Preparation and characterization of an AC–Fe3O4–Au hybrid for the simultaneous removal of Cd2+, Pb2+, Cr3+ and Ni2+ ions from aqueous solution via complexation with 2-((2,4-dichloro-benzylidene)-amino)-benzenethiol: Taguchi optimization
F. Nasiri Azada,
M. Ghaedi*a,
K. Dashtiana,
A. Jamshidi*bc,
G. Hassanid,
M. Montazerozohoria,
S. Hajatie,
M. Rajabif and
A. A. Bazrafshana
aChemistry Department, Yasouj University, Yasouj 75918-74831, Iran. E-mail: m_ghaedi@yu.ac.ir; Fax: +98-74-33223048; Tel: +98-74-33223048
bSocial determinates of health research center, Yasouj University of medical sciences, Yasouj, Iran
cDepartment of Environmental health engineering, Faculty of public health, Yasouj University of medical sciences, Yasouj, Iran. E-mail: jamshidiarsalan@yahoo.com
dDepartment of Environmental Health Engineering, Faculty of Health, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
eDepartment of Physics, Yasouj University, Yasouj, 75918-74831, Iran
fChemistry Department, Semnan University, Semnan, Iran
First published on 5th February 2016
Abstract
Activated carbon (AC) was magnetized with Fe3O4 nanoparticles (AC–Fe3O4-NPs) and loaded with Au nanoparticles (AC–Fe3O4–Au-NPs), and was fully characterized using different techniques such as XRD, XPS, VSM, TEM and SEM. 2-((2,4-Dichloro-benzylidene)-amino)-benzenethiol (DBABT), a complexing agent, was synthesized and characterized using 1H-NMR, ES-MS and FT-IR analysis. Subsequently, AC–Fe3O4-NPs was modified with DBABT and applied for the ultrasound-assisted removal of Cd2+, Pb2+, Cr3+ and Ni2+ ions via complexation with DBABT. The influences of variables such as reaction time and adsorbent mass (equilibrium) were optimized simultaneously. The method under optimum conditions was set at pH 5, concentrations of 5, 15, 25 and 25 mg L−1 for the Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively, 0.02 g for the adsorbent mass, 5 min sonication time and 6 mg L−1 for the concentration of DBABT, producing removal percentages of 80.59, 93.85, 68.52 and 81.68 for Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively. Analysis of real experimental equilibrium data at various concentrations of analytes reveals the efficiency of the Langmuir model for good representation of experimental data, with maximum mono-layer adsorption capacities of 185.22, 135.14, 188.70 and 133.34 mg g−1 for Cd2+, Pb2+, Cr3+ and Ni2+ ions respectively. The experimental data at various real times reveal that in most situations the systems reach equilibrium at contact times lower than 20 min, while the data fitted well to a combination of a pseudo second order kinetic model and intraparticle diffusion.
1. Introduction
Heavy metal ions present in higher content are toxic for the human body via damaging the nervous, reproductive and blood circulation systems.1–3 Reactive centers such as COOH, amine groups and sulfur atoms in body cells are candidates for attacking metal ions (transition and/or heavy metal ions). Source for their arrival in the environment are sources like industrial, agricultural and domestic wastes.4,5 In particular, Cd2+, Pb2+, Cr3+ and Ni2+ as more hazardous agents for humans namely as environmental contaminants which cannot be degraded or destroyed naturally.6,7 Therefore, the detection and removal of these pollutants need preliminary novel and efficient methods designed to mitigate medical and environmental risks.8 Their efficient removal from various media to concentrations lower than their threshold limits is an urgent requirement. The threshold limits of the metal ions being studied here according to important criteria for evaluating drinking water quality such as the World Health Organization (WHO: 0.003 mg L−1, 0.015 mg L−1, 0.05 mg L−1 and 0.03 mg L−1 for Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively), US Environmental Protection Agency (EPA: 0.005 mg L−1, 0.01 mg L−1, 0.05 mg L−1 and 0.06 mg L−1 for Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively) and Iranian National Standards Organization (INSO: 0.005 mg L−1, 0.05 mg L−1, 0.05 mg L−1 and 0.07 mg L−1 for Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively) were identified. Various removal and clean up approaches like chemical precipitation, ion-exchange, membrane separation, reverse osmosis and adsorption9,10 are good choices for this purpose that have attracted great attention in the purification and separation of metal ions in wastewater treatment.11 Adsorption techniques due to their simplicity, rapidity and ecofriendly nature are highly recommended to achieve this.12 Graphene and carbon based material, bio sorbents, silicates, zeolites, and chitosan have been extensively applied for metal ion sorption and/or preconcentration.13,14 Activated carbon (AC) with (without) modification is a powerful sorbent used in clean up protocols and its ability for adsorption and removal of metal ions is enhanced following activation using a strong acid that is associated with formation of variations functional groups like NH2, COOH, N, OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.15,16 The type and number of reactive centers is strongly effected by the variation of the loading which helps the agent on the AC surface.17 In addition, some of the most influencial properties of each adsorbent in such procedures is the presence of various functional groups, high surface area and a porous structure of the adsorbent.18 Therefore, application of nanostructure based adsorbents leads to enhancement of the adsorption properties. The presence of metallic centers like soft Au-NP centers leads to greater binding of organic chelating agents on adsorbents.19,20 One of the main problems is that separation based technique have problems associated with phase separation that can be simply overcome by usage of magnetic adsorbents based on Fe3O4.21,22 In such systems circumrotation by simple exposure to a magnetic field achieves phase separation without tedious extra work. Composites of AC with other nanostructures as a sole adsorbent lack sufficient sites for efficient binding and accumulation of the metal ions being studied here. This drawback was simply overcome through modification of the adsorbent surface using a novel Schiff base chelating agent.23,24 Magnetic nanoparticles have continued to draw considerable interest because of their great potential applications in magnetic fluids, catalysis, biotechnology/biomedicine, magnetic resonance imaging and magnetic recording devices in view of their paramagnetism, biocompatibility and safety.25–27 Magnetic separation benefits from features like good recovery, high efficiency, low cost and easy phase separation allowing for convenient and economical magnetic separation that eliminates centrifuge and/or filtration steps.28,29 Hybrid materials show the inherent properties of the adsorbents, in particular flexibility and strength, and also the high adsorption properties of the surface bonded nanoparticles.30 In addition, gold nanoparticles due to soft–soft interaction with S–H groups can be modified to favor separation of metal ions from preconcentrated environmental samples.31,32 Therefore, in this work, the hybrid AC–Fe3O4–Au-NPs was synthesized, characterized using SEM, XPS, VSM, TEM and XRD and used for simultaneous ultrasound assisted removal of Cd2+, Pb2+, Cr3+ and Ni2+ ions from aqueous media. Chelating agents loaded on AC–Fe3O4–Au-NPs through soft–soft interactions (π–Au metal), hydrogen bonding with AC functional groups or Fe3O4 centers and surface plasmons of Au-NP improved the removal efficiency. The effects of parameters were investigated with a Taguchi design and combined to search for and find optimum conditions that permit the maximum simultaneous removal process.
O.15,16 The type and number of reactive centers is strongly effected by the variation of the loading which helps the agent on the AC surface.17 In addition, some of the most influencial properties of each adsorbent in such procedures is the presence of various functional groups, high surface area and a porous structure of the adsorbent.18 Therefore, application of nanostructure based adsorbents leads to enhancement of the adsorption properties. The presence of metallic centers like soft Au-NP centers leads to greater binding of organic chelating agents on adsorbents.19,20 One of the main problems is that separation based technique have problems associated with phase separation that can be simply overcome by usage of magnetic adsorbents based on Fe3O4.21,22 In such systems circumrotation by simple exposure to a magnetic field achieves phase separation without tedious extra work. Composites of AC with other nanostructures as a sole adsorbent lack sufficient sites for efficient binding and accumulation of the metal ions being studied here. This drawback was simply overcome through modification of the adsorbent surface using a novel Schiff base chelating agent.23,24 Magnetic nanoparticles have continued to draw considerable interest because of their great potential applications in magnetic fluids, catalysis, biotechnology/biomedicine, magnetic resonance imaging and magnetic recording devices in view of their paramagnetism, biocompatibility and safety.25–27 Magnetic separation benefits from features like good recovery, high efficiency, low cost and easy phase separation allowing for convenient and economical magnetic separation that eliminates centrifuge and/or filtration steps.28,29 Hybrid materials show the inherent properties of the adsorbents, in particular flexibility and strength, and also the high adsorption properties of the surface bonded nanoparticles.30 In addition, gold nanoparticles due to soft–soft interaction with S–H groups can be modified to favor separation of metal ions from preconcentrated environmental samples.31,32 Therefore, in this work, the hybrid AC–Fe3O4–Au-NPs was synthesized, characterized using SEM, XPS, VSM, TEM and XRD and used for simultaneous ultrasound assisted removal of Cd2+, Pb2+, Cr3+ and Ni2+ ions from aqueous media. Chelating agents loaded on AC–Fe3O4–Au-NPs through soft–soft interactions (π–Au metal), hydrogen bonding with AC functional groups or Fe3O4 centers and surface plasmons of Au-NP improved the removal efficiency. The effects of parameters were investigated with a Taguchi design and combined to search for and find optimum conditions that permit the maximum simultaneous removal process.
2. Experimental
2.1. Materials and apparatus
All chemicals were used as received without further purification from Merck, Dermasdat, Germany. Equipment was used according to the manufacturer’s recommendations corresponding to previous publications.33–36 The concentrations of the metal (ions) being studied here were determined using a Varian model atomic absorption spectrophotometer.2.2. Preparation of 2-((2,4-dichloro-benzylidene)-amino)-benzenethiol
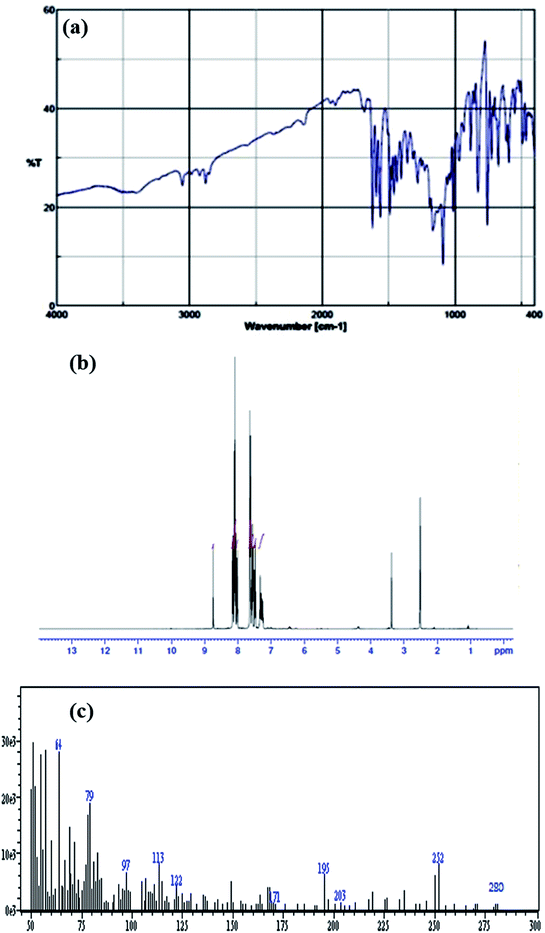
A solution of 2-aminothiophenol (0.376 g, 3 mmol) in absolute methanol (10 mL) was added to a solution of 2,4-dichlorobenzaldehyde (0.525 g, 3 mmol) in absolute methanol (10 mL) and stirred for 4 h. The product was produced as a greenish white precipitate (chemical structure is shown in Scheme 1). The compound was filtered and washed with methanol twice to obtain it in 83% yield. IR (Fig. 1a) (KBr, cm−1): 3405 (m), 3053 (w), 2922 (w), 2878 (w), 2142 (w), 1899 (w), 1682 (w), 1620 (s), 1591 (m), 1562 (s), 1488 (m), 1474 (w), 1458 (m), 1435 (m), 1404 (m), 1358 (m), 1315 (w), 1280 (m), 1250 (w), 1227 (w), 1187 (w), 1166 (m), 1089 (s), 1053 (w), 1034 (w), 1013 (m), 965 (m), 932 (w), 880 (m), 853 (s), 826 (m), 754 (s) 723 (m), 708 (w), 673 (m), 611 (m), 593 (m), 548 (m), 510 (w), 489 (m), 462 (m).1H-NMR spectrum (Fig. 1b) (in DMSO-d6), δ (ppm): 8.74 (s, 1H), 8.15 (d, 1H, J = 7.6 Hz), 8.10 (d, 1H, J = 8.8 Hz), 7.02 (d, 1H, J = 8.4 Hz), 7.63 (d, 2H, J = 8.4 Hz), 7.57 (t, 1H, J = 7.6 Hz, J = 7.2 Hz), 7.48 (t, 1H, J = 7.6 Hz, J = 7.2 Hz). ES-MS (m/z) (Fig. 1c): 280 (M + 1), 252, 203, 195, 171, 122, 113, 97, 79 and 64.
The thiolic Schiff base ligand has two coordinator atoms (S (soft atom) and N (relatively hard atom)). These two donor atoms can easily bind to metal ions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios as proposed in Scheme 1, the soft atom facilitates coordination to soft metal ions such as Cd2+ and Pd2+ while the hard donor atom of nitrogen intends to bind to the hard to moderate metal ions such as Ni2+ and Cr2+. Geometries of the formed complexes seemed to be tetrahedral or octahedral coordination compounds in which the coordinated ligands are the bidentate Schiff base and water molecules.
1 molar ratios as proposed in Scheme 1, the soft atom facilitates coordination to soft metal ions such as Cd2+ and Pd2+ while the hard donor atom of nitrogen intends to bind to the hard to moderate metal ions such as Ni2+ and Cr2+. Geometries of the formed complexes seemed to be tetrahedral or octahedral coordination compounds in which the coordinated ligands are the bidentate Schiff base and water molecules.
2.3. Preparation of AC–Fe3O4–Au-NPs
The preparation of a precursor solution for fabrication of the AC–Fe3O4–Au-NPs was as follows: 0.001 mol of FeCl2 and 0.002 mol of FeCl3 were mixed thoroughly with 200 mL of de-ionized water for 1 hour under sonication in a nitrogen atmosphere. In the next step, NH3·H2O was added to the above reaction mixture in an Erlenmeyer flask for 1 h at 50 °C in a nitrogen atmosphere. Subsequently, 1.0 g of AC was dispersed in 100 mL of absolute ethanol solution which was mixed thoroughly at room temperature for 18 h with 10 mL of the Fe3O4 nanoparticle suspension (22.0 mg mL−1). Then, the reaction mixture was deposited using a magnet and washed several times with ethanol and distillated water and dried at room temperature. Finally, the Au nanoparticles were synthesized as follows: 100 mL of a 2.5 × 10−5 M HAuCl4 aqueous solution, while stirring was heated to 90 °C. Then, 0.15 g of citric acid was added into the above solution and vigorously stirred for 40 min at 90 °C. Subsequently, the cloudy solution was added to 0.9 g of AC–Fe3O4-NPs and vigorously stirred for 20 h at room temperature. Finally, the reaction mixture was allowed to settle and rinsed several times with distillated water and dried in an oven at 50 °C.2.4. Simultaneous adsorption experiments
Ultrasonic-assisted adsorption experiments were performed in batch mode to simultaneously increase the diffusion coefficients of ions and the mass transfer of aggregates to the surface and the vacant sites of AC–Fe3O4–Au-NPs as follows: according to experimental runs in the TD, different concentrations of metal ions and DBABT at pH 6 (optimum value) were added into 50 mL Erlenmeyer flasks containing specific amounts of AC–Fe3O4–Au-NPs which were dispersed thoroughly over 5 min in an ultrasonic bath at room temperature. Finally, AC–Fe3O4–Au-NPs was separated from the solution using an external magnetic field (1.3 T) in less than one minute and subsequently the total concentrations of metal ions in the effluent liquid phase were measured using a flame atomic absorption spectrometer. To evaluate the performance of the method, removal percentage and (R%metal ions) were calculated.2.5. Taguchi design
The Taguchi method can lower variations in a process through the design of experiments based on their simultaneous variation rather than changing one factor at a time. This design allows fast and accurate estimation of the individual main effects and their best combination. In this paper, Taguchi was applied to screen 8.0 important factors based on one 2-level factor and seven 3-level factor (12 × 73) and the respective matrixes and responses are shown in Table 1. Data analysis was performed using statistical analysis of variance (ANOVA) and optimum conditions were found through the combination with a desirability function approach and validated using a confirmation test (n = 3 replicates at optimum). 3D response surfaces were plotted to visualize the variation of the response versus applied terms.| Run | Block | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | R%Cd(II) | R%Pb(II) | R%Cr(III) | R%Ni(II) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a X1, pH. X2, Cd(II) concentration (mg L−1). X3, Pb(II) concentration (mg L−1). X4, Cr(III) concentration (mg L−1). X5, Ni(II) concentration (mg L−1). X6, adsorbent mass (g). X7, sonication time (contact time) (min). X8, DBABT concentration (mg L−1). | |||||||||||||
| 1 | 1 | 7 | 15 | 15 | 5 | 5 | 0.03 | 7 | 6 | 44.56 ± 1.3 | 74.89 ± 1.9 | 84.2 ± 0.8 | 38.73 ± 1.2 |
| 2 | 1 | 7 | 5 | 15 | 25 | 15 | 0.02 | 3 | 9 | 73.12 ± 1.0 | 84.00 ± 1.4 | 46.2 ± 1.3 | 45.61 ± 1.9 |
| 3 | 1 | 5 | 15 | 15 | 25 | 25 | 0.01 | 5 | 6 | 70.36 ± 1.8 | 43.29 ± 2.3 | 47.6 ± 1.7 | 18.15 ± 2.0 |
| 4 | 1 | 7 | 25 | 15 | 5 | 5 | 0.01 | 5 | 9 | 44.19 ± 1.1 | 46.86 ± 2.4 | 60.31 ± 2.1 | 88.19 ± 2.8 |
| 5 | 1 | 5 | 15 | 25 | 25 | 5 | 0.03 | 7 | 9 | 23.26 ± 2.1 | 78.36 ± 2.3 | 21.32 ± 1.9 | 83.56 ± 2.3 |
| 6 | 1 | 5 | 15 | 5 | 25 | 15 | 0.01 | 7 | 3 | 31.19 ± 1.3 | 35.96 ± 1.7 | 60.81 ± 2.0 | 25.30 ± 1.9 |
| 7 | 1 | 5 | 15 | 5 | 5 | 15 | 0.02 | 7 | 9 | 61.59 ± 1.2 | 34.86 ± 1.5 | 28.19 ± 1.6 | 54.39 ± 1.6 |
| 8 | 1 | 7 | 5 | 25 | 15 | 25 | 0.01 | 7 | 6 | 84.21 ± 2.0 | 11.76 ± 1.3 | 31.96 ± 1.5 | 35.79 ± 1.9 |
| 9 | 1 | 7 | 5 | 15 | 25 | 5 | 0.02 | 5 | 3 | 75.96 ± 2.0 | 83.71 ± 1.9 | 36.12 ± 2.5 | 98.60 ± 1.2 |
| 10 | 1 | 7 | 15 | 5 | 25 | 15 | 0.03 | 3 | 6 | 90.35 ± 1.3 | 37.43 ± 1.6 | 41.19 ± 2.2 | 21.89 ± 1.1 |
| 11 | 1 | 5 | 25 | 25 | 5 | 25 | 0.02 | 3 | 6 | 35.12 ± 1.1 | 50.86 ± 2.3 | 39.20 ± 0.9 | 32.96 ± 1.9 |
| 12 | 1 | 5 | 25 | 5 | 15 | 5 | 0.03 | 5 | 9 | 28.6 ± 1.2 | 24.82 ± 1.1 | 48.56 ± 2.1 | 41.60 ± 1.0 |
| 13 | 1 | 5 | 15 | 15 | 15 | 25 | 0.03 | 3 | 3 | 10.21 ± 1.6 | 86.97 ± 1.0 | 44.39 ± 1.9 | 17.06 ± 0.9 |
| 14 | 1 | 5 | 5 | 5 | 5 | 5 | 0.01 | 3 | 3 | 38.31 ± 1.5 | 77.65 ± 1.7 | 53.26 ± 1.4 | 41.10 ± 1.2 |
| 15 | 1 | 7 | 25 | 25 | 15 | 5 | 0.02 | 7 | 3 | 10.26 ± 1.3 | 16.03 ± 2.5 | 43.76 ± 1.1 | 13.39 ± 2.9 |
| 16 | 1 | 7 | 15 | 25 | 5 | 15 | 0.03 | 5 | 3 | 35.01 ± 1.4 | 50.74 ± 1.2 | 40.13 ± 1.8 | 29.30 ± 2.1 |
| 17 | 1 | 7 | 5 | 25 | 15 | 15 | 0.01 | 3 | 9 | 31.26 ± 1.8 | 55.43 ± 2.1 | 38.20 ± 1.3 | 37.16 ± 0.7 |
| 18 | 1 | 5 | 5 | 15 | 15 | 15 | 0.02 | 5 | 6 | 40.01 ± 1.9 | 68.96 ± 1.3 | 86.19 ± 2.2 | 27.19 ± 1.3 |
3. Results and discussion
3.1. Characterization of samples
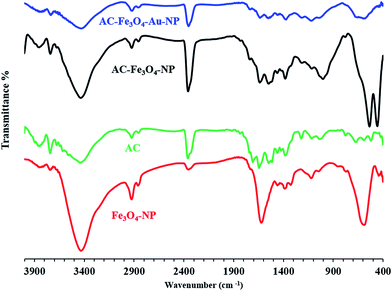
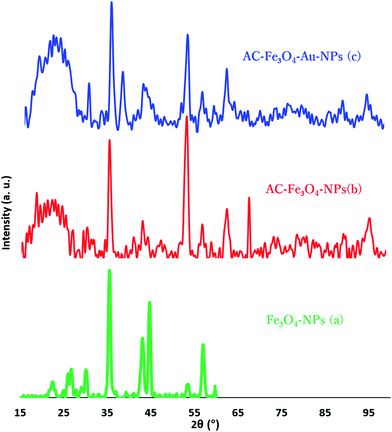
FT-IR spectra of Fe3O4-NPs, AC, AC–Fe3O4-NPs and AC–Fe3O4–Au-NPs (Fig. 2) were recorded and it can be seen that the Fe3O4 spectrum features a broad peak at about 3300 cm−1 which is attributed to the O–H stretching mode of water that was adsorbed from the air on the surface of Fe3O4, and the vibration peak of the Fe–O bond was observed at 590 cm−1. The spectra of AC, AC–Fe3O4-NPs and AC–Fe3O4–Au-NPs exhibit a large broad band at 3000–3400 cm−1 corresponding to O–H bond stretching of surface active carbon, the presence of a peak at around 1100 cm−1 corresponds to the vibrational mode of H–O–H and the peak at around 1600 cm−1 is due to H–O–H bending.37 The peak at around 590 cm−1 for AC–Fe3O4-NPs and AC–Fe3O4–Au-NPs indicates successful synthesis of the adsorbent in this study.The XRD pattern for the Fe3O4 nanoparticles (Fig. 3a) consists of eight characteristic peaks at 2θ = 23.2, 27, 30.1, 35.4, 43.0, 44.5, 53.5 and 57.0, while that for AC–Fe3O4-NPs (Fig. 3b) exhibits a new broad peak at 25° (002) corresponding to the interlayer spacing of the AC. The peaks at 43° (100), 53° (004) and 78° (100) correspond to diffractions and reflections from the carbon atoms.30 Modification of AC–Fe3O4-NPs with Au-NPs (Fig. 3c) causes the appearance of peaks at 38° (111) which belong to planes of the pure face-centered crystalline structure of Au. While peaks corresponding to Au at 44.4° (200), 64.6° (220) and 77.5° (311) overlap with the peaks of AC–Fe3O4-NPs that reflect successful modification and loading of Au-NPs onto AC–Fe3O4-NPs.
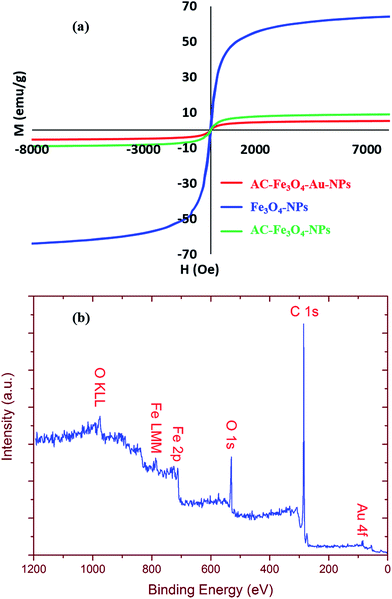
According to the saturation magnetization curve (Fig. 4a) it is obvious that there is no hysteresis in the magnetization sweep. Therefore, the magnetic coercivity of the nanostructures is low and suggests the presence of superparamagnetic Fe3O4.
The XPS results in Fig. 4b show the main differences in the surface composition of the four different nanoparticles in the C 1s, O 1s, Fe 2p and Au 4f regions.
The SEM images of AC, Fe3O4, AC–Fe3O4 and AC–Fe3O4–Au (Fig. 5) show that AC (Fig. 5a) has a superficial area with a lightly ordered structure among the shape distribution. The morphology of Fe3O4 (Fig. 5b and c) shows a regular, ordered structure and a spherical shape that following combination with AC (Fig. 5d) leads to a change in the morphology of the AC which reflects successful modification. Finally, modification of the surface of AC–Fe3O4-NPs with Au-NPs (Fig .5e) leads to a significant change in morphology which is related to intermixing and immobilization of AU-NPs on AC–Fe3O4-NsP.
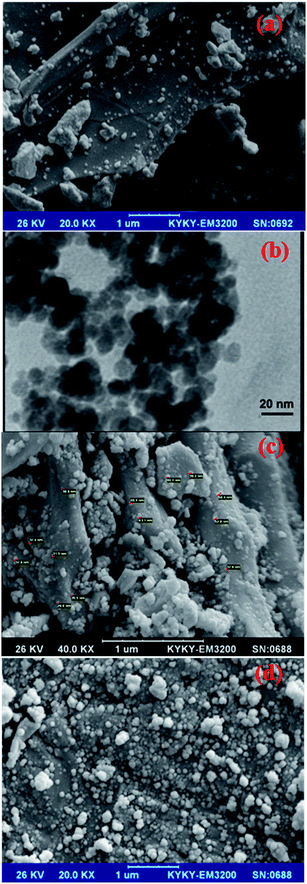 | ||
| Fig. 5 SEM image of AC (a), TEM image of Fe3O4-NPs (b), SEM images of AC–Fe3O4-NPs (c) and AC–Fe3O4–Au-NPs (d). | ||
3.2. Analysis of ANOVA under a Taguchi design
ANOVA was employed to test the statistical significant factors (see Table 2) based on their F and P values and comparison with respective threshold limits. Good model F values were obtained for the R% of the Cd2+, Pb2+, Cr3+ and Ni2+ ions (1.34 × 105, 211.43, 3613.41 and 2610.81) while their P values were <0.0001, 0.0047, 0.0003 and 0.0004, respectively. All these data confirm the high accurately and efficiency of the constructed equation and model to correlate the R% values in this study to the 8.0 factors being investigated. However, the P values corresponding to terms like X1, X2, X3, X4, X5, X6, X7 and X8, for R% of all of the studied metal ions are less than 0.05 and justify their significant and serious contribution to the experimental response. Therefore, the final equations in terms of coded factors for R% of all the metal ions being studied are:| R%Cd(II) = −45.98 + 8.35X1 − 3.75X2 + 17.19X3 − 2.85X4 − 1.77X5 + 3.94X6 + 0.42X7 − 12.49X8 | (1) |
| R%Pb(II) = 53.48 − 2.27X1 + 19.69X2 + 8.44X3 + 2.5X4 − 0.03X5 − 8.32X6 + 11.91X7 + 5.03X8 | (2) |
| R%Cr(III) = 47.31 − 0.41X1 + 5.9X2 − 7.43X3 + 3.57X4 + 6.62X5 + 1.38X6 − 3.57X7 − 0.90X8 | (3) |
| R%Ni(II) = 41.67 + 3.71X1 + 12.73X2 + 7.23X3 + 5.78X4 − 8.57X5 − 0.72X6 − 9.03X7 − 4.21X8 | (4) |
| Source | DFa | Cd(II) | Pb(II) | Cr(III) | Ni(II) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SSb | MSc | F-Value | p-Value | SS | MS | F-Value | p-Value | SS | MS | F-Value | p-Value | SS | MS | F-Value | p-Value | ||
| a Degree of freedom.b Sum of square.c Mean square. | |||||||||||||||||
| Model | 15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050.72 050.72 |
670.05 | 1.34 × 105 | <0.0001 | 9903.43 | 660.23 | 211.43 | 0.0047 | 4922.67 | 328.18 | 3613.41 | 0.0003 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456.96 456.96 |
697.13 | 2610.81 | 0.0004 |
| X1 | 1 | 1254.5 | 1254.5 | 2.50 × 105 | <0.0001 | 92.84 | 92.84 | 29.73 | 0.032 | 3.08 | 3.08 | 33.95 | 0.0282 | 252 | 252 | 943.77 | 0.0011 |
| X2 | 2 | 875.29 | 437.64 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237.91 237.91 |
<0.0001 | 4316.94 | 2158.47 | 691.22 | 0.0014 | 569.16 | 284.58 | 3133.35 | 0.0003 | 1501.53 | 750.76 | 2811.68 | 0.0004 |
| X3 | 2 | 2781.07 | 1390.53 | 2.77 × 105 | <0.0001 | 1523.73 | 761.87 | 243.98 | 0.0041 | 2419.47 | 1209.73 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319.79 319.79 |
<0.0001 | 533.75 | 266.88 | 999.47 | 0.001 |
| X4 | 2 | 2197.98 | 1098.99 | 2.19 × 105 | <0.0001 | 869.37 | 434.69 | 139.2 | 0.0071 | 246.91 | 123.46 | 1359.31 | 0.0007 | 1519.15 | 759.57 | 2844.67 | 0.0004 |
| X5 | 2 | 50.92 | 25.46 | 5074.64 | 0.0002 | 470.51 | 235.25 | 75.34 | 0.0131 | 708.62 | 354.31 | 3901.13 | 0.0003 | 2818.95 | 1409.48 | 5278.61 | 0.0002 |
| X6 | 2 | 482.07 | 241.03 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046.72 046.72 |
<0.0001 | 640.98 | 320.49 | 102.63 | 0.0096 | 17.13 | 8.56 | 94.29 | 0.0105 | 137.96 | 68.98 | 258.33 | 0.0039 |
| X7 | 2 | 128.72 | 64.36 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 829.19 829.19 |
<0.0001 | 1646.09 | 823.05 | 263.57 | 0.0038 | 312.14 | 156.07 | 1718.4 | 0.0006 | 958.89 | 479.44 | 1795.56 | 0.0006 |
| X8 | 2 | 2280.19 | 1140.09 | 2.27 × 105 | <0.0001 | 342.96 | 171.48 | 54.91 | 0.0179 | 646.17 | 323.08 | 3557.32 | 0.0003 | 2734.73 | 1367.37 | 5120.91 | 0.0002 |
| Residual | 2 | 0.01 | 5.02 × 10−03 | 6.25 | 3.12 | 0.18 | 0.091 | 0.53 | 0.27 | ||||||||
| Cor total | 17 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050.73 050.73 |
9909.67 | 4922.85 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 457.49 457.49 |
||||||||||||
For the Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively, the values of the determination coefficient R2 were 1.0, 0.9994, 1.0 and 0.9999, predicted R2 were 0.9999, 0.9490, 0.9970 and 0.9959, and the adjusted R2 were 1.0, 0.9946, 0.9997 and 0.9996. The Adeq. precision ratios of the models for the Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively, were 1200.376, 44.144, 228.310 and 175.235 indicating that all of the above values for predicted and adjusted R2 and Adeq strongly prove the high applicability and efficiency of the models for the prediction of real sorption data and also suggest the sufficiency of the developed models being studied for predictions of the simultaneous removal of these metal ions.
3.3. Optimized conditions
After screening the effects of different factors, DF (from STATISTICAL 10.0 software) as one of the most effective statistical methods was applied to find the optimum conditions of the variables. The minimum, middle and maximum values of desirability were configured as 0.0, 0.5 and 1.0, respectively. A value closer to 1.0 strongly supports closeness of each point to the real optimum conditions. Optimum values were obtained for all of the investigated variables and the corresponding desirability and R% value for each metal ion are shown in Table 3.| Variable | Optimum value |
|---|---|
| pH | 6.0 |
| Cd2+ concentration (mg L−1) | 5.0 |
| Pb2+ concentration (mg L−1) | 15 |
| Cr3+ concentration (mg L−1) | 25 |
| Ni2+ concentration (mg L−1) | 25 |
| Adsorbent mass (g) | 0.02 |
| Sonication time (min) | 5.0 |
| DBABT concentration (mg L−1) | 6.0 |
| Desirability Value | 0.85 |
|---|---|
| R% of Cd2+ | 80.6 |
| R% of Pb2+ | 93.9 |
| R% of Cr3+ | 68.6 |
| R% of Ni2+ | 82.0 |
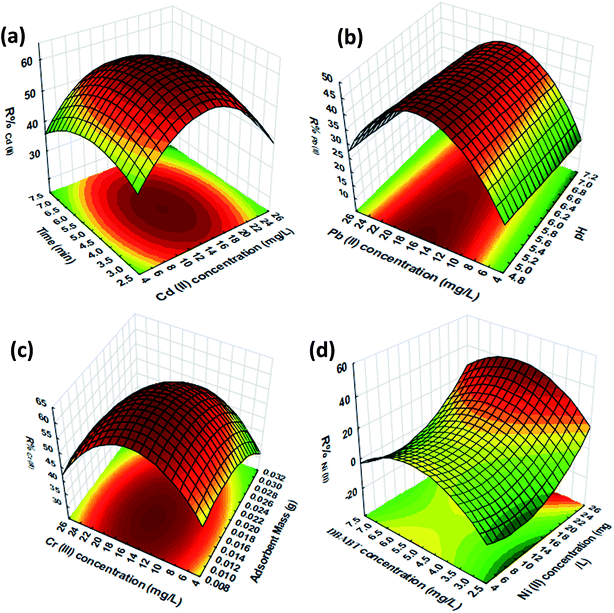
3.4. 3D response surface plots
The 3D response surface plots were used to illustrate combined effects and identify the major interactions between variables and R% of the metal ions being studied. In this work, four typical 3D response surface plots are shown in Fig. 6.The typical effect and interaction of sonication time and Cd2+ ion concentration on R%Cd(II) shows that the maximum Cd2+ ion removal percentage is achieved in a short sonication time (contact time) that emerged from the unique role of ultrasound in accelerating mass transfer, good mixing and the contact of analytes and the adsorbent, while simultaneously enhancing the diffusion coefficient. In addition, the effect of the initial Cd2+ ion concentration on R%Cd(II) clearly indicates that the high removal percentage at lower concentration strongly decreased at higher concentration which probably is due to saturation of the reactive center of the adsorbent or may be related to repulsive forces between bulk Cd2+ ions and those adsorbed on the adsorbent surface that was proven by the Langmuir isotherm.
Typically the effect and interaction of pH and the influence of the initial Pb2+ ion concentration on R%Pb(II) (Fig. 6b) shows that the adsorbent and ligand has a constant contributory response without a significant correlation with pH. This fact may be related to the absence of ligand proton exchange or Pb2+ ion distribution at various pH and the absence of strong formation of Pb(OH)+ and/or Pb(OH)2(s) over pH 5–7. The presence of amine and SH groups accelerate and facilate complex formation among the soft metal ions, while OH, COOH and NH groups and O atoms of the chelating agent and AC and/or Fe3O4 possibly bind and accumulate on the sorbent. At pH < 5.0 the removal percentage of the metal ions being studied is related to protonation of the chelating agent reactive sites such as the imine nitrogen or thiol groups. A decrease in the response at pH > 7.0 is attributed to the formation of metal hydroxide species such as soluble M(OH)+ and/or M(OH)mn± and insoluble precipitate of M(OH)2. Therefore, the working pH was selected as 5–7. In addition, the effect of the initial Pb2+ ion concentration on R%Pb(II) has a similar trend to the other metal ions.
Fig. 6d shows the effect of DBABT and Ni2+ concentration on R%Ni(II) and is found to have a similar trend for all of the analytes. The investigation of the effect of initial DBABT concentration on R%Pb(II) revealed that increasing DBABT can enhance the magnitude of complex formation among the metal ions being studied. It is known that the stoichiometry and concentration of the complex is strongly affected by ligand content. At lower DBABT content, probably due to insufficient reactive centers and also completion among AC functional groups for binding metal ions (in an irreversible pathway), leads to a reduction in the metal ion removal percentage. Increasing chelating agent content is associated with enhancement of complexation sites and leads to an enhancement in metal ion sorption/recovery. A higher content of chelating agent may possibly have changed the stoichiometry and also the polarity and lipophilicity of the complexes, with such deviation at high content being expected and may account for such a trend.
3.5. Isotherm study
The equilibrium adsorption isotherm was used to give useful information about the mechanism, properties and the tendency of the adsorbent for the metal ions being studied. The equilibrium data were fitted to isotherm equations such as Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D–R). The constant parameters and correlation coefficients (R2) obtained from the plots of known equations for the above models are summarized in Table 4. Based on the linear form of the Langmuir isotherm model (according to Table 4), the values of Ka (the Langmuir adsorption constant (L mg−1)) and Qm (theoretical maximum mono-layer adsorption capacity (mg g−1)) were obtained from the intercept and slope of the plot of Ce/qe vs. Ce, respectively. The high correlation coefficient at the optimum amount of the adsorbent mass shows the applicability of the Langmuir model for interpretation of the experimental data over the whole concentration range. The parameters of the Freundlich isotherm model such as KF (L mg−1) and n (the capacity and intensity of the adsorption) were calculated from the intercept and slope of the linear plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus ln
qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce, respectively. The values of 1/n for the Freundlich isotherm were 0.73, 0.56, 0.71 and 0.56 for Cd2+, Pb2+, Cr3+ and Ni2+, respectively, and justify high metal ion adsorption onto AC–Fe3O4–Au-NPs, while lower R2 values imply low suitability of this model for good prediction and representation of the experimental data.
Ce, respectively. The values of 1/n for the Freundlich isotherm were 0.73, 0.56, 0.71 and 0.56 for Cd2+, Pb2+, Cr3+ and Ni2+, respectively, and justify high metal ion adsorption onto AC–Fe3O4–Au-NPs, while lower R2 values imply low suitability of this model for good prediction and representation of the experimental data.
| Isotherm | Parameters | Value | |||
|---|---|---|---|---|---|
| Cd(II) | Pb(II) | Cr(III) | Ni(II) | ||
| 0.01 g | 0.01 g | 0.01 g | 0.01 g | ||
| Langmuir | Qm (mg g−1) | 185.22 | 135.14 | 188.70 | 133.34 |
| Ka (L mg−1) | 0.1380 | 0.4999 | 0.2030 | 0.4687 | |
| R2 | 0.9964 | 0.9984 | 0.9981 | 0.9976 | |
| Freundlich | 1/n | 0.7377 | 0.5651 | 0.7134 | 0.5627 |
| KF (L mg−1) | 3.90 | 5.06 | 4.471 | 4.96 | |
| R2 | 0.9911 | 0.9745 | 0.9946 | 0.9865 | |
| Temkin | B1 | 34.53 | 29.59 | 36.01 | 28.94 |
| KT (L mg−1) | 1.78 | 5.00 | 2.57 | 4.80 | |
| R2 | 0.9851 | 0.9976 | 0.9794 | 0.9881 | |
| Dubinin–Radushkevich | Qs (mg g−1) | 77.39 | 85.21 | 83.76 | 81.39 |
| B × 10−7 | −4 | −1 | −2 | −1 | |
| E | 1123.59 | 2272.72 | 1587.30 | 2272.72 | |
| R2 | 0.9172 | 0.9437 | 0.9156 | 0.9099 | |
The values of the Temkin constants and the correlation coefficient are lower than the Langmuir values. Therefore, the Temkin isotherm presents a lower ability to fit the experimental equilibrium data than the Freundlich and Langmuir isotherms which have the best correlation and efficiency to predict the experimental data.
The D–R model was employed to estimate the porosity apparent free energy and the adsorption characteristics. The slope of the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus ε2 gives K, and the intercept yields the Qm value.
qe versus ε2 gives K, and the intercept yields the Qm value.
The value of the D–R correlation is lower than the other isotherm values, suggesting poor ability to present and describe the experimental equilibrium data.
3.6. Comparison with the literature
Comparison of the performance of AC–Fe3O4–Au-NPs in terms of adsorption capacity, pH value and adsorption time toward adsorbents for the removal of metal ions is presented in Table 5. As can be seen, the contact time for this method (5 min) is comparable and superior to all of the mentioned methods. This observation confirms the unique role of ultrasound for enhancement and rapid adsorption of the metal ions being studied. On the other hand, the adsorption capacity for this adsorbent (0.02 g) is preferable and superior to other adsorbents (Table 5). This observation confirms the unique role of metallic nanoparticles and also the high contribution of the DBABT ligand on the collectivity of metal ions on the adsorbent. It shows the high performance of the proposed method for the removal of the metal ions being studied here: Cd2+, Pb2+, Cr3+ and Ni2+ ions.34–44 In other words, this table reveals that AC–Fe3O4–Au-NPs rather than AC and AC–Fe3O4-NPs showed better removal efficiency for the metal ions being studied here.| Sorbent | Ion | pH | Adsorption capacity (mg g−1) | Detection method | Ref. |
|---|---|---|---|---|---|
| Innovative electronic | Cd(II) | 4.0 | 236.4 | ICP-AES | 38 |
| Polyaniline grafted chitosan | Cd(II) | 6.0 | 12.87 | — | 39 |
| α-Ketoglutaric acid-modified magnetic chitosan | Cd(II) | 6.0 | 255.7 | FAAS | 40 |
| BiOBr microspheres | Cd(II) | — | 11.7 | Spectrophotometry | 41 |
| Dead T. viride | Cd(II) | 6.0 | 10.95 | Spectrophotometry | 42 |
| Polyvinyl alcohol-chelating sponge | Cd(II) | 5.5 | 17.83 | FAAS | 43 |
| AC | Cd(II) | 6.0 | 53.0 | FAAS | This work |
| AC–Fe3O4-NPs | Cd(II) | 6.0 | 110.11 | FAAS | This work |
| AC–Fe3O4–Au-NPs | Cd(II) | 6.0 | 185.22 | FAAS | This work |
| Polyaniline grafted chitosan | Pb(II) | 6.0 | 13.23 | — | 39 |
| BiOBr microspheres | Pb(II) | — | 6.5 | Spectrophotometry | 41 |
| Agaricus bisporus | Pb(II) | 5.5 | 86.4 | FAAS | 44 |
| Mn3O4-coated activated carbon | Pb(II) | 5.0 | 14.91 | ICP-AES | 45 |
| Few-layered graphene oxide nanosheets | Pb(II) | 6.5 | 1850 | Spectrophotometry | 46 |
| DPDB immobilized onto mesoporous silica | Pb(II) | 5.0 | 195.31 | FAAS | 47 |
| AC | Pb(II) | 6.0 | 17.8 | FAAS | This work |
| AC–Fe3O4-NPs | Pb(II) | 6.0 | 82.1 | FAAS | This work |
| AC–Fe3O4–Au-NPs | Pb(II) | 6.0 | 135.14 | FAAS | This work |
| Mn3O4-coated activated carbon | Cr(III) | 5.0 | 5.30 | ICP-AES | 45 |
| BiOBr microspheres | Cr(III) | — | 16.9 | Spectrophotometry | 41 |
| Gelatin–montmorillonite | Cr(III) | 5.5 | 52.91 | FAAS | 48 |
| Garden grass | Cr(III) | 4.0 | 19.4 | FAAS | 49 |
| Polyacrylonitrile nanofibers | Cr(III) | 2.0 | 137.6 | ICP-OES | |
| AC | Cr(III) | 6.0 | 60.0 | FAAS | This work |
| AC–Fe3O4-NPs | Cr(III) | 6.0 | 123.5 | FAAS | This work |
| AC–Fe3O4–Au-NPs | Cr(II) | 6.0 | 188.70 | FAAS | This work |
| Hemp-based material | Ni(II) | 50. | 160 | FAAS | 50 |
| Polyvinyl alcohol-chelating sponge | Ni(II) | 5.5 | 17.83 | FAAS | 43 and 51 |
| Fe3O4 nanoparticles | Ni(II) | 8.0 | 362.31 | Spectrophotometry | 52 |
| Activated carbon from Choerospondias axillaris | Ni(II) | 5.0 | 28.25 | FAAS | 53 |
| Orange peel | Ni(II) | 5.0 | 16.60 | FAAS | 54 |
| Histidine modified chitosan | Ni(II) | 5.0 | 55.6 | FAAS | 48 |
| AC | Ni(II) | 6.0 | 15.3 | FAAS | This work |
| AC–Fe3O4-NPs | Ni(II) | 6.0 | 79.7 | FAAS | This work |
| AC–Fe3O4–Au-NPs | Ni(II) | 6.0 | 133.34 | FAAS | This work |
3.7. Comparison with legal standard values and regeneration of the adsorbent
Comparison of the performance of AC–Fe3O4–Au-NPs for the removal of the metal ions studied here in terms of the average concentration after removal treatment with the WHO, USEPA and INSO legal concentrations is presented in Table 6. The results show that the average measured concentrations after removal treatment under optimal conditions are close to the standard levels and no significant difference is observed between them.| Metal ion | Legal concentration according to WHO (mg L−1) | Legal concentration according to USÈPA (mg L−1) | Legal concentration according to WHO (mg L−1) | Average measured concentration (mg L−1) |
|---|---|---|---|---|
| Cd2+ ions | 0.003 | 0.005 | 0.005 | 0.97 |
| Pb2+ ions | 0.015 | 0.01 | 0.05 | 0.91 |
| Cr3+ ions | 0.05 | 0.05 | 0.05 | 7.85 |
| Ni2+ ions | 0.03 | 0.06 | 0.07 | 4.5 |
The AC–Fe3O4–Au-NPs was efficiently regenerated with 50 mL of 0.50 M HCl and subsequently neutralized by washing with double distillated water. Subsequently, it was successfully reused in the adsorption–desorption process 5.0 times with very little change in the adsorption efficiency, supporting its regeneration.
4. Conclusion
In this work, activated carbon (AC) was successfully magnetized with Fe3O4 nanoparticles, modified with Au nanoparticles and then fully characterized with different techniques such as XRD and SEM, TEM, XPS and VSM. 2-((2,4-Dichloro-benzylidene)-amino)-benzenethiol was successfully synthesized and characterized using different techniques such as 1H-NMR, ES-MS and FT-IR analysis. Subsequently, AC–Fe3O4–Au-NPs was used for the ultrasound-assisted removal of Pb2+, Cr3+, Cd2+ and Ni2+ ions following complexation with DBABT. The Taguchi design was employed for selection of the statistically significant variables and in combination with DF was used to search for the optimum conditions and significant terms in the empirical equation, while the interactions and main effects of the investigated variables were estimated using ANOVA and 3D surface plots. Analysis of real experimental equilibrium data at various concentrations of the analytes reveal the efficiency of the Langmuir model for good representation of the experimental data with maximum mono-layer adsorption capacities of 185.22, 135.14, 188.70 and 133.34 mg g−1 for the Cd2+, Pb2+, Cr3+ and Ni2+ ions, respectively. The adsorption capacities for AC and AC–Fe3O4-NPs were much lower than these values. On the other hand, the proposed method was revealed to have good potential for the efficient removal of these metal ions from aqueous solution compared to several other adsorbents and methods. In addition, comparison to the legal standard values showed that the average measured concentrations after removal treatment under the optimal conditions were close to the standard levels and no significant difference was observed between them.Acknowledgements
The authors thank the Research Council of the Yasouj University and the Iran National Science Foundation for financially supporting this work.References
- M. Jamshidi, M. Ghaedi, K. Dashtian, S. Hajati and A. Bazrafshan, RSC Adv., 2015, 5, 59522–59532 RSC.
- F. N. Azad, M. Ghaedi, K. Dashtian, M. Montazerozohori, S. Hajati and E. Alipanahpour, RSC Adv., 2015, 5, 61060–61069 RSC.
- H. Mazaheri, M. Ghaedi, S. Hajati, K. Dashtian and M. Purkait, RSC Adv., 2015, 5, 83427–83435 RSC.
- F. Zare, M. Ghaedi, A. Daneshfar, S. Agarwal, I. Tyagi, T. A. Saleh and V. K. Gupta, Chem. Eng. J., 2015, 273, 296–306 CrossRef CAS.
- M. Ghaedi, H. Mazaheri, S. Khodadoust, S. Hajati and M. Purkait, Spectrochim. Acta, Part A, 2015, 135, 479–490 CrossRef CAS PubMed.
- B. Barfi, M. Rajabi, M. M. Zadeh, M. Ghaedi, M. Salavati-Niasari and R. Sahraei, Microchim. Acta, 2015, 182, 1187–1196 CrossRef CAS.
- M. Ghaedi, M. Montazerozohori, M. N. Biyareh, K. Mortazavi and M. Soylak, Int. J. Environ. Anal. Chem., 2013, 93, 528–542 CrossRef CAS.
- S. Hajati, M. Ghaedi and S. Yaghoubi, J. Ind. Eng. Chem., 2015, 21, 760–767 CrossRef CAS.
- M. Hébrant, M. Rose-Hélène and A. Walcarius, Colloids Surf., A, 2013, 417, 65–72 CrossRef.
- F. Ge, M.-M. Li, H. Ye and B.-X. Zhao, J. Hazard. Mater., 2012, 211, 366–372 CrossRef PubMed.
- E. Da’na and A. Sayari, Desalination, 2012, 285, 62–67 CrossRef.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Dalton Trans., 2013, 42, 5682–5689 RSC.
- J. Aguado, J. M. Arsuaga, A. Arencibia, M. Lindo and V. Gascón, J. Hazard. Mater., 2009, 163, 213–221 CrossRef CAS PubMed.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- M. Ghaedi, M. reza Rahimi, A. Ghaedi, I. Tyagi, S. Agarwal and V. K. Gupta, J. Colloid Interface Sci., 2016, 461, 425–434 CrossRef CAS PubMed.
- Y. Li, Q. Du, T. Liu, X. Peng, J. Wang, J. Sun, Y. Wang, S. Wu, Z. Wang and Y. Xia, Chem. Eng. Res. Des., 2013, 91, 361–368 CrossRef CAS.
- K. A. Krishnan, K. Sreejalekshmi, V. Vimexen and V. V. Dev, Ecotoxicol. Environ. Saf., 2016, 124, 418–425 CrossRef PubMed.
- G. Sethia and A. Sayari, Carbon, 2016, 99, 289–294 CrossRef CAS.
- M. Roosta, M. Ghaedi, N. Shokri, A. Daneshfar, R. Sahraei and A. Asghari, Spectrochim. Acta, Part A, 2014, 118, 55–65 CrossRef CAS PubMed.
- G. Karimipour, M. Ghaedi, R. Sahraei, A. Daneshfar and M. N. Biyareh, Biol. Trace Elem. Res., 2012, 145, 109–117 CrossRef CAS PubMed.
- Y. Wang, P. He, X. Zhao, W. Lei and F. Dong, J. Solid State Electrochem., 2014, 18, 665–672 CrossRef CAS.
- Q. Cai, F. Hu, S.-T. Lee, F. Liao, Y. Li and M. Shao, Appl. Phys. Lett., 2015, 106, 023107 CrossRef.
- G. Zheng, L. Polavarapu, L. M. Liz-Marzán, I. Pastoriza-Santos and J. Pérez-Juste, Chem. Commun., 2015, 51, 4572–4575 RSC.
- J. Liu, M. Ye, Z. Wei, F. Hu, J. Wang, G. Ge, Z. Hu, M. Shao and S.-T. Lee, Nanoscale, 2015, 7, 13427–13437 RSC.
- F. X. Ma, H. Hu, H. B. Wu, C. Y. Xu, Z. Xu and L. Zhen, Adv. Mater., 2015, 27, 4097–4101 CrossRef CAS PubMed.
- L. Xu and J. Wang, Environ. Sci. Technol., 2012, 46, 10145–10153 CAS.
- T. Wang, Z. Liu, M. Lu, B. Wen, Q. Ouyang, Y. Chen, C. Zhu, P. Gao, C. Li and M. Cao, J. Appl. Phys., 2013, 113, 024314 CrossRef.
- S. Patra, E. Roy, R. Madhuri and P. K. Sharma, Environ. Sci. Technol., 2015, 49, 6117–6126 CrossRef CAS PubMed.
- C. Karadaş and D. Kara, Water, Air, Soil Pollut., 2014, 225, 1–10 Search PubMed.
- S. Kumar, B. Kumar, A. Baruah and V. Shanker, J. Phys. Chem. C, 2013, 117, 26135–26143 CAS.
- F. N. Azad, M. Ghaedi, K. Dashtian, S. Hajati and V. Pezeshkpour, Ultrason. Sonochem., 2016, 31, 383–393 CrossRef CAS.
- Y. Cui, D. Hu, Y. Fang and J. Ma, Sci. China, Ser. B: Chem., 2001, 44, 404–410 CrossRef CAS.
- C. Namasivayam and D. Kavitha, Dyes Pigm., 2002, 54, 47–58 CrossRef CAS.
- V. Gupta, B. Gupta, A. Rastogi, S. Agarwal and A. Nayak, Water Res., 2011, 45, 4047–4055 CrossRef CAS PubMed.
- M. Gonçalves, M. C. Guerreiro, L. C. A. de Oliveira and C. S. de Castro, J. Environ. Manage., 2013, 127, 206–211 CrossRef PubMed.
- M. Ghaedi, H. A. Larki, S. N. Kokhdan, F. Marahel, R. Sahraei, A. Daneshfar and M. Purkait, Environ. Prog. Sustainable Energy, 2013, 32, 535–542 CrossRef CAS.
- A. Goudarzi, A. D. Namghi and C.-S. Ha, RSC Adv., 2014, 4, 59764–59771 RSC.
- M. Xu, P. Hadi, G. Chen and G. McKay, J. Hazard. Mater., 2014, 273, 118–123 CrossRef CAS PubMed.
- R. Karthik and S. Meenakshi, Chem. Eng. J., 2015, 263, 168–177 CrossRef CAS.
- G. Yang, L. Tang, X. Lei, G. Zeng, Y. Cai, X. Wei, Y. Zhou, S. Li, Y. Fang and Y. Zhang, Appl. Surf. Sci., 2014, 292, 710–716 CrossRef CAS.
- X. Wang, W. Liu, J. Tian, Z. Zhao, P. Hao, X. Kang, Y. Sang and H. Liu, J. Mater. Chem. A, 2014, 2, 2599–2608 CAS.
- R. M. Hlihor, M. Diaconu, F. Leon, S. Curteanu, T. Tavares and M. Gavrilescu, New Biotechnol., 2015, 32, 358–368 CrossRef CAS PubMed.
- C. Cheng, J. Wang, X. Yang, A. Li and C. Philippe, J. Hazard. Mater., 2014, 264, 332–341 CrossRef CAS PubMed.
- Y. Long, D. Lei, J. Ni, Z. Ren, C. Chen and H. Xu, Bioresour. Technol., 2014, 152, 457–463 CrossRef CAS PubMed.
- M.-E. Lee, J. H. Park, J. W. Chung, C.-Y. Lee and S. Kang, J. Ind. Eng. Chem., 2015, 21, 470–475 CrossRef CAS.
- G. Zhao, X. Ren, X. Gao, X. Tan, J. Li, C. Chen, Y. Huang and X. Wang, Dalton Trans., 2011, 40, 10945–10952 RSC.
- M. R. Awual and M. M. Hasan, Microporous Mesoporous Mater., 2014, 196, 261–269 CrossRef CAS.
- A. Eser, V. N. Tirtom, T. Aydemir, S. Becerik and A. Dinçer, Chem. Eng. J., 2012, 210, 590–596 CrossRef CAS.
- A. H. Sulaymon, A. A. Mohammed and T. J. Al-Musawi, Int. J. Chem. React. Eng., 2014, 12, 477–486 Search PubMed.
- G. Z. Kyzas, Z. Terzopoulou, V. Nikolaidis, E. Alexopoulou and D. N. Bikiaris, J. Mol. Liq., 2015, 209, 209–218 CrossRef CAS.
- G. Z. Kyzas, P. I. Siafaka, E. G. Pavlidou, K. J. Chrissafis and D. N. Bikiaris, Chem. Eng. J., 2015, 259, 438–448 CrossRef CAS.
- R. K. Gautam, P. K. Gautam, S. Banerjee, S. Soni, S. K. Singh and M. C. Chattopadhyaya, J. Mol. Liq., 2015, 204, 60–69 CrossRef CAS.
- R. M. Shrestha, M. Varga, I. Varga, A. P. Yadav, B. P. Pokharel and R. R. Pradhananga, J. Inst. Eng., 2014, 9, 166–174 Search PubMed.
- F. Gönen and D. S. Serin, Afr. J. Biotechnol., 2014, 11, 1250–1258 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |