Enhanced luminescence efficiency of aqueous dispersible NaYF4:Yb/Er nanoparticles and the effect of surface coating
Anees A. Ansari*a,
Ranvijay Yadavb and
S. B. Raib
aKing Abdullah Institute for Nanotechnology, King Saud University, Riyadh-11451, Saudi Arabia. E-mail: aneesaansari@gmail.com; Fax: +966-1-4670662; Tel: +966-1-4676838
bDepartment of Physics, Banaras Hindu University, Varanasi, 221005 India
First published on 17th February 2016
Abstract
In a general approach, we designed and synthesized monodisperse, well-defined, highly emissive and aqueous dispersible NaYF4:Yb/Er upconversion nanoparticles, and thereafter their surfaces were coated with inert NaYF4 and silica layers. The crystalline phase, morphology, composition and optical properties of the as-synthesized samples were well characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), UV-Vis absorption (UV-Vis), optical band gap energy, Fourier-transform infrared spectroscopy (FT-IR) and upconversion luminescence spectra, respectively. It is found that the synthesized hexagonal phase nanoparticles have a highly crystalline spherical shape, and are monodisperse with narrow size distribution. They can easily disperse in nonpolar cyclohexane solvent to form transparent colloid solutions. The optical band gap energy clearly shows the effect of surface coating of inert inorganic and porous silica layers surrounding the surface of seed core-nanoparticles due to the increase the crystalline size. The upconversion luminescence intensity was remarkably improved after the formation of a passive NaYF4 layer due to the decrease of non-radiative rate arising from the surface/defects of the particles in the form of surface dangling bonds and capping agents. The growth of the silica shell after inert shell formation and the emission intensity of Er3+ transitions were little affected with respect to inert shell coated core/shell nanoparticles, indicating that silica has been effectively grafted surrounding the core/shell nanoparticles. Our results indicate that surface coating of inactive and silica shells is a key step in producing upconversion nanocrystals with increased brightness for a variety of upconversion luminescence bioimaging and biosensing applications.
Introduction
Upconversion nanoparticles have been the subject of much interest over the past decade owing to the possibility of developing visible super-fluorescent fiber sources and visible fiber lasers excited in the near infrared range.1–6 Among various upconversion host matrixes, lanthanide ion-doped hexagonal-phase sodium yttrium fluoride (NaYF4) has turned out to be the best host matrix for Yb3+/Er3+ co-doped infrared-to-visible photo-conversion,1–4 due to its inherent low phonon energies (∼350 cm−1), large anti-stokes shift, long lifetimes, narrow emission bandwidth, high chemical stability, deep tissue penetration, low background interference, good biocompatibility and weak toxicity, which have provoked great research interest in biological sciences.3–5 These upconversion nanoparticles absorb in near-infrared (NIR) spectral region (∼980 nm), which is a biologically transparent window, and emit high energy visible photons through an upconversion process.1–6 Additionally, upconversion materials convert low energy, near infrared photons into high energy ultraviolet-visible (UV-Vis) photons with stable fluorescence and require very low excitation power densities (5–10 W cm−2) to emit photons.3–5 Until now, several approaches including microemulsion method, hydrothermal/solvothermal method, co-precipitation, microwave irradiation procedure and polyol method have been developed for the synthesis of different controlled size and shape upconversion nanoparticles.7,8 However, most of these controlled size upconversion nanoparticles are synthesized in organic solvents or at high temperature and obtained nanoparticles are hydrophobic and show poor dispersibility in water. The direct use of these nanoparticles for biological applications is limited owing to the low solubility in water and unsuitable surface property, which do not have functional chemical groups or appropriate sites for attachment of biomolecules.7,8 Besides, the used toxic organometallic precursors and hazardous coordinating solvents have become matters of substantial environmental concern. Therefore, developing an effective and user-friendly procedure to synthesize upconversion nanoparticles with well-defined crystal sizes and good dispersibility is still a challenge. Although, the luminescence efficiency of the upconversion nanoparticles is low (<1%) and this aspect remains a concern. Thus, designing a protective layer on the upconversion nanoparticles should be an effective strategy to improve the luminescent efficiency. Surface modification of upconversion nanoparticles offers a promising way to enhance the luminescence of these nanoparticles.7–9Up to now much effort have been expanded to enhanced overall photoluminescence efficiency of upconversion nanoparticles.8–11 Recently, we and some other research groups have been effectively grown a shell of inert inorganic crystalline materials surrounding the lanthanide ions-doped core-nanoparticles to improve the photoluminescence efficiency by enhancing the energy transfer from sensitizer to the activator.8–13 These upconversion core/shell nanostructure shows luminescence enhancement because the shell can shield dopant ions and inhibit defect sites on the particle surface from luminescence quenching.8,13 However, most of the inorganic inert shell materials are hydrophobic in aqueous environment. Therefore, direct use of these hydrophobic core/shell nanoparticles in biological applications is limited on account their weak dispersibility in aqueous environment and poor biological conjugation activity. It is therefore necessary to modify the surfaces of these core/shell nanoparticles with active functional groups or hydrophilic ligands to improve their solubility in aqueous environment so that these nanoparticles can be bonded to biomolecules as required. Previously, some literature reports demonstrated that the presence of some functional groups (such as –COOH, –NH2, or –SH) on the surface of upconversion nanoparticles could not only increase their water dispersity but also allow further conjugation with biologically active molecules.14–20 Additionally, these upconversion nanoparticles not only exhibit upconversion luminescence efficiency, but also have appropriate surface properties that can be used to conjugate with biomolecules through terminal functional groups, such as antibodies and DNA with. Among the various employed surface coating materials mesoporous silica has been paid much attention because they have several attractive features, such as a stable mesoporous structure, tunable pore sizes with a narrow distribution, high specific surface area and pore volume, nontoxic nature, good biocompatibility, and well-defined surface properties. Some reports in literature have been discussed with silica coating being a protective matrix because of its reliable chemical stability, optical transparency, chemical inertness, cheapness, biocompatibility, non-toxicity, photochemical stability even under laser photolysis, and easily transferred into a wide range of solvent.17–20
In this report, we propose the strategy to successful synthesis of well-defined NaYF4:Yb/Er (core)-nanoparticles with an average size of 100–115 nm, which are well monodispersed and demonstrated high aqueous dispersibility in aqueous and non-aqueous solvents. These upconversion core nanoparticles were coated with crystalline inactive NaYF4 layer and thereafter with amorphous silica shell around the surface of core nanoparticles to improve the luminescent efficiency and aqueous dispersibility, respectively. Comparative crystalline nature, morphology and optical properties of core, core/shell and core/shell/shell nanoparticles were analyze systematically to investigate the influence of surface coating on optical and solubility on as-prepared upconversion nanomaterials. These luminescent core/shell/shell nanoparticles are promising for use as luminescent probes in biological labelling and imaging technology.
Experimental
Materials and methods
Yttrium oxide (99.99%, Alfa Aesar, Germany), ytterbium oxide (99.99%, Alfa Aesar, Germany), erbium nitrate (99.99%, BDH Chemicals Ltd, England), ethylene-diamine tetra-acetic acid (EDTA), sodium fluoride, tetraethyl orthosilicate (TEOS, 99 wt% analytical reagent A.R.), C2H5OH, NaOH, HNO3 and NH4OH were used as a starting materials without any further purification. Y(NO3)36H2O, Yb(NO3)36H2O and Er(NO3)36H2O were prepared by dissolving the corresponding oxide in diluted nitric acid. Nanopure water was used for preparation of the solutions. Ultrapure de-ionized water was prepared using a Milli-Q system (Millipore, Bedford, MA, USA). All other chemicals used were of reagent grade.Preparation of NaYF4:Yb/Er nanoparticles
In a typical synthesis procedure, for preparation of NaYF4:Yb3+/Er3+ nanoparticles EDTA was used as a chelating agent for complex formation with trivalent lanthanides. 7.8 mL of 2 M Y(NO3)36H2O, 2 mL of 2 M Yb(NO3)36H2O and 0.2 mL of 2 M Er(NO3)36H2O were dissolved together in dist. water and heated upto 80 °C. In another solution EDTA in equivalent amount was prepared in dist. water and injected into the metal solution for complex formation. Later on, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio amount of NaF dissolved in dist. water was mixed up in this forgoing reaction and kept for constant stirring with heating (80 °C) on hot plate for 1 h.21 This suspension was filled in Teflone-line stainless autoclave and sealed for different time interval for hydrothermal crystallization. After autoclave cooled at room temperature the final powder product was then collected by centrifugation, washed with distilled water and absolute ethanol, and dried in oven at 80 °C for 6 h for further characterization.
3 molar ratio amount of NaF dissolved in dist. water was mixed up in this forgoing reaction and kept for constant stirring with heating (80 °C) on hot plate for 1 h.21 This suspension was filled in Teflone-line stainless autoclave and sealed for different time interval for hydrothermal crystallization. After autoclave cooled at room temperature the final powder product was then collected by centrifugation, washed with distilled water and absolute ethanol, and dried in oven at 80 °C for 6 h for further characterization.
Preparation of NaYF4:Yb/Er@NaYF4 core/shell nanoparticles
For the preparation of NaYF4:Yb3+/Er3+@NaYF4 core/shell nanoparticles, 0.400 g core-nanoparticles was dispersed in minimum amount of dist. water along with ethylene glycol with the help of ultrasonic bath and then constant stirring for 30 min. Typically, 0.632 g Y(NO3)36H2O dissolved in dist. water and after that a solution of NaF dissolved in dist. water was introduced into foregoing mixed system, and the suspension was refluxed at 100 °C for 3 h until the precipitation was occurred. This white precipitate was centrifuged and washed with ethanol to remove excess un-reacted reactants. The core/shell nanoparticles were collected after centrifugation and allowed to dry in ambient temperature for further characterization.Preparation of silica coated NaYF4:Yb/Er@NaYF4@SiO2 core/shell/shell nanoparticles
The NaYF4:Yb3+, Er3+@NaYF4@SiO2 core/shell/shell nanoparticles were prepared through a versatile solution Stober method as follows.12,20 The synthesized core/shell nanoparticles (50 mg) were well dispersed in a mixed solution of deionized water (50 mL), ethanol (70 mL) and concentrated aqueous ammonia (1.0 mL) in a three-neck round-bottom flask. Afterward, 1.0 mL of tetraethyl orthosilicate (TEOS) was added drop-wise in 2 min, and the reaction was allowed to proceed for 5–6 h under continuous mechanical stirring. After 6 h of continuous stirring at room temperature, the silica-coated core/shell/shell nanoparticles were separated by centrifugation, washed several times with ethanol and dried at room temperature.Characterization
The crystallinity of the powder samples was examined by X-ray diffraction (XRD) at room temperature with the use of PANalytical X'Pert X-ray diffractometer equipped with a Ni filter using Cu Kα (λ = 1.54056 Å) radiations as X-ray source. The size and morphology of the samples were inspected using a field emission transmission electron microscope (FE-TEM) equipped with the EDX (FETEM, JEM-2100F, JEOL, Japan) operating at an accelerating voltage of 200 kV. EDX analysis was used to confirm the presence of the elements. The samples for TEM were prepared by depositing a drop of a colloidal ethanol solution of the powder sample onto a carbon-coated copper grid. The FTIR spectra were recorded on a Perkin-Elmer 580B IR spectrometer using KBr pellet technique in the range 4000 to 400 cm−1. The UV/Vis absorption spectra were measured in the Perkin-Elmer Lambda-40 spectrophotometer in the range 190–600 nm, with the sample contained in 1 cm3 stoppered quartz cell of 1 cm path length. The upconversion luminescence spectra were recorded using iHR320 (Horiba Jobin Yvon) spectrometer equipped with R928P photon counting photomultiplier tube and a 980 nm diode laser with 2.4 W maximum power as an excitation source. All measurements were performed at room temperature.Results and discussion
Crystal structure, size and morphologies of the nanoparticles
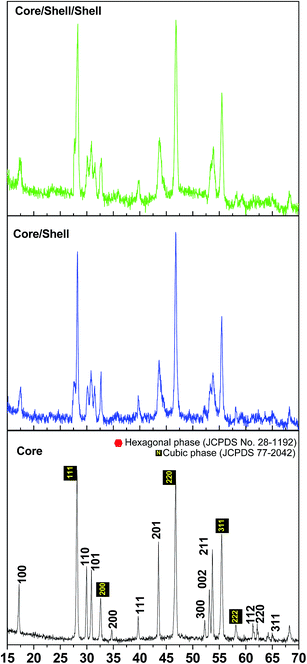
X-ray diffraction pattern was employed to examine the composition, phase purity and crystalline nature of the as-prepared nano-products. Fig. 1 illustrate the XRD pattern of the as obtained core, core/shell and core/shell/shell nanoparticles. The broad reflection peaks of XRD pattern indicate that the prepared samples are in small grain size with high crystallinity, which are in good agreement with face-centered cubic NaYF4 (space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) crystal structure data (JCPDS card no. 77-2042) and small amount of hexagonal phase NaYF4 crystal structure data (JCPDS card no. 28-1192).7 The diffraction peak positions and intensity are perfectly matched with the literature reports.7,22,23 As illustrated in Fig. 1c, the diffraction peaks of the silica surface modified core/shell/shell nanoparticles are broadened with decrease relative diffraction intensity with respect to the seed core-nanoparticles. It indicates that the structure of the seed core brings partial disorder to the ordered porous framework of the silica shell. Whereas, there is no defined characteristic peak for amorphous SiO2 is observed after one coating process due to the thinner property of the SiO2 layer. It suggests that the presence of the SiO2 coating significantly influence nanoparticle crystallinity, resulting the change in reflection intensity of XRD pattern. Although, it is difficult to discern the diffraction and territory of inert NaYF4 shell from the XRD data, since NaYF4 shell shows similar crystal structure and lattice constants to NaYF4.
m) crystal structure data (JCPDS card no. 77-2042) and small amount of hexagonal phase NaYF4 crystal structure data (JCPDS card no. 28-1192).7 The diffraction peak positions and intensity are perfectly matched with the literature reports.7,22,23 As illustrated in Fig. 1c, the diffraction peaks of the silica surface modified core/shell/shell nanoparticles are broadened with decrease relative diffraction intensity with respect to the seed core-nanoparticles. It indicates that the structure of the seed core brings partial disorder to the ordered porous framework of the silica shell. Whereas, there is no defined characteristic peak for amorphous SiO2 is observed after one coating process due to the thinner property of the SiO2 layer. It suggests that the presence of the SiO2 coating significantly influence nanoparticle crystallinity, resulting the change in reflection intensity of XRD pattern. Although, it is difficult to discern the diffraction and territory of inert NaYF4 shell from the XRD data, since NaYF4 shell shows similar crystal structure and lattice constants to NaYF4.
Transmission electron microscopy images give the direct evidence to investigate the formation of shell on the surface of upconversion core-nanoparticles. It is observed from the low resolution TEM micrographs that the as prepared core-nanoparticles are in spherical shape, fairly uniform monodispersed, large surface area, highly crystalline and well define size distribution with average particle sizes of 90–115 nm (Fig. 2a and b). As shown in Fig. 2b and c, the typical high resolution TEM image revealed their high crystalline nature and structural uniformity with porous surface. Moreover, the surface of the nanoparticles is quite rough, highly porous as apparently observed in the TEM images (in inset Fig. 2b). This porous surface of the luminescent nanoparticles are facilitating for the high dispersibility in organic solvents. The TEM image clearly shows that the obtained silica coated core/shell/shell nanoparticles still retain the uniform nanoparticle morphology, which are highly agglomerated (Fig. 2d). As seen in Fig. 2d, the silica core/shell/shell nanoparticles are highly agglomerated with different particle size distribution. In this micrograph, the dark areas are related to the high concentration of luminescent core-nanoparticles with aggregate nature and light grey color for amorphous silica surface coating. It is known that silica layer contain a large number of surface silanol groups which are highly soluble in aqueous solvents. We believe that these surface silanol groups promote to increase in aggregation process through hydrogen bonding in aqueous solvent. High-magnification TEM image shows that the thickness of the silica shell is very uniform and about 25–30 nm (in inset Fig. 2d), and the uniform ordered mesopores can be observed. No significant changes in the morphology and composition of silica coated core/shell nanoparticles are observed after the silica surface modifications, as evidenced by TEM imaging shown in Fig. 2d. The FE-TEM image in inset Fig. 2b shows the crystalline fringes of the nanoparticles. The electron diffraction pattern (inset of Fig. 2a) from the FE-TEM also demonstrated unambiguously the single-crystalline nature of the sample. The selected-area electron diffraction (SAED) pattern shows spotty polycrystalline diffraction rings corresponding to the (111), (200), (222), (220), (311) and (422) planes of the β-phase NaYF4 lattice, consistent with the XRD (JCPDS card no. 28-1192) results.7,22,23 These results suggest the high crystallinity of the as-prepared β-NaYF4 nanoparticles.
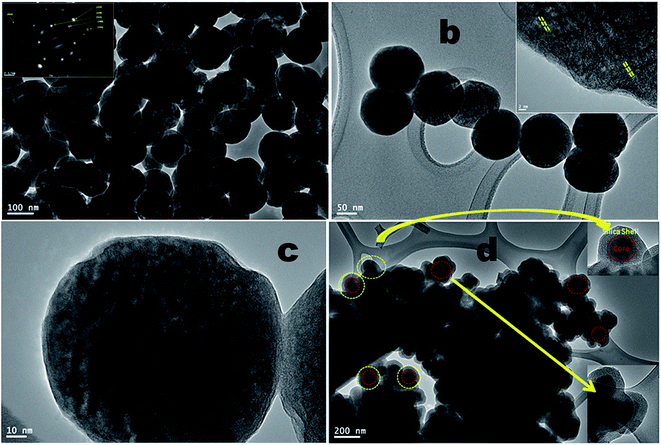 | ||
| Fig. 2 FE-TEM micrographs of (a–c) NaYF4:Yb/Er (core), inset shows the SAED patterns, and (d) NaYF4:Yb/Er@NaYF4@SiO2 core/shell/shell nanoparticles. | ||
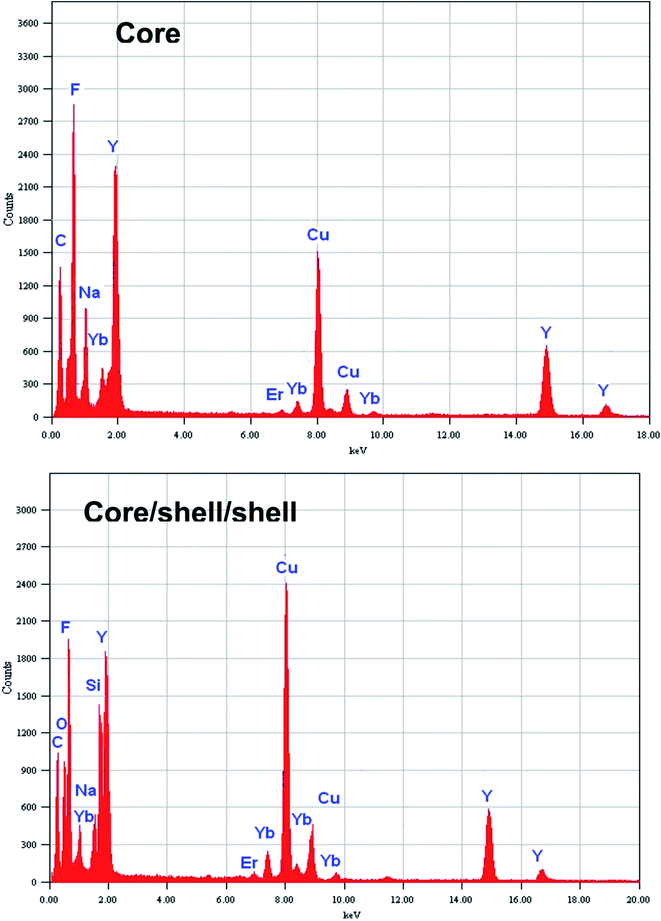
Energy dispersive X-ray analysis was used to determine the compositional changes before and after growing a layer of NaYF4 and amorphous silica around the surface of core nanoparticles. The EDX analysis demonstrated the existence of all expected basic chemical elements such as Na, Y, F, Yb, Er, O and Si, indicating that the doped elements are homogeneously distributed inside the crystal lattice of NaYF4 nanoparticles (Fig. 3). It should be noted that the origin of strong Cu peaks that appeared in the EDX spectra are from the copper micrometer-grids. The C peak also came from the carbon-coated Cu-TEM grid. No other impurities are evident in the figure implying that the resulting core and core/shell/shell nanoparticles are pure in chemical composition.
Comparative optical properties of core, core/shell and core/shell/shell nanoparticles
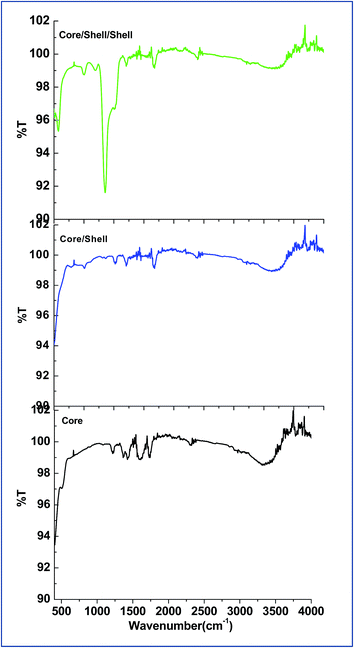
FTIR spectroscopy verifies the surface modification of the as-prepared samples before and after shell formation around the surface of core nanoparticles. As illustrated in Fig. 4, all samples exhibits a broad with low intensity infrared band in between 3100–3600 cm−1 and two weak intensity bands at around 1600 and 1217 cm−1, which ascribed to asymmetric, symmetric stretching and bending vibration modes of the surface attached hydroxyl groups, respectively.12,19,20 Another two weak intensity bands are also observed at around 1740 and 1430 cm−1 attributed to the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and (C–O) functional group of surface attached EDTA carboxylic acid.21 As seen in Fig. 4c, a strong doublet infrared absorption peak is observed at around 1080 cm−1 and two weak intensity peaks at 800 and 460 cm−1, which are arise from the asymmetric and symmetric stretching vibration modes of silica (Si–O and Si–O–Si).12,19,20 These are well known silica bands which confirmed the successful silica layer formation around the surface of core/shell nanoparticles.
O) and (C–O) functional group of surface attached EDTA carboxylic acid.21 As seen in Fig. 4c, a strong doublet infrared absorption peak is observed at around 1080 cm−1 and two weak intensity peaks at 800 and 460 cm−1, which are arise from the asymmetric and symmetric stretching vibration modes of silica (Si–O and Si–O–Si).12,19,20 These are well known silica bands which confirmed the successful silica layer formation around the surface of core/shell nanoparticles.
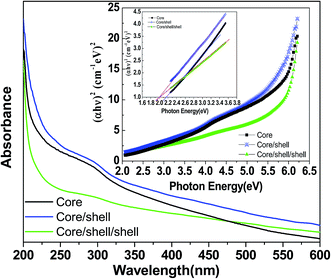
Optical properties and colloidal stability are examined by UV-visible absorption spectroscopy. The spectra of all three samples are recorded in dist. water over the 200–600 nm UV-Vis spectral regions. Fig. 5 displays the absorption spectra of core, core/shell and silica layer grown core/shell/shell nanoparticles. It is noticed that absorption spectra of all samples are closely similar except absorption band edge. It may be due to similar refractive index NaYF4 shell is covered around the surface of core nanoparticles. Whereas, a little difference such as red shift in band edge in the absorption spectra of silica coated core/shell/shell nanoparticles is observed, resulting the formation of optically active thinner amorphous silica layer around the surface of core/shell nanoparticles. It is worth noticing that the core-nanoparticles are coated with a hydrophobic layer of an inert NaYF4 shell on their surface and there are no appropriate functional groups (such as –COOH and –NH2) on their surface. So that, their solubility in aqueous solvent is poor, therefore, surface modification is necessary before their use in biological detections. So that they are coated by silica shell to enhancement their colloidal stability in aqueous solvent. The resultant silica surface modified core/shell/shell nanoparticles shows excellent solubility as well as colloidal stability in water to form a colloidal solution by sonication treatment, as shown in Fig. 5c. This is very important for amorphous silica coated core/shell/shell nanoparticles to fulfill bio-related applications, especially drug delivery and fluorescence imaging, because smaller nanoparticles (∼100 nm diameter) can escape from phagocytes in the reticuloendothelial (RES) system, such as the liver and spleen, and circulate through blood vessels with a long blood half-life; however, bigger nanoparticles result in rapid uptake by the RES. No obvious agglomeration or settling is found after storing the sample for a period of weeks under ambient conditions. The presence of silanol molecules on their surfaces not only results in high solubility in water, but also allows further conjugation with various biomolecules, which paves the way for further bio-applications of the core/shell/shell nanoparticles.
The electronic band structure or band gap energy is estimated from the energy difference between the highest occupied and lowest unoccupied molecular orbitals. The Tauc and Menths method is used to determine the band gap energy (Eg) of the prepared samples.24 According to these authors, the Eg is associated with absorbance and photon energy by the following equation:
| αhν = (hν − Eg)n | (1) |
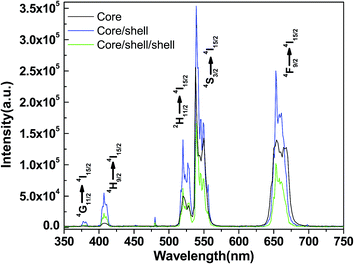
In order to evaluate the up-conversion quality of the as-prepared upconversion nanoparticles, we demonstrated a comparative spectral study with surface coated core/shell/shell nanoparticles and other well-known upconversion phosphors. The upconversion emission spectra of core, core/shell and silica modified core/shell/shell nanoparticles under excitation of a 980 nm NIR diode laser measured at room temperature. The upconversion spectrum shows five emission peaks located at 378, 406, 512–533, 536–558 and 643–675 nm, which correspond to 4G11/2 → 4I15/2, 4H9/2 → 4I15/2, 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, and 4F9/2 → 4I15/2 transitions of Er3+ ions, respectively. The green emission between 512–533 nm and 536–558 nm originates from the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2, transitions of Er3+.7,25,26 A dominant red emission assigned to the 4F9/2 → 4I15/2 transition of Er3+ is observed at 643–675 nm. As seen in Fig. 6, all three samples exhibit similar up-conversion spectral profile except a change in luminescence intensity is observed after assembly of shell around seed core-nanoparticles, which may be attributed to the alteration of crystallinity as well as influence of shell. The observed similar transition positions confirming that the local crystalline environments are identical before and after growth of shell. It is revealed in the spectrum that the luminescence efficiency of core-nanoparticles is generally lower than that of the reported bulk material.27,28 Yan and co-workers pointed out that this may result from the energy transfer processes to the surface through adjacent dopant ions or because luminescence of surface dopant ions is quenched.27 Yi and Chow also suggested that the organic surfactant with high vibrational energy on the nanoparticle surface (such as –COOH, –OH anions in this work) quenched the fluorescence.28 However, both of them demonstrated that this problem can be improved by coating an inert NaYF4 layer around the core-nanoparticles.
Interestingly, we observed remarkable enhancement in luminescence intensity after epitaxial growth of inert NaYF4 shell compared to their counter seed core-nanoparticles. Since pure NaYF4 do not exhibit upconversion luminescence, the increased upconversion emission observed undoubtedly originated from the core-nanoparticles that became enhanced after being covered by a shell. This phenomenon can be interpreted by the fact that formation of a shell could greatly decrease the surface defects and ligand influence of the core nanoparticles, which makes the luminescent ions located on the surface capable of functioning just as well as those within the crystal matrix itself.27 The emission intensity for the core/shell nanoparticles is 50 times higher as compared to the core-nanoparticles. The comparison of core and core/shell nanoparticles is done for the same weight percent of nanoparticles. This results in the fact that the number of core/shell particles is larger than the number of core nanoparticles. Because of this, the increase in luminescence intensity of core/shell nanoparticles is thus an under estimation. Based on the upconversion pump efficiency formula I ∝ Pn (n ≥ 2),29 where I stands for the upconversion intensity, P stands for the excitation pump power, and n is the number of absorbed photons. From the power dependence curve for green and red emission, both the 536–558 and 643–675 nm emissions are two-photon processes. The increase in the luminescence intensity for core/shell nanoparticles compared to core-nanoparticles is attributed to the presence of a shell, which protects the dopants in the core, especially those near the surface, from quenching arising from surface anchored surfactant hydroxyl groups. The reduction in quenching improves the overall upconversion quantum yield of the core-nanoparticles.12,13,28,30 Furthermore, our group has shown strong evidence for the formation of core/shell lanthanide nanoparticles using the aforementioned synthesis procedure12,13 and we conclude the same is true for these core/shell nanoparticles. Nevertheless, this is not the only observed effect of the Er3+/Yb3+-doped shell. According to the previously literature reports, the growth of this shell has triggered a general enhancement of the Tm3+ emission intensities from the Tm3+/Yb3+-doped core.13–17,31 This effect can be explained considering that the high-energy vibrational modes of the adsorbed species on the surface of the nanoparticles including the EDTA, sodium citrate, ethylene glycol, oleic acid and oleylamine capping ligands are known to induce quenching of the luminescence of lanthanide ions.13–17 To avoid this reduction in the luminescence intensity, this is a common option to passivate the surface of the nanoparticles by growing a protecting shell of undoped host material.12,13 In the present situation, grown an inert shell around the active Er3+/Yb3+-doped NaYF4 core-nanoparticles can have this effect and, thus, trigger the enhancement observed in Fig. 6b. Since this enhancement is linked to a core/shell nano-architecture, its presence can be considered as additional proof of the success in growing this kind of structure.
After the core/shell nanoparticles are coated with a silica layer their luminescence decreased to some extent because of the light-scattering effect on both emission and incident light by the silica layer. It is well recognized that the emission of luminescent lanthanide nanoparticles will be quenched to some extent in the environments that have a high phonon frequency.12,17 This phenomenon can be attributed to the involvement of large vibrational modes, such as –OH and –COOH in nanoparticles, which leads to the increase of nonradiative transition from 4S3/2 to 4F9/2 transitions.13–17,31,32 It is suggested that in amorphous silica modified core/shell nanoparticles, the non-radiative transition rate of Yb3+ increased greatly because of the large involvement of Si–OH bonds, leading to the obvious quenching of the Er3+ emission transitions. Moreover, the surface silanol (Si–OH) groups have tremendous vibration frequencies from 1000 to 3600 cm−1 will quench the emission of Er3+ to a great extent in silica modified core/shell nanoparticles. Despite the decrease in luminescence, the silica coated core/shell/shell nanoparticles still show naked-eye emissions under a NIR laser, which remains strong enough for various biomedical applications such as imaging, detection, and sensing. The presence of the silica layer is confirmed by TEM images and FTIR spectroscopy.
Conclusions
In conclusion, the fabricated highly emissive and aqueous dispersible NaYF4:Yb/Er, NaYF4:Yb/Er@NaYF4 and NaYF4:Yb/Er@NaYF4@SiO2 core/shell/shell nanoparticles with upconversion luminescent properties were prepared via general hydrothermal approach. The decrease band gap energy from core to core/shell/shell nanoparticles, which attributed to the quantum-size effect due to the growth of the inactive NaYF4 and amorphous silica shell, which decrease the crystallinity of the nanomaterials, respectively. In case of an passive layer deposited surrounding the core sample the emission intensity is significantly enhanced (∼10 times) in respect to the seed core-nanoparticles, which may be due to inert shell protect the luminescent core from the incident and emission light from the surface defects and suppress the non-radiative rate arising from surface/defect of particles. A slight decrease in upconversion efficiency of core/shell/shell nanoparticles was observed because of amorphous silica surface modification. However, silica surface modified core/shell/shell nanoparticles still possess strong upconversion luminescence property, similar to their parent core-nanoparticles. Comparative optical analysis of the core, core/shell and core/shell/shell nanoparticles indicate that the surface property of the upconversion core-nanoparticles can be changed after being coated with inert shell and porous silica. Our work has demonstrated a successful approach to prepare innovative very bright porous upconversion core, core/shell nanoparticles for broad photonic based biotechnological applications by using surface defect reduction strategies.Acknowledgements
This project was funded by national plan for science, technology and innovation (MAARIFAH) King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, award Number (13-Bio1246-02).References
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449 RSC.
- X. Chen, D. Peng, Q. Ju and F. Wang, Chem. Soc. Rev., 2015, 44, 1318 RSC.
- G. Chen, H. Ågren, T. Y. Ohulchanskyy and P. N. Prasad, Chem. Soc. Rev., 2015, 44, 1680 RSC.
- Y. I. Park, K. T. Lee, Y. D. Suh and T. Hyeon, Chem. Soc. Rev., 2015, 44, 1302 RSC.
- H. P. Zhou, C. H. Xu, W. Sun and C. H. Yan, Adv. Funct. Mater., 2009, 19, 3892–3900 CrossRef CAS.
- K. Zheng, L. Wang, D. Zhang, D. Zhao and W. Qin, Opt. Express, 2010, 18, 2934 CrossRef CAS PubMed.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426 CrossRef CAS PubMed; Z. Li and Y. Zhang, Nanotechnology, 2008, 19, 345606 CrossRef PubMed.
- A. Kar and A. Patra, Nanoscale, 2012, 4, 3608 RSC; R. Rajendran, L. K. Shrestha, K. Minami, M. Subramanian, R. Jayavel and K. Ariga, J. Mater. Chem. A, 2014, 2, 18480 Search PubMed.
- F. Vetrone, R. Naccache, V. Mahalingam, C. G. Morgan and J. A. Capobianco, Adv. Funct. Mater., 2009, 19, 2924 CrossRef CAS.
- F. Shi, J. Wang, D. Zhang, G. Qin and W. Qin, J. Mater. Chem., 2011, 21, 13413 RSC.
- K. Prorok, A. Bednarkiewicz, B. Cichy, A. Gnach, M. Misiak, M. Sobczyk and W. Strek, Nanoscale, 2014, 6, 1855 RSC.
- A. A. Ansari, A. K. Parchur, M. Alam and A. Azzeer, Spectrochim. Acta, Part A, 2014, 131, 30 CrossRef CAS PubMed.
- A. K. Parchur, A. I. Prasad, A. A. Ansari, S. B. Rai and R. S. Ningthoujam, Dalton Trans., 2012, 41, 11032 RSC.
- S. Cui, H. Chen, H. Zhu, J. Tian, X. Chi, Z. Qian, S. Achilefu and Y. Gu, J. Mater. Chem., 2012, 22, 4861 RSC.
- Y. Bao, Q. A. N. Luu, Y. Zhao, H. Fong, P. S. Maya and C. Jiang, Nanoscale, 2012, 4, 7369 RSC.
- W. Xu, Y. Zhu, X. Chen, J. Wang, L. Tao, S. Xu, T. Liu and H. Song, Nano Res., 2013, 6, 795 CrossRef CAS.
- X. Kang, Z. Cheng, C. Li, D. Yang, M. Shang, P. Ma, G. Li, N. Liu and J. Lin, J. Phys. Chem. C, 2011, 115, 15801 CAS.
- A. A. Ansari, T. N. Hasan, N. A. Syed, J. P. Labis, A. K. Parchur, G. Shafi and A. A. Alshatwi, Nanomedicine: Nanotechnology, Biology and Medicine, 2013, 9, 1328 CrossRef CAS PubMed.
- A. A. Ansari and J. P. Labis, J. Mater. Chem., 2012, 22, 16649 RSC.
- A. A. Ansari, S. P. Singh, N. Singh and B. D. Malhotra, Spectrochim. Acta, Part A, 2012, 86, 432 CrossRef CAS PubMed.
- G. Gao, C. Zhang, Z. Zhou, X. Zhang, J. Ma, C. Li, W. Jin and D. Cui, Nanoscale, 2013, 5, 351 RSC.
- F. Zhang, J. Li, J. Shan, L. Xu and D. Zhao, Chem.–Eur. J., 2009, 15, 11010 CrossRef CAS PubMed.
- L. Wang and Y. Li, Chem. Mater., 2007, 19, 727 CrossRef CAS.
- J. Tauc and A. Menth, J. Non-Cryst. Solids, 1972, 8/9, 569 CrossRef.
- Y. Dai, H. Xiao, J. Liu, Q. Yuan, P. Ma, D. Yang, C. Li, Z. Cheng, Z. Hou, P. Yang and J. Lin, J. Am. Chem. Soc., 2013, 135, 18920 CrossRef CAS PubMed.
- L. Tian, Z. Xu, S. Zhao, Y. Cui, Z. Liang, J. Zhang and X. Xu, Materials, 2014, 7, 7289 CrossRef.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CAS.
- G. S. Yi and G. M. Chow, Chem. Mater., 2007, 19, 341 CrossRef CAS.
- N. M. Sangeetha and F. C. J. M. van Veggel, J. Phys. Chem. C, 2009, 113, 14702 CAS.
- H. X. Mai, Y. W. Zhang, L. D. Sun and C. H. Yan, J. Phys. Chem. C, 2007, 111, 13721 CAS.
- Y. Ren, M. Chen, L. Hu, X. Fang and L. Wu, J. Mater. Chem., 2012, 22, 944 RSC.
- D. Hu, M. Chen, Y. Gao, F. Li and L. Wu, J. Mater. Chem., 2011, 21, 11276 RSC.
| This journal is © The Royal Society of Chemistry 2016 |