| Lactose: 50 g L−1 (deproteinized whey) | Cell immobilization | Lb. casei SU no. 22 and Lc. lactis WS 1042 | 17.5 | 0.7 | 7.0 | 0.4 | 22 |
| Lactose: 40 g L−1 (CSe) | Cell immobilization | Lb. casei subsp. casei DSM 20244 | 33.0 | 1.0 | 5.5 | 0.22 | 23 |
| Lactose: 37.4 g L−1 (whey permeate) | Conventional | Lb. helveticus strain milano | 30.0 | 0.875 | 9.7 | 0.352 | 24 |
| Starch: 20 g L−1 (cassava starch) | Cell concentration and cell recycling | Lb. plantarum SW14 | 20.0 | NDf | 8.0 | 0.40 | 25 |
| Starch: 20 g L−1 (sago starch) | Cell concentration and cell recycling | E. faecium No. 78 | 11.7 | 0.76 | 3.04 | 0.26 | 14 |
| Sucrose: 52.1 g L−1 (beet molasses) | Conventional | Lb. delbrueckii IFO 3202 | 41.1 | NDf | 4.11 | 0.1 | 26 |
| Sucrose: 50 g L−1 (molasses) | Cell immobilization | Sp. cellulosolvens NCIMB 12173 | 11.7 | NDf | 3.29 | 0.25 | 27 |
| Xylose: 69.9 g L−1 (CSe) | Conventional | Lc. lactis IO-1 | 20.6 | 0.728 | 1.03 | 0.05 | 9 |
| Xylose: 50 g L−1 (CSe) | Cell concentration and cell recycling | E. mundtii QU 25 | 41.0 | 1.01 | 6.15 | 0.155 | This work |
4. Conclusion
This study is the first to report on continuous fermentation with cell recycling for LA production from xylose. In conventional continuous fermentation, 50 g L−1 xylose in the feeding medium and a dilution rate of 0.15 h−1 were optimal for LA production. Cell recycling at pH 6.2 provided further enhancement of LA concentration, yield and productivity. Finally, 41.0 g L−1 LA with a yield of 1.01 g g−1 and a productivity of 6.15 g L−1 h−1 was achieved using a feeding medium containing corn steep liquor and yeast extract at a dilution rate of 0.155 h−1 with cell concentration and cell recycling.Acknowledgements
This study has been supported by a Grant-in-Aid (P12088) for JSPS Fellows to Mohamed Ali Abdel-Rahman from the Japan Society for the Promotion of Science, Japan.References
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, Biotechnol. Adv., 2013, 31, 877–902 CrossRef CAS PubMed.
- R. P. John, K. M. Nampoothiri and A. Pandey, Appl. Microbiol. Biotechnol., 2007, 74, 524–534 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, J. Biotechnol., 2011, 156, 286–301 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo and K. Sonomoto, RSC Adv., 2013, 3, 8437–8445 RSC.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo, K. Shibata and K. Sonomoto, Appl. Microbiol. Biotechnol., 2011, 89, 1039–1049 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo, K. Hanada, K. Shibata and K. Sonomoto, Appl. Environ. Microbiol., 2011, 77, 1892–1895 CrossRef CAS PubMed.
- Y. J. Wee and H. W. Ryu, Bioresour. Technol., 2009, 100, 4262–4270 CrossRef CAS PubMed.
- T. Gao and K. P. Ho, J. Biotechnol., 2013, 168, 646–651 CrossRef CAS PubMed.
- K. Tanaka, A. Komiyama, K. Sonomoto, A. Ishizaki, S. J. Hall and P. F. Stanbury, Appl. Microbiol. Biotechnol., 2002, 60, 160–167 CrossRef CAS PubMed.
- B. Choudhury and T. Swaminathan, Appl. Biochem. Biotechnol., 2006, 128, 171–183 CrossRef CAS PubMed.
- Y. Tashiro, W. Kaneko, Y. Sun, K. Shibata, K. Inokuma, T. Zendo and K. Sonomoto, Appl. Microbiol. Biotechnol., 2011, 89, 1741–1750 CrossRef CAS PubMed.
- Y. Shiwa, H. Yanase, Y. Hirose, S. Satomi, T. Araya-Kojima, S. Watanabe, T. Zendo, T. Chibazakura, M. Shimizu-Kadota, H. Yoshikawa and K. Sonomoto, DNA Res., 2014, 21, 369–377 CrossRef CAS PubMed.
- I.-K. Yoo, H. N. Chang, E. G. Lee, Y. K. Chang and S.-H. Moon, Biotechnol. Lett., 1997, 19, 237–240 CrossRef CAS.
- K. Shibata, D. M. Flores, G. Kobayashi and K. Sonomoto, Enzyme Microb. Technol., 2007, 41, 149–155 CrossRef CAS.
- G. Bustos, N. de la Torre, A. B. Moldes, J. M. Cruz and J. M. Dominguez, J. Food Eng., 2007, 78, 405–412 CrossRef CAS.
- S. G. Karp, A. H. Igashiyama, P. F. Siqueira, J. C. Carvalho, L. P. Vandenberghe, V. Thomaz-Soccol, J. Coral, J.-L. Tholozan, A. Pandey and C. R. Soccol, Bioresour. Technol., 2011, 102, 1765–1772 CrossRef CAS PubMed.
- S. Tejayadi and M. Cheryan, Appl. Microbiol. Biotechnol., 1995, 43, 242–248 CrossRef CAS.
- J. P. Guyot, M. Calderon and J. Morlon-Guyot, J. Appl. Microbiol., 2000, 88, 176–182 CrossRef CAS PubMed.
- S. Hjorleifsdottir, S. Seevaratnam, O. Holst and B. Mattiasson, Curr. Microbiol., 1990, 20, 287–292 CrossRef.
- J. Saxena and R. S. Tanner, World J. Microbiol. Biotechnol., 2012, 28, 1553–1561 CrossRef CAS PubMed.
- C. Nolasco-Hipolito, T. Matsunaka, G. Kobayashi, K. Sonomoto and A. Ishizaki, J. Biosci. Bioeng., 2002, 93, 281–287 CrossRef CAS PubMed.
- T. Roukas and P. Kotzekidou, Food Biotechnol., 1996, 10, 231–242 CrossRef CAS.
- W. Krischke, M. Schroder and W. Trosch, Appl. Microbiol. Biotechnol., 1991, 34, 573–578 CrossRef CAS.
- D. Roy, J. Goulet and A. Le Duy, J. Dairy Sci., 1987, 70, 506–513 CrossRef.
- W. Bomrungnok, K. Sonomoto, S. Pinitglang and A. Wongwicharn, APCBEE Proc., 2012, 2, 97–103 CrossRef CAS.
- Y. Göksungur and U. Güvenç, J. Chem. Technol. Biotechnol., 1997, 69, 399–404 CrossRef.
- S. S. Kanwar, B. S. Chadha, H. K. Tewari and V. K. Sharma, World J. Microbiol. Biotechnol., 1995, 11, 687–688 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |