Efficient degradation of methylene blue over boron-doped g-C3N4/Zn0.8Cd0.2S photocatalysts under simulated solar irradiation
Wei Tiana,
Quanhao Shena,
Naixu Li*a and
Jiancheng Zhou*abc
aSchool of Chemistry and Chemical Engineering, Southeast University, Nanjing 211189, Jiangsu, P. R. China. E-mail: naixuli@seu.edu.cn
bDepartment of Chemical and Pharmaceutical Engineering, Southeast University Chengxian College, Nanjing 210088, Jiangsu, P. R. China
cJiangsu Province Hi-Tech Key Laboratory for Bio-medical Research, Southeast University, Nanjing 211189, P. R. China. E-mail: jczhou@seu.edu.cn
First published on 2nd March 2016
Abstract
Boron-doped g-C3N4/Zn0.8Cd0.2S (BCN/ZS) has been prepared through a facile two-pot method and acted as an efficient photocatalysis material for degradation of methylene blue (MB) exposed to the Xe lamp. Construction of the ZS solid solution and BCN graphite-like structure, boron introduction into carbon sites in CN framework and formation of the hybrid structure between BCN and ZS are fully confirmed via various physical and chemical techniques. The last two structural factors are closely related to the enhanced photocatalytic function of the hybrids. Moreover, h+ and ˙O2− are testified as the major reactive species in MB photodegradation. Finally, the possible reaction path was proposed.
1. Introduction
Semiconductors, as one of the most efficient promoters for the conversion of solar irradiation to chemical energy, have found extensive application in various fields ranging from (photo)transformation of CO2 into high value-added chemicals, solar H2 evolution to photodegradation of environmental pollutants.1–6 Among these highly explored materials, TiO2 has been acknowledged as the most commonly utilized photocatalytic material due to its alluring properties such as its relatively low cost, the high photostability and nontoxicity. Nevertheless, it is merely UV-responsive and does not refer to visible-light stimulated reactions originated from unsatisfactory light harvesting performance on account of its excessively wide bandgap (3.2 eV) and low quantum efficiency. Despite intensive attempts on its various modifications invested to tackle the issues, limited progresses have been made.7–11As a highly appreciated alternative, graphitic carbon nitride (CN) has been proposed for use as a metal-free organic conjugated semiconductor for its extraordinary properties involving desirable thermal and chemical durability, suitable bandgap energy and position, unique optical and electronic properties (e.g. its response to visible light) as well as facile preparation from abundantly available precursors such as urea and melamine.12–15 Thereby, there is beyond dispute that it has risen as a shining star on the horizon of the path on which scientists have been actively pursuing new materials for environmental decontamination and artificial photosynthesis. However, it has been known to be restricted by some imperfections such as inescapable recombination of photoexcited charge carriers and low visible-light-active capability, leading to confined application of this material. In this regard, improvement and modification of CN are of scientific and technological importance and noble mission among aggressive scholars. In practice, structurally and chemically functionalized CNs are often dealt with to counteract aforementioned bottlenecks.16,17
Structural functionalization is widely reported to be dominated by introduction of nanostructures including quantum dots, nanoleaves, nanorods and nanoporous textures and large surface areas into CN framework via chemical cleavage or templating methods that opens new opportunities for the remarkably altered synthesis, structure and properties of CN toward an efficient visible light-driven material for catalysis.13,18–21 Despite all this, most researchers still direct their attentions to chemical modification mainly consisting of chemical doping22–25 and formation of heterojunctions.26–29
Among chemical modification with foreign atoms, boron-doped CN (BCN) has been broadly covered to amplify (photo)activity of CN for the targeted reaction by perfecting optical, electronical and surface properties. Especially, selection of boron source may result in different doping sites and further affect Lewis acidity on the surface of CN, which alters separation efficiency of charge carriers to some extent. Hence, reasonable choice of boron reagents can promote (photo)catalytic function. For instance, Wang's group fabricated BCN by participation of Ph4BNa30,31 and BH3NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 as boron reagents in thermally triggered polycondensation of urea or dicyandiamide as a CN precursor. With the exception of expected activities of as-prepared BCN samples, these authors highlighted that the boron atoms entered carbon sites and functioned as strong Lewis acid sites, which enlarged catalytic performances by accelerated separation of electrons from holes. By contrast, when boron atoms replace some hydrogen in primary or secondary amino groups, the aforementioned effect did not spring up possibly due to different boron precursors.33
32 as boron reagents in thermally triggered polycondensation of urea or dicyandiamide as a CN precursor. With the exception of expected activities of as-prepared BCN samples, these authors highlighted that the boron atoms entered carbon sites and functioned as strong Lewis acid sites, which enlarged catalytic performances by accelerated separation of electrons from holes. By contrast, when boron atoms replace some hydrogen in primary or secondary amino groups, the aforementioned effect did not spring up possibly due to different boron precursors.33
Apart from chemical doping, coupling CN or doped CN with a second semiconductor with a suitable bandgap or a cocatalyst to construct a hybrid semiconductor equally provides a powerful tool in optimizing the charge carriers handling and hindering the recombination process and thereby impels enhanced property for photocatalysis.34 Amongst the semiconductors or cocatalysts commonly available, ZnxCd1−xS solid solutions rise to fame for their eminent characteristics. They are endowed with an adjustable optical response region and separation efficiency of electron–hole pairs by x value. Besides, they are readily synthesized with various protocols from earth-abundant feedstocks. More importantly, similar to other semiconductors, they are so widely tunable through chemical and morphological modification that it allows the rational engineering and preparation of a broad set of ZnxCd1−xS-based promoters and thus hold great promise for versatile applications in photocatalysis or catalysis.35–40 With regard to structurally modified ZnxCd1−xS, a typical case is from Liu et al.40 He prepared Cd0.5Zn0.5S with unique nano-twin structures by precipitate-hydrothermal method and pointed out its photocatalytic superiority (1.79 mmol h−1) in H2 evolution over those of its counterparts (below 0.96 mmol h−1) obtained by three other methods including hydrothermal, precipitation and thermal sulfuration methods. Notably, the author highlighted that the formation of twin-boundary-dependent potential associated with nano-twin structures was favourable for separation of charge carriers and thus led to enhanced catalytic function under visible light irradiation. Another case in point is twinned Cd0.5Zn0.5S nanorods by microwave-assistant hydrothermal method.39 These nanorods accommodated a series of homojunctions with near periodical and dense distribution, which proved very efficient in separating photo-generated charges and prolonging lifetime of both photo-generated electrons and holes, leading to improved photoactivity for solar H2 generation. To conclude, structural alterations work well in improved catalytic performance. Besides, chemical modification of these compounds has been widely reported to play an indispensable part in improvement of photocatalysis. Li et al.35 synthesized NiS functionalized Cd0.5Zn0.5S nanocrystals by a microwave-assistant hydrothermal method. Research results indicated that compared with those of naked Cd0.5Zn0.5S, NiS loading enabled the initial H2 evolution rate up to 1.4 mmol h−1 from below 0.4 mmol h−1 and AQY to 33.9% from 9.2% due to remarkably enhanced optical absorption capacity and better separation of excitons for the composite. More importantly, the author confirmed NiS as an excellent electron acceptor and active site carrier for proton reduction that might equal or even surpass noble metals. Similar conclusions could be come to by Lu et al.38 as well.
Herein, BCN/ZS heterostructures were facilely prepared via a two-step route. First, BCN was prepared through co-calcination of H3BO3 and urea (0.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) at 550 °C for 4 h with a heated rate of 4.5 °C min−1. Second, BCN was efficiently covered by ZS by an in situ precipitation method. Afterwards, they were subjected to structural and compositional examination via various physical and chemical techniques, namely XRD, UV-vis, FT-IR, PL, TEM, EDS and XPS and employed as the catalysts for degradation of MB under simulated sunlight. In previously reported results,41 ZS (x = 0.8) demonstrated as the most active and environment-friendly photocatalyst (56.1%) among ZnxCd1−xS solid solutions for removal of MB. Accordingly, it was selected as the optimal candidate for assembly of CN-based samples in this paper. Besides, formation of heterojunctions between BCN and ZS, boron introduction into carbon sites in CN, presence of ZS solid solution and BCN graphite-like structure are well determined. The former two factors play a dominating role in increased photocatalytic activity. What's more, h+ and ˙O2− are identified as the major reactive species in photodegradation of MB. Finally, the possible reaction path was proposed.
1, w/w) at 550 °C for 4 h with a heated rate of 4.5 °C min−1. Second, BCN was efficiently covered by ZS by an in situ precipitation method. Afterwards, they were subjected to structural and compositional examination via various physical and chemical techniques, namely XRD, UV-vis, FT-IR, PL, TEM, EDS and XPS and employed as the catalysts for degradation of MB under simulated sunlight. In previously reported results,41 ZS (x = 0.8) demonstrated as the most active and environment-friendly photocatalyst (56.1%) among ZnxCd1−xS solid solutions for removal of MB. Accordingly, it was selected as the optimal candidate for assembly of CN-based samples in this paper. Besides, formation of heterojunctions between BCN and ZS, boron introduction into carbon sites in CN, presence of ZS solid solution and BCN graphite-like structure are well determined. The former two factors play a dominating role in increased photocatalytic activity. What's more, h+ and ˙O2− are identified as the major reactive species in photodegradation of MB. Finally, the possible reaction path was proposed.
