Preparation of photodegradable polyacrylamide hydrogels via micellar copolymerization and determination of their phototunable elasticity and swelling behaviors†
Fatma
Selen
a,
Volkan
Can
*bc and
Gokhan
Temel
*a
aYalova University, Polymer Engineering Department, Yalova, 77100, Turkey. E-mail: gtemel@yalova.edu.tr; Tel: +90 226 815 54 14
bHelmholtz Institute, Lisa-Meitner Campus, Hahn-Meitner Platz 1, Berlin, 14109, Germany. E-mail: volkan.can@helmholtz-berlin.de; Tel: +49 30 8062 43198
cIstanbul Technical University, Department of Chemistry, Maslak, Istanbul, 34469, Turkey
First published on 22nd March 2016
Abstract
Polyacrylamide (PAAm) hydrogels were prepared by micellar copolymerization methodology in the presence of acrylamide and photo-decomposable hydrophobic crosslinker (diacrylated Irgacure-2959). After the synthesis of hydrophobically functionalized hydrogels via free radical copolymerization, gel samples were exposed to UV irradiation for different time intervals between 0 and 17 hours in aqueous medium. According to the results of swelling and compression tests, the physical properties of the hydrogels were altered by UV exposure time. The swelling degree of the hydrogels increased gradually with the irradiation time. Moreover, the 17 h irradiated hydrogel sample was entirely decomposed at the end of the swelling test. According to Scanning Electron Microscopy images, UV irradiated hydrogel samples exhibited an apparent degradation behavior.
1. Introduction
Photoinduced polymerization is one of the most preferable and facile techniques to reduce energy consumption and improve green chemistry applications.1,2 Besides, the radical photopolymerization technique has been used in several commercial areas such as curing of coatings, fabrication of smart materials, nanocomposites, etc.3,4 Even though various polymeric materials have been produced by UV polymerization techniques, new kinds of macromolecular structures such as molecular weight controlled polymers,5,6 hydrogels,7–9 macromolecular photoinitiators,10–12 star and block copolymers13,14 can be synthesized by photoinduced polymerization strategies. Norrish Type I (α-cleavage) photoinitiators such as irgacure, acetophenone and acylphosphine oxide derivatives are known to be able to directly initiate the free radical photopolymerization under UV light. Hence they have found wide applications in the UV curing and coating industry.2,15–17Hydrophilic three dimensional crosslinked structures are able to absorb high degree of water and these materials show resemblance with natural tissues due to their soft and flexible structure. Since they have a high degree of water uptake characteristic, biocompatibility and non-toxic properties, these water swellable structures have found wide spread application areas in agriculture, drug-release researches, wound treatment etc.18–21 Especially photo-responsive or light-triggered hydrogels are promising materials and are used for cell migration, light-stimuli drug delivery and tissue engineering.22–24 On the other hand, degradable hydrogels have great potential in controlled release and tissue-engineering applications. Besides, photodegradation is one of the most popular method to gain control over the degradability of the polymeric materials and for this purpose, photodegradable groups are inserted into the macromolecular structure.25,26 There are several approaches on this matter that utilize different polymerization methods, such as reversible addition fragmentation chain transfer (RAFT), step-growth/chain-growth polymerizations and click chemistry techniques.25–28 However, there is constant need of new strategies to obtain photodegradable hydrogels for practical applications.
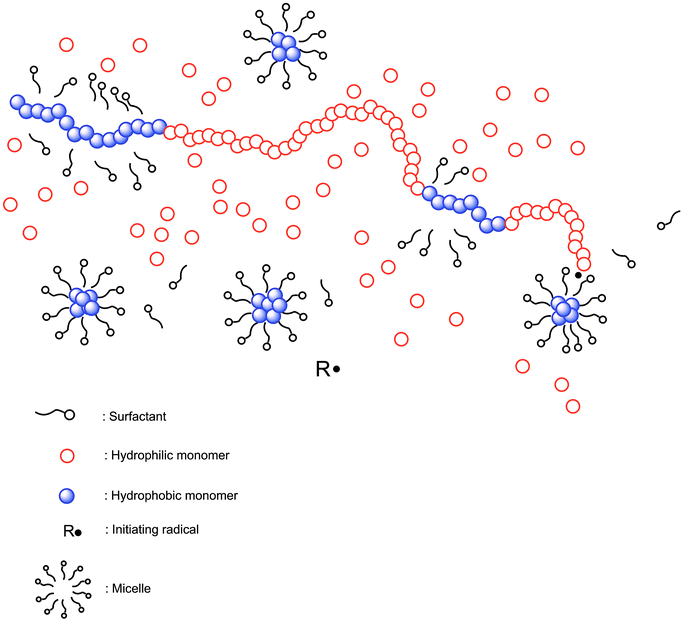
In this report, micellar copolymerization, which is a powerful method to obtain multi-block copolymers of both hydrophilic and hydrophobic monomers, was used to achieve hydrophobically modified hydrogel structure (Scheme 1).29–36 We introduce a new route for the synthesis of a photodegradable hydrogel via micellar copolymerization of a diacrylate functionalized photoinitiator and acrylamide in the presence of redox initiator in ambient temperature. A commercially available photoinitiator, (Irgacure-2959), was converted to a photocleavable crosslinker with a simple esterification and this hydrophobic compound was successfully incorporated into the hydrophilic polyacrylamide chains via micellar copolymerization methodology. Resulting hydrophobically modified hydrogel was undergone photo-degradation in various UV irradiation doses and degradation behavior was followed in the means of swelling and elasticity.
2. Experimental
2.1. Materials
Irgacure-2959 (98% Ciba), acryloyl chloride (97% contains <210 ppm MEHQ as stabilizer, Aldrich), dichloromethane (DCM, ≥99.8%, Aldrich), triethylamine (99%, Merck), acrylamide (AAm, Merck), sodium dodecylsulfate (SDS, Sigma), ammonium persulfate (APS, Fluka), and N,N,N′,N′-tetramethylethylenediamine (TEMED, Fluka) were used as received.2.2. Synthesis of diacrylated Irgacure-2959 (DAI)
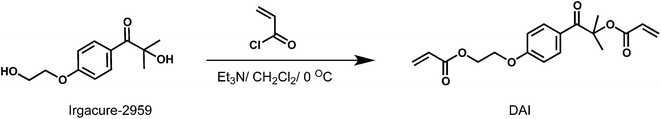
Acryloyl chloride (2.16 mL, 26.79 mmol) in DCM (3 mL) was added dropwise at 0 °C to a solution of Irgacure-2959 (2.0 g, 8.93 mmol) and triethylamine (2.51 mL, 19 mmol) in DCM (15 mL). After the addition of acryloyl chloride solution, the resulting mixture was stirred at room temperature overnight. Then the mixture was washed several times with 5% NaOH aqueous solution (200 mL), three times with distilled water (100 mL) and a saturated aqueous solution of NaCl (100 mL). The organic layers were dried over anhydrous Na2SO4, filtered, and then the solvent was evaporated. Crude product was purified by column chromatography (EtAc/Hex: 1/4) yield: 40%. 1H NMR (500 MHz, CDCl3), 7.96–6.79 ppm (m, 4H, Ar H), 6.37–5.72 ppm (m, 6H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 4.44–4.42 ppm (t, 2H, O
CH2), 4.44–4.42 ppm (t, 2H, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CO–CH2), 4.17–4.15 ppm (t, 2H, Ph–O–CH2), 1.65 ppm (s, 6H, C–(CH3)2). FT-IR (ATR): ν = 3083, 3039, 2947, 1723, 1710, 1676, 1602, 1509, 1131 cm−1. GC-MS (EI, 70 eV): 219, 99, 76, 55.
CO–CH2), 4.17–4.15 ppm (t, 2H, Ph–O–CH2), 1.65 ppm (s, 6H, C–(CH3)2). FT-IR (ATR): ν = 3083, 3039, 2947, 1723, 1710, 1676, 1602, 1509, 1131 cm−1. GC-MS (EI, 70 eV): 219, 99, 76, 55.
2.3. Gel preparation
The hydrogels were prepared by free radical micellar crosslinking copolymerization of AAm and DAI in aqueous SDS solutions at 30 °C. The concentration of SDS in the reaction solution was fixed at 7 w/v%, which is much above its critical micelle concentration (0.24 w/v%) at the same temperature. The initial concentration of the total monomer (AAm + DAI) was set to 5 w/v% throughout the experiments while the DAI content in the monomer mixture was 2.5 mol% and APS (3.5 mM). Stock solutions of APS and TEMED were prepared by dissolving 0.16 g of APS and 0.50 mL of TEMED each in 10 mL of distilled water.General procedure of photodegradable hydrogel preparation: DAI (0.0562 g) was added to the solution of SDS (0.7 g) in 7.0 mL of water and stirred to obtain a transparent solution. After addition of AAm (0.444 g) and stock solutions of TEMED (0.5 mL), solution was stirred until homogenous mixture obtained. Then, right after the stock solution of APS (0.5 mL) was added to initiate the reaction, the volume is rapidly completed to 10 mL in a flask. For the swelling and mechanical measurements, the solution was transferred into several plastic syringes of 4 and 10 mm internal diameters and the polymerization was conducted for 20 h at 30 °C.
2.4. General procedure of UV irradiation of hydrogels
Due to the strong adhesion properties of hydrogels, hydrophobically modified hydrogels were synthesized in plastic syringes. At the end of the reaction period, samples were extracted on to a moisturized and flat surface and cut into appropriate cylindrical pieces. Cut samples transferred into Pyrex tubes and 2 mL of distilled water was added into tubes to prevent hydrogels dry during the UV exposure period.2.5. Tensile mechanical tests
Uniaxial compression measurements were performed on cylindrical hydrogel samples just after preparation and in equilibrium swollen states by using a Zwick Roell, 10 N test machine at 25 °C under the following conditions: the hydrogel samples subjected to the mechanical tests right after the synthesis were about 4.1 ± 0.3 mm of diameter and 3 ± 0.2 mm of height. Dimensions of the network samples in equilibrium swollen states was varied between 10 and 16 mm of diameter with the similar diameter/height ratios as the samples at right after synthesis state. Before the test, an initial compressive force of 0.001 N was applied to ensure a complete contact between the gel and the plates. The tests were conducted at a constant speed of 0.3 mm min−1. Load and displacement data were collected during the experiment. The Young's modulus, E, was calculated from the slope of stress–strain curves between 5 and 15% compressions. For reproducibility, at least five samples were measured for each gel and the results were averaged.3. Results and discussion
3.1. Synthesis and characterizations
Micellar copolymerization is highly efficient and facile technique to prepare hydrophobically modified polymeric structures in aqueous media, and resulting polymer exhibits multi-block copolymeric structure possessing both hydrophobic and hydrophilic moieties (Scheme 1).29–36 In this contribution, micellar copolymerization approach was utilized for the first time to prepare hydrogels which contain photo-decomposable crosslinker units. For this purpose, diacrylated Irgacure-2959 (DAI) was synthesized by simple esterification method under mild conditions (Scheme 2). Mono and diacrylated Irgacure-2959 mixture was formed after acrylation reaction and desired difunctional crosslinker (DAI) was obtained after purification procedure. According to GC-MS measurement, resulting photo-decomposable crosslinker is in high purity and MS peaks confirm the exact structure of obtained product. 1H NMR and FT-IR results also confirm that diacrylation of Irgacure-2959 was carried out successfully (See ESI†). Thereby obtained photoactive hydrophobic compound (DAI) was used to achieve photo-cleavable polyacrylamide based hydrogel.3.2. Determination of gel fractions before and after UV irradiation
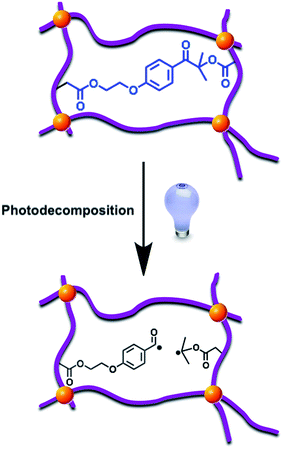
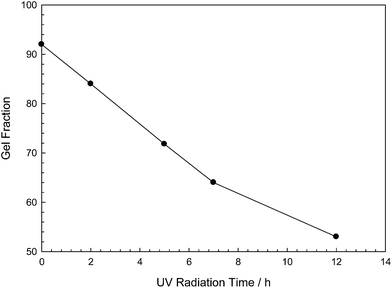
Polyacrylamide based photodegradable hydrogels were prepared as explained in Experimental section and the resulting hydrogel samples were exposed to varying doses of UV light (2, 5, 7, 12, 17 h) in order to understand the effect of the photodecomposable crosslinker on the hydrogel properties (Scheme 3).Gel fractions were investigated by gravimetric method. Fig. 1 denotes the gel fractions of hydrogel samples that are exposed to UV irradiation at different time intervals. As it can be seen from the figure, non-irradiated hydrogel has 91% gel fraction where, the gel fraction of UV irradiated samples decreases by increase of irradiation time. In addition, 12 h irradiated sample degraded approximately by 50% that causes the network to dissolve after this point. Hence, 17 hours irradiated sample was completely dissolved during swelling experiments and gel fraction of corresponding hydrogel could not be determined. These results can be directly attributed to the altered micro-structure of hydrogels due to the UV exposure.
3.3. Swelling and mechanical behaviors of hydrogels
Fig. 2 depicts the variation of swelling ratio with swelling time of photo-decomposable hydrogels. Samples show a swelling–deswelling pattern due to the ionic contribution of SDS moiety. Initially, gels contain an approximate concentration of 7% SDS, which causes the gel to act as an ionic network. However, as the swelling process continues, SDS molecules are washed out to decrease the ionic content of the gel. Therefore, gels swell up to a maximum value then start to deswell and reach lower equilibria. While non-irradiated sample has the lowest swelling ratio, water absorption capacities of UV irradiated samples are found to increase with the UV exposure time due to the decomposition of photodegradable crosslink units. On the other hand, maximum irradiated sample, 17 h, starts falling apart into pieces during the swelling process and no deswelling could be observed.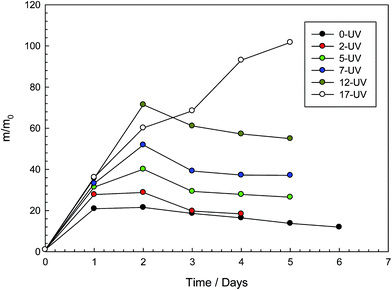 | ||
| Fig. 2 Swelling ratio m/m0 of photo-decomposable hydrogels with different time of UV irradiation shown as a function of the swelling time. | ||
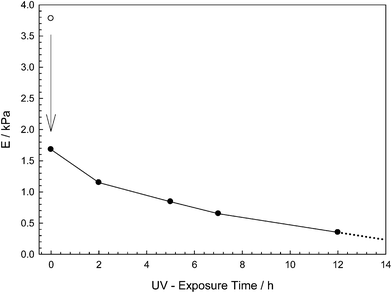
As the variation of the elastic moduli investigated, the non-irradiated sample was found to exhibit the highest elastic modulus value, since it does not undergo any photodecomposition process (Fig. 3). While the same sample reached equilibrium swollen state, elastic modulus value dramatically decreased as expected. Furthermore, elastic moduli continued to decrease as UV exposure time of hydrogel samples were increased. In addition, modulus of 17 hours irradiated hydrogel could not be investigated since sample was decomposed before end of the swelling experiment.
3.4. Macroscopic and morphologic studies of hydrogels
The morphologies of non-irradiated and irradiated hydrogels were investigated using scanning electron microscopy (SEM). In Fig. 4, non-irradiated hydrogel sample has porous structure due to the lyophilization process and it shows thick walled room structure (Fig. 4a). However, UV exposed hydrogel samples demonstrate thinner interface and irregular porous structures. Another point seen from SEM images is the decrease of wall thickness with increasing UV-exposure time (Fig. 4b and c). 12 hours UV exposed hydrogel exhibits much more decomposed material and separation of polymeric layers can be clearly observed in Fig. 4c.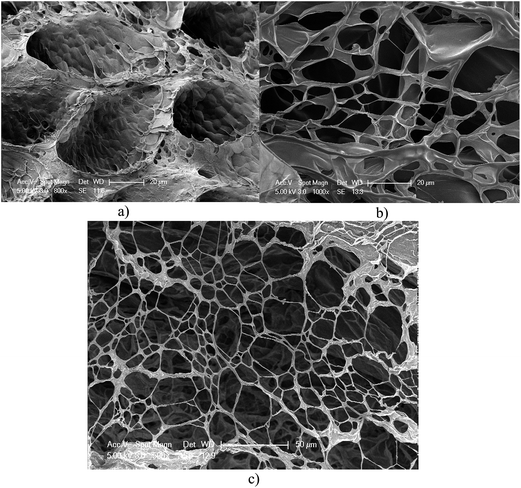 | ||
| Fig. 4 Scanning electron microscopy images of (a) non-irradiated, (b) 7 h irradiated and (c) 12 h irradiated hydrogel samples. | ||
Fig. 5 shows the hydrogel samples at swollen equilibrium states that were at the same dimensions initially and exposed to UV irradiation at different time intervals. It is obvious that swelling degree of the hydrogels increase significantly with the UV exposure time. 12 h exposed hydrogels are quite weak and 17 h irradiated hydrogels decompose almost completely at the end of swelling process. Figure exhibits a good representation of the macroscopic behavior of the photodegradable crosslinked hydrogels.
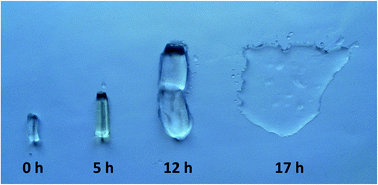 | ||
| Fig. 5 Equilibrium swollen state photograph of photodegradable hydrogel samples before and after irradiation. | ||
4. Conclusion
Photo-decomposable crosslinker was readily synthesized by end-functionalization of commercially available Irgcure-2959 photoinitiator with acrylate units. Afterwards, the resultant photodegradable crosslinker was successfully polymerized with acrylamide monomer via free radical micellar copolymerization in order to achieve hydrogels with phototunable properties. The study showed that physical and swelling properties of obtained hydrogels were strongly depended on the dose of UV light exposure. Furthermore, the samples that are exposed to UV more than 12 hours are found to be almost completely decomposed. We believe that these kinds of hydrogels may find many potential applications in various areas such as photo-triggered materials preparation, drug delivery systems and degradable thermosets.Acknowledgements
This study was supported by Yalova University Scientific Research Foundation (2013/YL/032).References
- S. Protti and M. Fagnoni, Photochem. Photobiol. Sci., 2009, 8, 1499–1516 CAS.
- J. P. Fouassier and J. Lalevee, Photoinitiators for Polymer Synthesis-Scope, Reactivity, and Efficiency, Wiley-VCH VerlagGmbH & Co. KGaA, Weinheim, 2012 Search PubMed.
- J. P. Fouassier, in Photoinitiation, Photopolymerization and Photocuring, Fundamentals and Applications, Hanser Verlag, Munich, 1995 Search PubMed.
- Y. Yagci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245–6260 CrossRef CAS.
- M. A. Tasdelen, M. Uygun and Y. Yagci, Macromol. Chem. Phys., 2011, 212, 2036–2042 CrossRef CAS.
- D. Konkolewicz, K. Schroeder, J. Buback, S. Bernhard and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 1219–1223 CrossRef CAS.
- N. Karaca, G. Temel, D. K. Balta, M. Aydin and N. Arsu, J. Photochem. Photobiol., A, 2010, 209, 1–6 CrossRef CAS.
- H. W. Ooi, K. S. Jack, A. K. Whittaker and H. Peng, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 4626–4636 CAS.
- J. Matsui, Y. Ochi and K. Tamaki, Chem. Lett., 2006, 35, 80–81 CrossRef CAS.
- G. Temel, N. Karaca and N. Arsu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5306–5312 CrossRef CAS.
- B. Gacal, H. Akat, D. K. Balta, N. Arsu and Y. Yagci, Macromolecules, 2008, 41, 2401–2405 CrossRef CAS.
- G. Temel, B. Aydogan, N. Arsu and Y. Yagci, Macromolecules, 2009, 42, 6098–6106 CrossRef CAS.
- J. T. Goldbach, T. P. Russell and J. Penelle, Macromolecules, 2002, 35, 4271–4276 CrossRef CAS.
- G. Temel, B. Aydogan, N. Arsu and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2938–2947 CrossRef CAS.
- D. S. Esen, N. Arsu, J. P. Da Silva, S. Jockusch and N. J. Turro, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1865–1871 CrossRef CAS.
- E. Frick, H. A. Ernst, D. Voll, T. J. A. Wolf, A.-N. Unterreiner and C. Barner-Kowollik, Polym. Chem., 2014, 5, 5053–5068 RSC.
- Z. Q. Li, J. Torgersen, A. Ajami, S. Muhleder, X. H. Qin, W. Husinsky, W. Holnthoner, A. Ovsianikov, J. Stampfl and R. Liska, RSC Adv., 2013, 3, 15939–15946 RSC.
- S. J. Buwalda, K. W. M. Boere, P. J. Dijkstra, J. Feijen, T. Vermonden and W. E. Hennink, J. Controlled Release, 2014, 190, 254–273 CrossRef CAS PubMed.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2012, 64, 18–23 CrossRef.
- D. Seliktar, Science, 2012, 336, 1124–1128 CrossRef CAS PubMed.
- T. Vermonden and B. Klumperman, Eur. Polym. J., 2015, 72, 341–343 CrossRef CAS.
- C. Garcia-Astrain, C. Pena-Rodriguez, A. Retegi, A. Eceiza, M. A. Corcuera and N. Gabilondo, Mater. Lett., 2015, 160, 142–145 CrossRef CAS.
- L. Yu and J. Ding, Chem. Soc. Rev., 2008, 37, 1473–1481 RSC.
- M. Arslan, T. N. Gevrek, R. Sanyal and A. Sanyal, Eur. Polym. J., 2015, 62, 426–434 CrossRef CAS.
- M. A. Azagarsamy, D. D. McKinnon, D. L. Age and K. S. Anseth, ACS Macro Lett., 2014, 3, 515–519 CrossRef CAS.
- D. Klinger and K. Landfester, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1062–1075 CrossRef CAS.
- F. Ercole, H. Thissen, K. Tsang, R. A. Evans and J. S. Forsythe, Macromolecules, 2012, 45, 8387–8400 CrossRef CAS.
- J. Liu, I. Y. Rad, F. Sun and J. W. Stansbury, Polym. Chem., 2014, 5, 227–233 RSC.
- S. Abdurrahmanoglu, V. Can and O. Okay, Polymer, 2009, 50, 5449–5455 CrossRef CAS.
- M. P. Algi and O. Okay, Eur. Polym. J., 2014, 59, 113–121 CrossRef CAS.
- G. Bastiat, B. Grassl, A. Khoukh and J. Francois, Langmuir, 2004, 20, 5759–5769 CrossRef CAS PubMed.
- M. Pabon, J. M. Corpart, J. Selb and F. Candau, J. Appl. Polym. Sci., 2004, 91, 916–924 CrossRef CAS.
- A. Hill, F. Candau and J. Selb, Macromolecules, 1993, 26, 4521–4532 CrossRef CAS.
- F. Candau, E. Volpert, I. Lacik and J. Selb, Macromol. Symp., 1996, 111, 85–94 CrossRef CAS.
- E. Volpert, J. Selb and F. Candau, Macromolecules, 1996, 29, 1452–1463 CrossRef CAS.
- K. C. Dowling and J. K. Thomas, Macromolecules, 1990, 23, 1059–1064 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra00556j |
| This journal is © The Royal Society of Chemistry 2016 |