2. Experimental
2.1. Materials
All chemicals were analytical reagent and commercially available and used without further purification.2.2. Synthesis of BCN
Synthetic route of BCN powder was similar to that of PCN with some alterations.41 Briefly, a mixture of boric acid (1.2 g), urea (20 g) and a certain amount of anhydrous alcohol was magnetically stirred at 80 °C for a period of time and dried at 100 °C in an oven to remove alcohol completely. Subsequently, the obtained white solids were well-ground and thermally treated at 550 °C for 4 h with a ramp of 4.5 °C min−1 in a muffle furnace. Finally, the resulting yellow powder was thoroughly rinsed with anhydrous alcohol for several times, then dried enough and labelled as BCN for further use. For comparison, CN was prepared via the same heat treatment in the absence of boric acid and alcohol.2.3. Fabrication of BCN/ZS composite photocatalysts
The composites were fabricated through the similar procedure as that of PCN/ZS with small modification.41 Typically, a mixture of Zn(Ac)2·2H2O, Cd(Ac)2·2H2O (Zn/Cd = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BCN and deionized water was sonicated for 2 h, followed by magnetic stirring of these compounds with a slightly excessive thiourea at 80 °C for 4 h to fully yield BCN/ZS. Afterwards, the crude product was well-washed with deionized water, sufficiently dried at 100 °C and well-ground for the following use. For simplicity, the BCN samples modified with different amount of ZS were denoted as BCN/ZS-y (y = 0, 0.1, 0.25, 0.5 and 2 wt%), where y represented a mass ratio of BCN to ZS. For reference, CN/ZS-0.25, BCN/ZnS-0.25 and BCN/CdS-0.25 were also synthesized via the similar route as that of BCN/ZS.
1), BCN and deionized water was sonicated for 2 h, followed by magnetic stirring of these compounds with a slightly excessive thiourea at 80 °C for 4 h to fully yield BCN/ZS. Afterwards, the crude product was well-washed with deionized water, sufficiently dried at 100 °C and well-ground for the following use. For simplicity, the BCN samples modified with different amount of ZS were denoted as BCN/ZS-y (y = 0, 0.1, 0.25, 0.5 and 2 wt%), where y represented a mass ratio of BCN to ZS. For reference, CN/ZS-0.25, BCN/ZnS-0.25 and BCN/CdS-0.25 were also synthesized via the similar route as that of BCN/ZS.
2.4. Characterization
X-ray diffraction (XRD) analysis was carried out on Bruker D8-Discover diffractometer with Cu Kα radiation (λ = 1.54178 Å). The transmission electron microscopy (TEM) and the energy-dispersive X-ray spectrometry (EDS) were conducted on Hitachi H-600 operating at an accelerating voltage of 200 kV. Ultraviolet-visible (UV-vis) spectra were recorded on a Shimadzu UV-3600 spectrometer in a wavelength range of 240–800 nm. X-ray photoelectron spectroscopy (XPS) was performed on 2000 XPS system with a monochromatic Al Kα source. Fourier transform infrared spectra (FT-IR) were collected on a TENSOR27 PMA 50 FT-IR spectrometer. Photoluminescence (PL) spectra were obtained at room temperature on a fluorospectrophotometer (Fluoromax-4) using a Xe lamp as an excitation source.2.5. Photocatalytic reaction for MB degradation
The degradation process was fairly parallel to that reported in previous paper with tiny adjustment.41 Shortly, the photocatalytic performances of BCN/ZS samples were estimated in an assay of MB degradation reactions under the 500 W Xe lamp illumination. The aqueous MB solution (250 mL, 0.12 mmol L−1) and the composite photocatalyst (0.3 g) were added into a 250 mL Pyrex flask and magnetically stirred in the dark for about 1 h to form an adsorption–desorption equilibrium between them. Then, the reaction was allowed for 2 h under consecutive agitation and light irradiation. The temperature of the whole system was maintained at the ambient temperature by a flow of cooling water. At set intervals, 4 mL aliquots were withdrawn and centrifuged to remove the photocatalyst particles. The absorbance of MB was measured on a UV-vis spectrophotometer (λmax = 664 nm). The degradation efficiency (D, %) of MB was calculated according to the following equation:| D = (1 − Ct/C0) × 100% | (1) |
3. Results and discussion
3.1. XRD investigation
XRD patterns of as-prepared samples displayed in Fig. 1 unambiguously elucidate their structural peculiarities. Simultaneously, standard patterns of cubic ZnS and hexagonal CdS are provided for comparison. The typical peaks located at 13.1° and 27.5° in Fig. 1a are assigned to the (100) and (002) diffraction planes and related to the in-plane structural packing motif and interlayer stacking of aromatic segments of CN, separately. The calculated interplanar distance of aromatic units is 0.324 nm. In the case of BCN (Fig. 1b), similar positions and evidently reduced intensities relative to CN suggest BCN with a 2D graphite-like structure analogous to it and retarded crystal growth by B doping, as is well known for the melon network.31,32 Also, BCN has a lattice spacing of 0.325 nm, which is in line with CN and further states the structural preservation of CN after its modification with boron. For pristine ZS (Fig. 1c), its appealing feature of solid solution is fully judged from the three following points:35,38,41,42 (a) the curve shape is parallel to that reported by many authors;39,40 (b) the (111), (200) and (311) characteristic peaks located at 26.9°, 44.5° and 52.7° position between that of (100), (220) and (311) for the standard cubic ZnS and that of (002), (110) and (112) for the standard hexagonal CdS, respectively (the regions labelled with the two corresponding dash lines); (c) a combined XRD pattern of ZnS and CdS is not observed, precluding the possibility of the formation of their composite. Besides, the lattice spacing for the (111) plane of ZS is estimated to be 0.331 nm, which conforms to the following TEM result. As for composite samples (Fig. 1d–g), the similarity of their patterns to ZS indicates decoration of ZS with BCN leads to largely unaffected phase structure and crystallinity. Notably, a painstaking comparison on XRD curves of BCN/ZS-0.25 (Fig. 1e) and CN/ZS-0.25 (Fig. 1h) supports that B doping is independent of structural variation of the composites.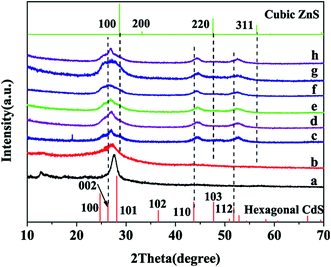 | ||
| Fig. 1 XRD patterns of as-prepared samples (a) CN, (b) BCN, (c) ZS, (d) BCN/ZS-0.1, (e) BCN/ZS-0.25, (f) BCN/ZS-0.5, (g) BCN/ZS-2 and (h) CN/ZS-0.25. | ||
3.2. XPS investigation
XPS features as a powerful probe for qualitative and quantitative analysis of the surface chemical composition and electronic state of the as-prepared samples, which necessitates XPS analysis of selected samples. XPS spectra of BCN/ZS-0.25 are exhibited in Fig. 2 and those of BCN provided for comparison. The peak at 191.68 eV in B 1s spectrum (Fig. 2a) provides the convincing evidence for boron insertion in BCN frame in the form of N–B–N coordination, which implies boron as a substitute for some carton atoms in the CN framework. The boron atoms work as Lewis acid centers and thus favor prompt separation of excitons, leading to elevated light-induced catalytic function,30,32 which matches well with the PL result (Fig. 6). The high resolution C 1s spectrum in Fig. 2b shows the sharp peaks positioned at 288.39 and 284.80 eV are respectively attributed to an sp2-bonded carbon (C–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and to carbon impurities.30 In the N 1s spectrum (Fig. 2c), the binding energy of 398.56, 400.06 and 400.9 eV are separately ascribed to C
N) and to carbon impurities.30 In the N 1s spectrum (Fig. 2c), the binding energy of 398.56, 400.06 and 400.9 eV are separately ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C coordination, tertiary nitrogen N–(C)3 groups and the amino groups C–N–H associated with structural defects and incomplete condensation.43 On the other hand, the peak at 399.12 eV may result from some chemical interaction of BCN and ZS. Notably, a slight chemical shift occurs to counterparts of BCN compared with some XPS peaks of B 1s, C 1s and N 1s in BCN/ZS-0.25, which infers a strong chemical interaction between BCN and ZS.38 As can be seen from Fig. 2d, the characteristic doublet of Zn 2p at 1044.98 eV and 1021.78 eV makes clear that Zn exists in the chemical state of Zn2+. Also, the XPS survey spectrum presented in the inset manifests the presence of B, C, N, Zn, Cd and S. The peaks recorded at 404.98 eV and 411.78 eV in the Cd region (Fig. 2e) are attributable to Cd2+, which corresponds to the spin–orbit split components. In the final figure, S 2p peaks at 162.47 and 161.21 eV support the presence of S2−. In light of the consistent binding energy of Zn2+, Cd2+ and S2− in ZS with the literature data related,35,37,44 the sample is identified as a solid solution for a second time.
N–C coordination, tertiary nitrogen N–(C)3 groups and the amino groups C–N–H associated with structural defects and incomplete condensation.43 On the other hand, the peak at 399.12 eV may result from some chemical interaction of BCN and ZS. Notably, a slight chemical shift occurs to counterparts of BCN compared with some XPS peaks of B 1s, C 1s and N 1s in BCN/ZS-0.25, which infers a strong chemical interaction between BCN and ZS.38 As can be seen from Fig. 2d, the characteristic doublet of Zn 2p at 1044.98 eV and 1021.78 eV makes clear that Zn exists in the chemical state of Zn2+. Also, the XPS survey spectrum presented in the inset manifests the presence of B, C, N, Zn, Cd and S. The peaks recorded at 404.98 eV and 411.78 eV in the Cd region (Fig. 2e) are attributable to Cd2+, which corresponds to the spin–orbit split components. In the final figure, S 2p peaks at 162.47 and 161.21 eV support the presence of S2−. In light of the consistent binding energy of Zn2+, Cd2+ and S2− in ZS with the literature data related,35,37,44 the sample is identified as a solid solution for a second time.
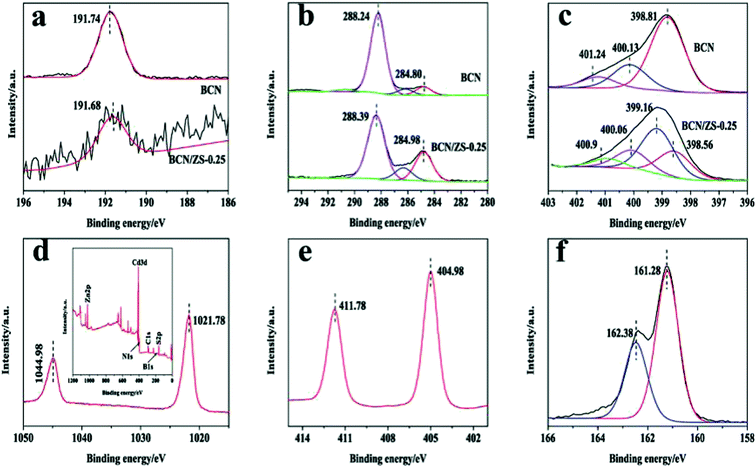 | ||
| Fig. 2 XPS spectra of (a) B 1s, (b) C 1s, (c) N 1s, (d) Zn 2p with an inset of survey spectrum, (e) Cd 3d and (f) S 2p in the BCN/ZS-0.25 sample. | ||
Additionally, XPS spectra offer the Zn2+ and Cd2+ content of 13.45 and 5.17 atomic% in the BCN/ZS-0.25, respectively. Thus, the real molar ratio of Zn2+ to Cd2+ is about 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In a word, “Zn0.8Cd0.2S” is in fact Zn0.72Cd0.28S, which matches roughly with its nominal composition.
1. In a word, “Zn0.8Cd0.2S” is in fact Zn0.72Cd0.28S, which matches roughly with its nominal composition.
3.3. TEM and EDS investigation
TEM and EDS characterization presented in Fig. 3 expresses useful information on the architecture and elemental constitution of BCN, ZS, and BCN/ZS-0.25. The typical graphite-like structure of BCN as evidenced by XRD is clearly visualized by Fig. 3a. Fig. 3b illustrates regular particle morphology of ZS with a uniform diameter of ca. 10 nm. Remarkably, the magnified TEM (Fig. 3c) confirms ZS as a solid solution by virtue of the ordered lattice fringes (lattice spacing) of 0.350 nm, corresponding to the (111) plane of ZS, which is in agreement with XRD and XPS results. In Fig. 3d, uneven distribution of ZS aggregates on BCN sheets is clearly observed. For another thing, no obvious structural change of BCN sheets can be found before and after the formation of ZS functionalized BCN (Fig. 3a and d). HRTEM image of BCN/ZS-0.25 in Fig. 3e substantiates construction of the heterostructure between BCN and ZS as XPS does. Interestingly, coincident lattice spacing of ZS in Fig. 3c (0.350 nm) and inset in Fig. 3e (0.351 nm) are in favor of unaffected framework of ZS via its cooperation with BCN. In a word, BCN and ZS remain their individual frameworks after their chemical combination. In addition, judging from the EDS pattern (Fig. 3f and its inset), it is beyond doubt that B, C, N, Zn, Cd and S constitute BCN/ZS-0.25, which receives a cross-identification from XPS. | ||
| Fig. 3 TEM graphs of the samples (a) pure BCN, (b) pure ZS and (d) BCN/ZS-0.25, HRTEM of (c) ZS and (e) BCN/ZS-0.25 and (f) EDS pattern of BCN/ZS-0.25. | ||
3.4. UV-vis investigation
A meticulous comparison in the UV-vis curves shown in Fig. 4 sheds light on the variation in light absorption properties of as-prepared samples. Apparently, in Fig. 4a, the absorption band of BCN exhibits a visual red-shift in comparison with that of CN, which is indicative of the amplified visible light absorption and narrowed bandgap by B doping.30,31 Next, it undergoes a pronounced movement to a long wavelength with its modification with ZS, implying the close connection at the interface in nanoscale level between them that allows more photons captured to run the light driven reaction, which agrees well with XPS and TEM analyses. Notably, a ZS loading-dependent successive red-shift in the sole steep absorption band of the composites as an indication of an intense band-edge absorption from band-to-band transition from VB to CB is equally associated with construction of heterostructures.35 The hybrid structure carries a low interface potential barrier to accelerate movement of electrons and thus benefits a higher catalytic function. Interestingly, BCN/ZS-0.25 provides approximate optical performance as CN/ZS-0.25 does possibly due to exceptionally low BCN content in BCN/ZS-0.25 covering effect of B doping, which can be more clearly observed in Fig. 4b. Furthermore, similar to those of the composites, ZS displays a classical sole and steep absorption band and thus is rendered a strong band-edge absorption as a solid solution.44 On the other hand, absence of a combined absorption edge of ZnS and CdS is observed, ruling out formation of their composite.Bandgap energy of a semiconductor is calculated according to the following expression:5,45,46
| Eg = 1240/λg (nm) | (2) |
3.5. IR investigation
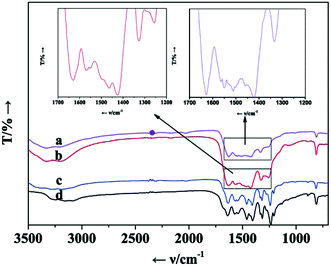
IR spectra of CN, BCN, BCN/ZS-0.25 and CN/ZS-0.25 are summarized in Fig. 5. The peaks at 814 cm−1 and in the range of 1200–1700 cm−1 are respectively ascribed to characteristic absorption of tri-s-triazine rings and aromatic CN heterocycles.30 Besides, the broad absorption band around 3200 cm−1 originates from the stretching vibration of N–H bond in surface uncondensed amino groups.41,46 These peaks are equally visual in IR spectra of three other samples, declaring the framework of CN remains untouched once again after its modification with boron and/or ZS. Notably, the typical peak at 1370 cm−1 closely related to N–B–N is hardly observed possibly owing to superposition of the band with that of the N–C–N stretch.30,323.6. PL investigation
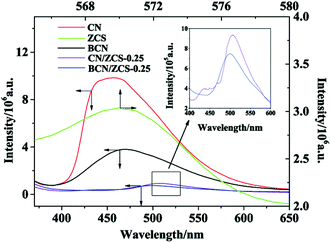
It is well known that PL emission originates from exciton recombination. Thus, PL intensity is used to reflect their separation efficiency and further assess catalytic function. Fig. 6 presents PL spectra of CN, BCN, ZS, CN/ZS-0.25 and BCN/ZS-0.25. The broad PL band of CN centered at 456 nm results from the band–band PL phenomenon with the light energy corresponding to Eg calculated by UV-vis absorption spectra. The highly quenched PL intensity of BCN states that B doping furthers good disassociation of light induced electrons from holes without any significant alteration in the PL band position. Also, the effect is further reinforced after its decoration with ZS to form BCN/ZS-0.25, which implies that the heterojunction between ZS and BCN hinders the recombination of the charge carriers and encourages their efficient separation, leaving more to participate in the photodegradation of MB. As seen from the inset in Fig. 6, the lower PL intensity of BCN/ZS-0.25 than CN/ZS-0.25 indicates accelerated separation efficiency by B doping works well in the composite, which is consistent with XPS analysis. Notably, an obvious red shift with regard to PL spectrum of BCN or CN takes place upon the formation of the composites (BCN/ZS-0.25 or CN/ZS-0.25). A possible explanation to this is that higher light absorption of the composites (Fig. 4) means the narrower bandgap and thus corresponding PL emission with lower light energy upon recombination of charge carriers. The similar observation was obtained in reported paper.413.7. Evaluation of photocatalytic performance
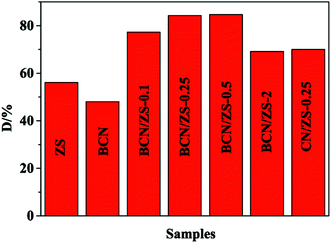
MB photodegradation over BCN/ZS composite samples serves as a model reaction to investigate y-dependent photocatalytic activity. As seen from Fig. 7, the composites (69.2–84.3%) are more photocatalytically active than the pure BCN (48.1%) and ZS (56.1%41) references in terms of D value, which benefits mainly from formation of the heterojunctions between plain references to capture more photons to prompt light-induced reaction and to enable the fast splitting of light-induced excitons, which has been confirmed by XPS (Fig. 2), UV-vis (Fig. 4) and PL (Fig. 6). Next, among composites, BCN/ZS-0.25 (84.3%) works as the optimal photocatalyst possibly due to the most efficient chemical contact between BCN and ZS. Finally, B doping equally plays a key role in elevation in light-driven catalytic function in view of enhanced D value of BCN/ZS-0.25 relative to that of CN/ZS-0.25 (70%), which is rationalized by evidently reduced PL intensity of BCN/ZS-0.25 (Fig. 6) as a convincing symbol for more efficient separation of excitons.In order to highlight the superiority of BCN/ZS-0.25 in catalytic performance, control experiments are of great importance and summarized in Table 1. Clearly, BCN/ZS-0.25 has the overwhelming catalytic advantage over BCN/ZnS-0.25 and BCN/CdS-0.25 (Table 1, Title 2 and 3), possibly due to much more photons collected to run the reaction and more powerful corrosion-resistant performance and much lower toxicity compared with the two references, respectively.47,48 Besides, the blank tests (Table 1, Title 4–6) confirm that MB photodegradation indeed is a photocatalytic process instead of a thermal catalytic or self-degradation one.
3.8. Photostability test
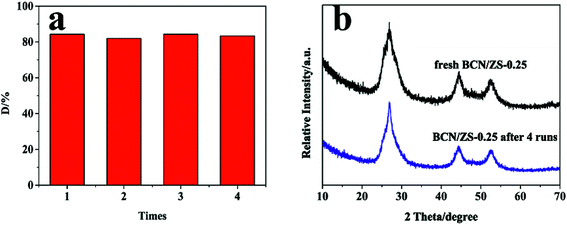
Generally speaking, excellent duration to the light is in great demand for a very promising catalytic material. Thereby, the photostability of BCN/ZS-0.25 is assessed. As illustrated respectively in Fig. 8a and b, BCN/ZS-0.25 remains actively and structurally constant, enunciating its outstanding chemical duration.3.9. The reaction mechanism
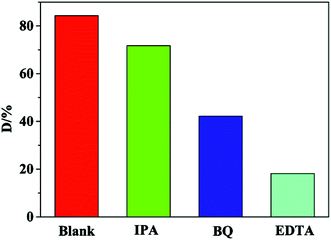
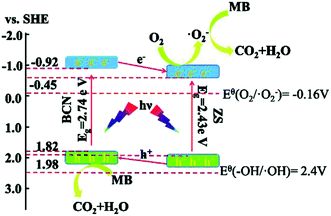
It is well-known that determination of primary active species in the degradation process underlies the proposal for reaction mechanism. Accordingly, the reactive species trapping experiments were conducted. Ethylene diamine tetracetic acid (EDTA), isopropanol (IPA) and benzoquinone (BQ) were used as the hole (h+) scavenger, hydroxyl radical (˙OH) scavenger and superoxide radical (˙O2−) scavenger, respectively. As noticed from Fig. 9, upon addition of EDTA and BQ into the original system (blank), the considerably diminished D values certificate h+ and ˙O2− as the main reactive species. Similarly, ˙OH turns out to be the minor species.The suitable band potentials have been reported to be highly desirable for two semiconductors intended for assembly of a hybrid. The valence band (VB) and conduction band (CB) potentials can be obtained from the two equations below:5,41,45
| EVB = X − Ee + 0.5Eg | (3) |
| ECB = EVB − Eg | (4) |
4. Conclusions
BCN/ZS samples were fabricated via a technically viable two-pot method and acted as efficient catalysts for MB photodegradation. As-prepared samples were subjected to various characterizations by means of XRD, TEM-EDS, XPS, UV-vis, FT-IR and PL. Research results highlight that construction of heterostructure between BCN and ZS holds the main part in increased photocatalytic activity. Also, no doubt that boron doping results in enhanced catalytic function of the composites as well by virtue of replacement of carbon sites in CN matrix by boron atoms as Lewis acid centers that accelerate splitting of excitons. Furthermore, ˙O2− and h+ are the major species in MB photodegradation. On the basis of this, the reaction path has been proposed.Acknowledgements
This work was financially supported by the Fundamental Research Funds for the Central Universities of China (No. 3207045403 and 3207045409), National Natural Science Foundation of China (No. 21576050) and Jiangsu Provincial Natural Science Foundation of China (BK20150604).Notes and references
- Y. J. Zhang, T. Mori and J. H. Ye, Sci. Adv. Mater., 2012, 4, 282–291 CrossRef CAS.
- Q. W. Tian, L. Zhang, J. H. Liu, N. X. Li, Q. H. Ma, J. C. Zhou and Y. M. Sun, RSC Adv., 2015, 5, 734–739 RSC.
- J. H. Liu, L. Zhang, N. X. Li, Q. W. Tian, J. C. Zhou and Y. M. Sun, J. Mater. Chem. A, 2015, 3, 706–712 CAS.
- H. L. Wang, L. S. Zhang, Z. G. Chen, J. Q. Hu, S. J. Li, Z. H. Wang, J. S. Liu and X. C. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- N. Li, H. Teng, L. Zhang, J. Zhou and M. Liu, RSC Adv., 2015, 5, 95394–95400 RSC.
- C. S. Pan and Y. F. Zhu, Catal. Sci. Technol., 2015, 5, 3071–3083 CAS.
- M. V. Dozzi and E. Selli, J. Photochem. Photobiol., C, 2013, 14, 13–28 CrossRef CAS.
- J. Tian, Z. Zhao, A. Kumar, R. I. Boughton and H. Liu, Chem. Soc. Rev., 2014, 43, 6920–6937 RSC.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- Y. Chen and L. Guo, J. Mater. Chem., 2012, 22, 7507–7514 RSC.
- Y. Chen, L. Wang, G. Lu, X. Yao and L. Guo, J. Mater. Chem., 2011, 21, 5134–5141 RSC.
- Z. X. Zhou, J. H. Wang, J. C. Yu, Y. F. Shen, Y. Li, A. R. Liu, S. Q. Liu and Y. J. Zhang, J. Am. Chem. Soc., 2015, 137, 2179–2182 CrossRef CAS PubMed.
- Z. Zhou, Y. Shen, Y. Li, A. Liu, S. Liu and Y. Zhang, ACS Nano, 2015, 9, 12480–12487 CrossRef CAS PubMed.
- Y. J. Zhang and M. Antonietti, Chem.–Asian J., 2010, 5, 1307–1311 CAS.
- Y. J. Zhang, A. Thomas, M. Antonietti and X. C. Wang, J. Am. Chem. Soc., 2009, 131, 50–51 CrossRef CAS PubMed.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- X. C. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596–1606 CrossRef CAS.
- Z. Yang, Y. Zhang and Z. Schnepp, J. Mater. Chem. A, 2015, 3, 14081–14092 CAS.
- J. Wang, C. Zhang, Y. Shen, Z. Zhou, J. Yu, Y. Li, W. Wei, S. Liu and Y. Zhang, J. Mater. Chem. A, 2015, 3, 5126–5131 CAS.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed.
- Y. J. Zhang, Z. Schnepp, J. Y. Cao, S. X. Ouyang, Y. Li, J. H. Ye and S. Q. Liu, Sci. Rep., 2013, 3, 1–5 Search PubMed.
- Y. J. Zhang, T. Mori, J. H. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- X. F. Chen, J. S. Zhang, X. Z. Fu, M. Antonietti and X. C. Wang, J. Am. Chem. Soc., 2009, 131, 11658–11659 CrossRef CAS PubMed.
- Z. J. Huang, F. B. Li, B. F. Chen, T. Lu, Y. Yuan and G. Q. Yuan, Appl. Catal., B, 2013, 136, 269–277 CrossRef.
- J. S. Zhang, M. W. Zhang, G. G. Zhang and X. C. Wang, ACS Catal., 2012, 2, 940–948 CrossRef CAS.
- Y. J. Zhang, T. Mori, L. Niu and J. H. Ye, Energy Environ. Sci., 2011, 4, 4517–4521 CAS.
- M. W. Zhang and X. C. Wang, Energy Environ. Sci., 2014, 7, 1902–1906 CAS.
- J. S. Zhang, M. W. Zhang, R. Q. Sun and X. C. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145–10149 CrossRef CAS PubMed.
- X. H. Zhang, L. J. Yu, C. S. Zhuang, T. Y. Peng, R. J. Li and X. G. Li, ACS Catal., 2014, 4, 162–170 CrossRef CAS.
- Z. Z. Lin and X. C. Wang, Angew. Chem., Int. Ed., 2013, 52, 1735–1738 CrossRef CAS PubMed.
- H. Q. Li, Y. X. Liu, X. Gao, C. Fu and X. C. Wang, ChemSusChem, 2015, 8, 1189–1196 CrossRef CAS PubMed.
- Y. Wang, H. R. Li, J. Yao, X. C. Wang and M. Antonietti, Chem. Sci., 2011, 2, 446–450 RSC.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- K. Sridharan, T. Kuriakose, R. Philip and T. J. Park, Appl. Surf. Sci., 2014, 308, 139–147 CrossRef CAS.
- N. X. Li, B. Y. Zhou, P. H. Guo, J. C. Zhou and D. W. Jing, Int. J. Hydrogen Energy, 2013, 38, 11268–11277 CrossRef CAS.
- N. X. Li, L. Z. Zhang, J. C. Zhou, D. W. Jing and Y. M. Sun, Dalton Trans., 2014, 43, 11533–11541 RSC.
- J. Zhong, Y. Zhang, C. Hu, R. Hou, H. Yin, H. Li and Y. Huo, J. Mater. Chem. A, 2014, 2, 19641–19647 CAS.
- Y. Lu, D. Wang, P. Yang, Y. Du and C. Lu, Catal. Sci. Technol., 2014, 4, 2650–2657 CAS.
- M. Liu, D. Jing, Z. Zhou and L. Guo, Nat. Commun., 2013, 4, 1–8 Search PubMed.
- M. Liu, L. Wang, G. Lu, X. Yao and L. Guo, Energy Environ. Sci., 2011, 4, 1372–1378 CAS.
- W. Tian, N. Li and J. Zhou, Appl. Surf. Sci., 2016, 361, 251–258 CrossRef CAS.
- Y. B. Wang, J. C. Wu, J. W. Zheng, R. R. Jiang and R. Xu, Catal. Sci. Technol., 2012, 2, 581–588 CAS.
- P. Qiu, H. Chen and F. Jiang, RSC Adv., 2014, 4, 39969–39977 RSC.
- J. Zhang, J. Yu, M. Jaroniec and J. R. Gong, Nano Lett., 2012, 12, 4584–4589 CrossRef CAS PubMed.
- N. X. Li, M. C. Liu, Z. H. Zhou, J. C. Zhou, Y. M. Sun and L. J. Guo, Nanoscale, 2014, 6, 9695–9702 RSC.
- G. G. Zhang and X. C. Wang, J. Catal., 2013, 307, 246–253 CrossRef CAS.
- Q. Z. Wang, Y. B. Shi, Z. Y. Du, J. J. He, J. B. Zhong, L. C. Zhao, H. D. She, G. Liu and B. T. Su, Eur. J. Inorg. Chem., 2015, 24, 4108–4115 CrossRef.
- Q. Z. Wang, J. H. Lian, J. J. Li, R. F. Wang, H. H. Huang, B. T. Su and Z. Q. Lei, Sci. Rep., 2015, 5, 1–9 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |