Oxidation of CO by N2O over Al- and Ti-doped graphene: a comparative study†
Mehdi D. Esrafili*,
Fariba Mohammadian-Sabet and
Parisa Nematollahi
Laboratory of Theoretical Chemistry, Department of Chemistry, University of Maragheh, Maragheh, P.O. Box: 5513864596, Iran. E-mail: esrafili@maragheh.ac.ir; Fax: +98-4212276060; Tel: +98-4212237955
First published on 28th June 2016
Abstract
In this work, we employ density functional theory calculations to investigate the CO oxidation mechanisms by N2O molecules over Al- or Ti-doped graphene (Al–/Ti–graphene). The reaction barriers and thermodynamic parameters are calculated using the M06-2X density functional with the 6-31G* basis set. The possible reaction pathway proposed for the oxidation of CO with N2O molecules is as follows: N2O → N2 + Oads and CO + Oads → CO2. Unlike Al–graphene, upon adsorption of the N2O molecules over Ti–graphene, they are quickly dissociated into N2 and Oads species via a barrier-less reaction. Then, the activated Oads reacts with CO molecules to form CO2 molecules. The calculated activation energies of the reaction CO + Oads → CO2 on Al– and Ti–graphene are calculated to be 0.06 and 0.16 eV, which are lower than those on traditional noble metal catalysts. Our results indicate that both Al– and Ti–graphene can be used as a potential catalyst for low-temperature CO oxidation by N2O molecules.
1. Introduction
Carbon monoxide (CO) is one of the most prominent atmospheric pollutants produced largely by incomplete burning of fuels in industrial and automotive combustion processes. The health significance of CO is due to the fact that it is able to combine irreversibly with the iron atom of haemoglobin to produce carboxyhaemoglobin, whose formation prevents blood from having its normal oxygen-carrying capacity.1 Hence, the catalytic oxidation of CO to carbon dioxide (CO2) has become one of the most efficient reactions to clean the exhausts from automobiles and industries. In recent years, numerous studies have been devoted to the CO oxidation reaction by O2 molecules as the most common oxidizing agent.2–9 Also, a few investigations have been reported with other oxidizing agents such as nitrous oxide (N2O).10–12 The N2O molecule is known as a major greenhouse gas with a larger global warming potential than CO2. It is emitted to the atmosphere mainly from agricultural and industrial activities. Thus, besides the oxidation of CO molecules, the reduction of N2O becomes one of the main important topics for scientific investigations. It should be noted that the direct reaction of N2O with CO molecules has an extremely high activation energy which hinders the reaction to take place at room temperature. Therefore, it can be catalyzed by different types of catalysts in the gas phase.13–15 According to previous studies,16,17 the catalytic oxidation of CO by N2O proceeds as the following reactions:| N2O → N2 + Oads | (1) |
| Oads + CO → CO2 | (2) |
A variety of investigations have been performed on the oxidation of CO by N2O molecules using transition metal cations.18–20 For instance, Kartha et al.21 indicated that substituting Fe atoms in lanthanum titanates can significantly enhance the catalytic activity of CO oxidation by N2O molecules. However, despite these extensive investigations performed on the CO oxidation by N2O molecules over various metal surfaces, recently there have been considerable efforts to do this oxidation/reduction reaction over cheaper and favourable carbon-based materials such as graphene22,23 or boron nitride nanotubes (BNNTs).24
Graphene is a novel form of carbon that can act as a potential support for metal atoms and clusters to realize new carbon–metal nanocomposite catalysts. Recently, graphene as a matrix of a heterogeneous catalyst has been extensively studied due to its huge surface-to-volume ratio that provides a large catalytic reaction area. The exclusive π-conjugated structure of graphene results in outstanding thermal, mechanical, and electrical properties25–28 and can be considered for the next generation of electronic materials.29 According to previous studies,30 the existence of the strong sp2 bonding between the carbon atoms of pure graphene sheets makes it chemically inert. In order to solve this problem, researchers have proposed that substituting the carbon atoms of graphene sheets with foreign atoms can extensively enhance its catalytic activity31 or its sensitivity toward the adsorption of gas molecules.32,33 Some of these metallic or metal-free atoms are Fe,34,35 Mn,36,37 Cu,38 Pd,39 Sn,9 N,40,41 P42 or Si.43–45 For example, Wang et al.46 studied the adsorption of CO and H2 molecules on Fe-, Co-, Ni- and Cu-embedded graphene theoretically. They found that the Fe- and Co-embedded graphene can preferably capture up to three CO molecules per metal atom, rather than H2 molecules. They finally estimated that Fe– and Co–graphene can be used as a filter membrane for removing CO efficiently in the feed gas of hydrogen fuel cells. Besides these light 2p (C, N) or 3p (Si, P) dopants, Al atoms as a 3p element can induced strong structural changes in graphene sheets due to their considerably larger atomic radius than those of 2p atoms.47–50 For instance, Jiang et al.51 investigated the oxidation of CO by O2 molecules over Al-doped graphene theoretically. They found that the amount of charge transfer from the O2 to the CO molecule via the embedded Al atom plays an important role in the oxidation of CO molecules. So, the low-cost Al-doped graphene can be used as an efficient catalyst toward CO oxidation. Another theoretical investigation performed by Dai et al.52 refers to the adsorption of some common gases over B-, N-, S- and Al-embedded graphene. They demonstrated that only NO and NO2 molecules can bind to B-doped graphene, while NO2 molecules bind to S-doped graphene. Therefore, they revealed that B- and S-doped graphene could be used as a favourable sensor for the adsorption of gas pollutants such as NO and NO2. Moreover, the effects of doped titanium (Ti) or N atoms on the interaction of CO, NO, SO2, and HCHO gases with graphene sheets were investigated by Zhang et al.53 According to their findings, the adsorption energy and structural analysis indicated that the doped Ti atom could greatly improve the interaction of these gas molecules with the graphene surface. They also declared that Ti-doped graphene can act as a selective gas absorber while N-doped graphene did not exhibit this capability apparently. In another work, Rojas and Leiva et al.54 investigated the interaction between some small gas molecules and Ti-doped graphene sheets. They found that the Ti-doped graphene surface can effectively adsorb H2 molecules. These results imply that Ti-doped graphene is more effective than N-doped graphene in detecting and removing gas molecules because of its high selectivity.
To date, numerous studies have been devoted to understanding the mechanism of CO oxidation or N2O reduction over different surfaces.11,23,55 Among these, supported noble metal catalysts like Pt56,57 or Ag58 exhibit outstanding performance, due to their large surface area and unique electronic properties. However, the problem is that these noble metal-based catalysts are expensive and have disadvantages in terms of toxicity and environmental consideration. Moreover, they usually require a high reaction temperature for a significant operation due to their high energy barrier. So, it is desirable to replace these costly catalysts with low cost materials for different industrial reactions at room temperature. Al-doped graphene has been widely studied as a low-cost metal-free catalyst for the oxidation of CO51,59 and the dissociation of H2O.60 Recently, Jiang et al.51 indicated that Al-doped graphene can exhibit a high catalytic activity for the oxidation of CO with reaction barriers comparable with those on noble metal-doped graphene sheets. On the other hand, the Ti atom has been known as one of the promising hydrogen adsorbing materials for many years.54,61 Also, it is found that Ti atoms with strong binding energy can be utilized as a functional material to uniformly coat graphene and they can strongly be adsorbed on the graphene sheet without diffusion.62,63 Thus, it is expected that Ti-doped graphene may provide an important model to evaluate the catalytic activity of single Ti atoms on graphene sheets. Besides, it can be regarded as a reference model for developing non-metal atomic catalysts for N2O oxidation.
In the present work, we explore and compare the geometry, electronic structure and catalytic properties of Al- and Ti-doped graphene (Al–/Ti–graphene) by means of density functional theory (DFT) calculations. It is important to mention that no previous study has been reported for the CO oxidation mechanism by N2O over Al–/Ti–graphene. In addition, our results can be useful for better understanding the chemical properties of Al–/Ti–graphene and are worthy for the development of an automobile catalytic converter in order to clean up more than one gas by a single process.
2. Computational details
All of the geometry optimizations and frequency calculations were performed at the M06-2X/6-31G* level of theory using the Gaussian 09 suite of programs.64 M06-2X is a global-hybrid meta-GGA functional which has been exclusively designed to treat medium range correlation energy, non-covalent interactions, main group thermochemistry, and reaction barriers.65,66 In order to determine the minimum energy pathways, intrinsic reaction coordinate (IRC) calculations were also performed in the forward and reverse directions. A hexagonal graphene supercell (4 × 4 graphene unit cell), containing 48 carbon atoms, was chosen as the basic model for the calculations. Al– and Ti–graphene were then modelled by substituting a single Al or Ti atom with one C atom on the surface. The adsorption energies (Eads) of the adsorbed molecules over Al–/Ti–graphene were calculated using the following equation:| Eads(A) = EA–M − EM − EA | (3) |
3. Results and discussion
3.1. Geometric structures of Al– and Ti–graphene
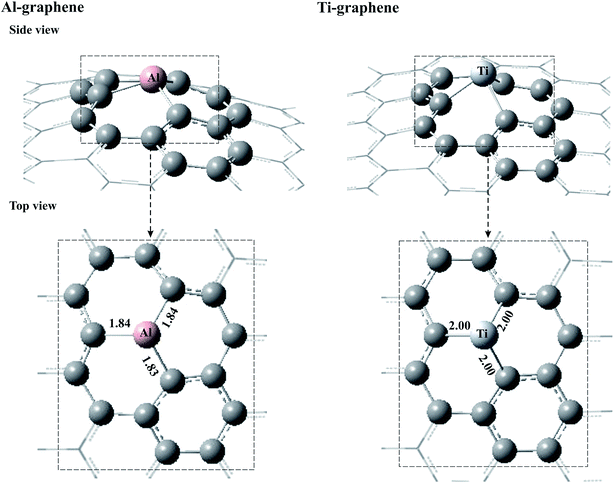
As noted above, the aim of the present theoretical study is to investigate the catalytic oxidation of CO molecules by N2O over Al–/Ti–graphene. To this aim, the interaction of CO and N2O molecules with these surfaces is investigated at the M06-2X/6-31G* level. Fig. 1 depicts the optimized structures of Al– and Ti–graphene. It is seen that after Al is embedded into graphene, the geometric structure of the graphene changes dramatically, where the Al atom projects out of the surface due to its larger size than C atoms and its preference to sp3 hybridization. Covalent Al–C bonds (≈1.84 Å) are formed in Al–graphene, that are quite a bit larger than the C–C bonds in the pure graphene sheet (1.42 Å).23 These obtained Al–C bond lengths are larger than those theoretically reported by Ao et al. (1.63 Å),69 but in good agreement with those mentioned by Wen and co-workers (1.85 Å).51 Note that the discrepancy between the calculated Al–C bond lengths in this study and those of ref. 69 can be attributed to the distinction between the used cluster and periodic slab models or to different DFT calculations. Meanwhile, about 0.3 e is transferred from the Al atom to the graphene sheet according to the NBO analysis, which may further confirm the strong interaction between Al and its nearest C atoms.Analogous with Al–graphene, when the Ti atom is replaced with one C atom of the graphene sheet, the Ti atom moves out of the plane with the average Ti–C bond length of 2.00 Å (Fig. 1), displacing also the positions of the first, second, and third out-of-plane neighbours. This value is smaller than that reported by Hu et al. (2.28 Å),62 but larger than that by Zhang (1.78 Å).70 According to the NBO charge density analysis, a sizable charge of about 1.0 e is transferred from the Ti atom to its nearest C atoms, which may reveal the ionic nature of the Ti–C bonds.
It should be noted that the aggregation of metal atoms, such as Al or Ti, in high concentrations over the catalyst is a considerable problem.51 Therefore, to evaluate this possibility, the diffusion of Al and Ti atoms to their nearest position on the graphene sheets is studied. As Fig. S1 of the ESI† indicates, the calculated diffusion barrier is obtained to be about 2.95 and 3.42 eV for the Al and Ti atoms, respectively. On the other hand, the relatively large adsorption energies of these dopants over the vacancy site of the graphene guarantees that the Al and Ti dopants can disperse on graphene quite stably without a clustering problem. Thus, it is concluded that both Al– and Ti–graphene are stable enough to be utilized in the catalytic oxidation of CO by N2O.
3.2. Adsorption of N2O and CO molecules over Al– and Ti–graphene
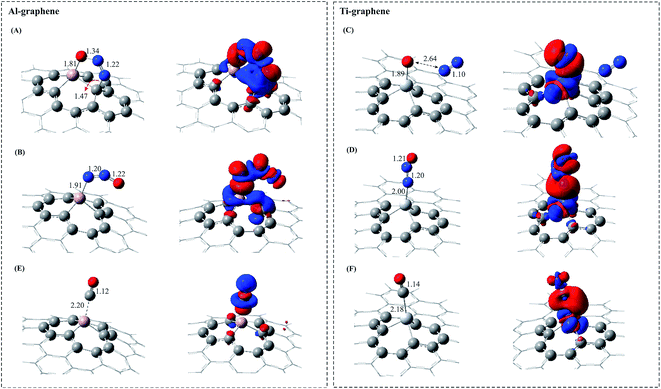
First, we explore the adsorption of the isolated N2O molecule on Al– and Ti–graphene. In order to find stable configurations of N2O adsorbed over these surfaces, different adsorption sites and patterns (including side-on and end-on) were considered. Among these different adsorbed complexes, the most favourable configurations were chosen for the study of N2O adsorption. The most stable adsorption complexes of N2O over these surfaces along with their geometric values are shown in Fig. 2. Besides, Table 1 lists the corresponding binding distances, net charge transfers (qCT), adsorption energies (Eads) and thermodynamic parameters (ΔG298 and ΔH298) of these complexes.| Complex | R (Å) | qCT (e) | Eads (eV) | ΔG298 (eV) | ΔH298 (eV) |
|---|---|---|---|---|---|
| Al–graphene | |||||
| A | 1.81 | 0.6 | −0.81 | −0.25 | −0.78 |
| B | 1.91 | 0.6 | −0.06 | 0.37 | −0.04 |
| E | 2.20 | 0.2 | −0.62 | −0.19 | −0.58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Ti–graphene | |||||
| C | 1.89 | 0.6 | −3.05 | −2.72 | −3.06 |
| D | 2.00 | 0.5 | −1.24 | −0.81 | −1.21 |
| F | 2.18 | 0.1 | −1.03 | −0.58 | −0.98 |
The attachment of a single N2O molecule to the Al–graphene sheet is initially considered by both the O and N end attacking in a cycloaddition configuration. In the case of the O end attacking, the N2O molecule uses its O and N atoms to bind the Al and C atoms of Al–graphene, respectively, forming a five-membered Al–O–N–N–C ring (Fig. 2 (complex A)). The Eads of this configuration is −0.81 eV which is larger than that reported by Lv et al. (−0.57 eV)71 as obtained at the PW91/6-31G** level. The difference may be due to the proper description of London dispersion effects with the M06-2X density functional, which is the main constituent part of the weak intermolecular interactions. The binding distance of Al–O is 1.81 Å, which is in the range of chemisorption and is larger than the Si–O bond in Si-embedded graphene (1.61 Å).22 Moreover, according to this adsorption, the O–N–N bond angle of N2O is decreased significantly from 180° in the free N2O to 117.6° in the adsorbed form. From the NBO analysis, a net charge of 0.6 e is transferred from the surface to the N2O molecule which causes the elongation of the N–O bond length from 1.19 Å in the gas form to 1.34 Å in the adsorbed configuration. Due to the N–O bond elongation, the N2O molecule is activated, therefore, its dissociation can occur easily. To investigate the electron density redistribution due to the adsorption of the N2O molecule over Al–graphene, an electron density difference (EDD) plot is also provided (Fig. 2). As can be seen, the blue colour in the EDD plot clearly indicates that there is a sizable electron density accumulation between the O atom of the N2O molecule and the Al atom of the surface, which confirms the chemisorption nature of the adsorption. It can be seen from thermochemistry results that the formation of complex A is exothermic (ΔH298 = −0.78 eV) with a negative ΔG298 value of about −0.25 eV (Table 1). Another stable configuration is achieved when the N2O molecule is attached to the Al atom by its end N atom in which it is placed in a tilted position over the Al–graphene sheet (complex B). In this case, compared to complex A, the N2O molecule is adsorbed weakly (Eads = −0.06 eV) on Al–graphene while the N atom is about 1.91 Å away from the Al atom (Table 1 and Fig. 2). Herein, the N–N–O angle and the N–O bond length are 139° and 1.22 Å, respectively, which are 41° and 0.01 Å smaller than those in the isolated state. The EDD plot confirms the great charge accumulation around the Al–N bond while the charge is depleted between the O atom of the N2O molecule and the C atom of the Al–graphene surface. Additionally, a total charge of about 0.6 e is transferred from the Al atom to the N atom of the N2O molecule. As is also evident from Table 1, the adsorption process to form complex B is an exothermic reaction (ΔH298 = −0.04 eV), with a positive ΔG298 value of 0.37 eV.
Continuously, the N2O adsorption over Ti–graphene is also studied and the corresponding favourable adsorption structures are depicted in Fig. 2. Analogous with Al–graphene, here, the N2O molecule can be attached to the Ti atom of the surface via its O or N end and forms two different adsorption configurations. When the N2O molecule approaches from its O site to the Ti atom, the dissociative complex C is achieved. In this configuration, upon the adsorption of the N2O molecule, it is easily dissociated into N2 and activated atomic oxygen (Oads) species. In addition, the N2O molecule has a larger Eads value (−3.05 eV) on Ti–graphene than on Al–graphene. Thus, it is evident that compared to the Al atom, the Ti atom in Ti–graphene plays a more important role in the adsorption and activation of the N2O molecule, which is most likely due to its large polarization and charge transfer effects. The binding distance between the remaining Oads and Ti atom is 1.89 Å and the N2 molecule is placed about 2.64 Å away from it. It is of particular interest that the physisorbed N2 molecule (Ti–N = 2.88 Å) can desorb easily from the surface and the activated Oads remains over the Ti atom. As is evident from the EDD map, there is a significant build up of electron density around the Ti–Oads bond, which indicates the strong binding of Oads to the Ti atom. In addition, the total net charge transfer from the Ti atom to Oads (0.6 e) is another proof for the strong interaction between the Ti and Oads atoms. It is interesting to know that according to NBO atomic charges, a major negative charge (−0.5 e) is on Oads and only 0.05 e is accumulated over the N2 molecule. The formation of this complex is extremely exothermic (ΔH = −3.06 eV) and has a large (more negative) ΔG298 value which reveals its easy formation process in ambient conditions (Table 1). Complex D is the other possible configuration considered for the adsorption of N2O molecules over Ti–graphene. In this configuration, the gas molecule adsorbs via its N atom over the Ti atom of the surface while it is completely in a vertical position right above the Ti atom of the surface. The Ti–N bond length and Eads are about 2.00 Å and −1.24 eV, respectively. It can be seen from Fig. 2 that the N2O molecule is weakly adsorbed over the surface and the EDD map also verifies this fact. Table 1 indicates that a net charge of 0.5 e is transferred from the surface to the N2O molecule. It is noticeable that the main negative charge (−0.5 e) is accumulated over the N atom that is directly bonded to the Ti atom of Ti–graphene. Like complex C, the formation of this complex is exothermic and is spontaneous at normal temperatures (Table 1).
On the other hand, seeking the most stable configurations of a single CO molecule adsorbed on Al–/Ti–graphene leads to the characterization of different possible adsorption complexes. The most stable configurations of CO adsorption over Al–/Ti–graphene are displayed in Fig. 2 (complexes E and F). Also, the corresponding binding distance, qCT, Eads, ΔH298 and ΔG298 values are summarized in Table 1. It is found that the CO molecule in the end-on position, in which the C atom of the CO molecule directly bonds to the Al or Ti atom of the surface, is the most energetically probable adsorption configuration. In these structures, the CO molecule is weakly adsorbed over the surface with the C–Al (C–Ti) bond length of 2.20 (2.18) Å. The corresponding Eads value of the adsorbed CO over Al– and Ti–graphene is −0.62 and −1.03 eV, respectively. One can see from the Table 1 results that analogous with the N2O adsorption, the absolute value of Eads for the CO molecule over Ti–graphene is larger than that of Al–graphene. This can be related to the higher catalytic activity of Ti–graphene than that of Al–graphene. Table 1 shows that in complexes E and F, a net charge of about 0.2 (0.04) e is transferred from the Al (Ti) atom to the 2π* orbitals of the CO molecule. The EDD map can verify the physical adsorption of the CO molecule over Al– and Ti–graphene (Fig. 2), where there exists a small electron density accumulation region around the Al–C or Ti–C bond. Generally, although the absolute values of the thermodynamic parameters (ΔH298, ΔG298) of the adsorbed CO over Ti–graphene are larger than those of Al–graphene, totally, the formation of both these complexes is exothermic and they are possible at room temperature (Table 1).
In summary, comparing the Eads values of the adsorbed N2O and CO molecules over Al–/Ti–graphene demonstrates that the N2O molecule binds more strongly than CO, so the doped graphene will be probably covered with the N2O species. Also, the N2O molecule can be easily decomposed into N2 and Oads species over the Ti–graphene sheet. Hence, it can be proposed that Ti–graphene might be a better catalyst for N2O decomposition than Al–graphene, which can be attributed to its larger polarization and charge transfer effects.
3.3. Proposed reaction pathways for CO oxidation over Al–/Ti–graphene
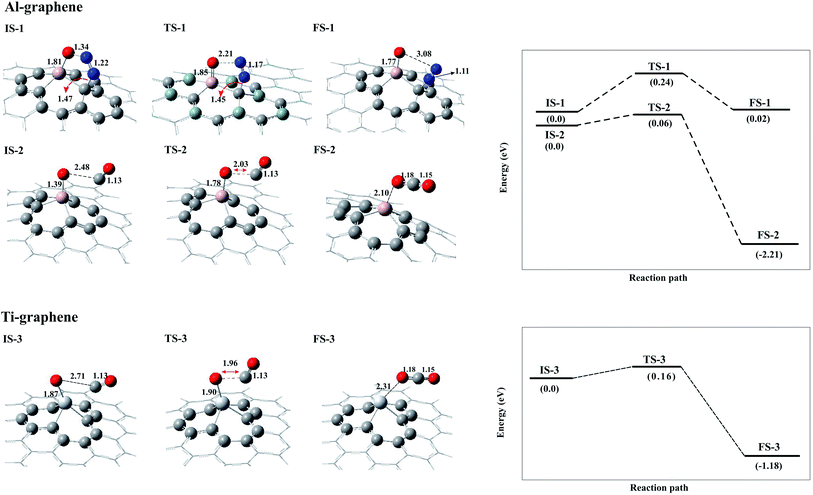
CO oxidation by N2O molecules is proposed in step-wise reactions, which are the decomposition of N2O followed by CO oxidation; i.e. N2O → N2 + Oads and Oads + CO → CO2. In order to investigate the reaction mechanisms of CO oxidation by N2O molecules over Al–graphene, we start from the favourable configuration A. The configurations of the initial state (IS), transition state (TS) and final state (FS) with their related geometric parameters and the reaction pathway are shown in Fig. 3. Additionally, the corresponding activation energy (Eact), ΔH298 and ΔG298 of these elementary reactions are reported in Table 2. As mentioned before, the stable complex A is chosen as the initial state (IS-1) in which the N2O molecule forms a five-membered ring over the Al–graphene sheet. In this configuration the N2 and Oads species are produced passing through the transition state TS-1 with an activation energy of 0.24 eV, which is smaller than those of noble metal catalysts72 or other metal-doped graphene sheets.11,71–73 This small Eact value shows that the N2O decomposition over Al–graphene is achieved quite easily. In TS-1, the Al–O and O–N bond lengths are increased to 1.85 and 2.21 Å, respectively. The N2 molecule is finally produced in FS-1 with the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond length of 1.11 Å while the Al–O bond is significantly decreased from 1.85 Å in TS-1 to 1.77 Å in FS-1. In this configuration, the N2 molecule and atomic oxygen atom (Oads) are completely separated from each other (Oads–N = 3.08 Å) and Oads remains over the Al atom of the surface to react with the CO molecule. It is noteworthy that the reaction step IS-1 → FS-1 is exothermic with a negative ΔH298 value and can be performed at ambient conditions (Table 2). Besides, the adsorption energy of Oads over Al–graphene is about −6.2 eV, which may indicate its strong interaction with the Al atom. In the next step (Oads + CO → CO2), the CO molecule can be oxidized by the activated Oads atom. Although the CO molecule can approach the Al atom of Al–graphene in different co-adsorption configurations, the reaction starts with the stable configuration as IS-2 (Fig. 3). In this configuration, the CO molecule is located about 2.48 Å away from Oads while it is a little tilted to the surface. In TS-2, the distance between the C atom of the CO molecule and Oads is reduced to 2.03 Å. Also, the Al–Oads bond length is elongated from 1.39 Å in IS-2 to 1.78 Å in TS-2, in order to facilitate the formation of the CO2 molecule in FS-2. In FS-2, the CO2 molecule is formed about 2.10 Å away from the Al atom and easily desorbs from the surface due to its small Eads of about −0.52 eV. The barrier energy of the IS-2 → FS-2 step is very small (0.06 eV) with the reaction energy of −2.21 eV. Note that the activation energy obtained for the oxidation of CO + Oads in the present study is smaller than those of noble metal catalysts such as Au-doped graphene74 or those using normal transition metals (e.g. Cu, Fe).34,75 Besides, as the results of Table 2 indicate, the reaction IS-2 → FS-2 has a negative value of ΔH298 and ΔG298 which confirms the feasibility of this reaction at room temperature (Table 2).
N bond length of 1.11 Å while the Al–O bond is significantly decreased from 1.85 Å in TS-1 to 1.77 Å in FS-1. In this configuration, the N2 molecule and atomic oxygen atom (Oads) are completely separated from each other (Oads–N = 3.08 Å) and Oads remains over the Al atom of the surface to react with the CO molecule. It is noteworthy that the reaction step IS-1 → FS-1 is exothermic with a negative ΔH298 value and can be performed at ambient conditions (Table 2). Besides, the adsorption energy of Oads over Al–graphene is about −6.2 eV, which may indicate its strong interaction with the Al atom. In the next step (Oads + CO → CO2), the CO molecule can be oxidized by the activated Oads atom. Although the CO molecule can approach the Al atom of Al–graphene in different co-adsorption configurations, the reaction starts with the stable configuration as IS-2 (Fig. 3). In this configuration, the CO molecule is located about 2.48 Å away from Oads while it is a little tilted to the surface. In TS-2, the distance between the C atom of the CO molecule and Oads is reduced to 2.03 Å. Also, the Al–Oads bond length is elongated from 1.39 Å in IS-2 to 1.78 Å in TS-2, in order to facilitate the formation of the CO2 molecule in FS-2. In FS-2, the CO2 molecule is formed about 2.10 Å away from the Al atom and easily desorbs from the surface due to its small Eads of about −0.52 eV. The barrier energy of the IS-2 → FS-2 step is very small (0.06 eV) with the reaction energy of −2.21 eV. Note that the activation energy obtained for the oxidation of CO + Oads in the present study is smaller than those of noble metal catalysts such as Au-doped graphene74 or those using normal transition metals (e.g. Cu, Fe).34,75 Besides, as the results of Table 2 indicate, the reaction IS-2 → FS-2 has a negative value of ΔH298 and ΔG298 which confirms the feasibility of this reaction at room temperature (Table 2).
| Reaction | Eact (eV) | ΔE (eV) | ΔG298 (eV) | ΔH298 (eV) |
|---|---|---|---|---|
| Al–graphene | ||||
| IS-1 → P-1 | 0.24 | 0.02 | −0.02 | 0.03 |
| IS-2 → P-2 | 0.06 | −2.21 | −2.19 | −2.24 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Ti–graphene | ||||
| IS-3 → P-3 | 0.16 | −1.18 | −1.90 | −1.23 |
Furthermore, the CO oxidation reaction over Ti–graphene is also studied. Like Al–graphene, a two-step reaction pathway is also taken into account for the CO oxidation reaction over this surface. However, as mentioned before, the N2O molecule in complex C is dissociated into N2 and Oads species. In other words, we can say that the C → Oads + N2 step occurs as a barrier-less reaction. In the second step, the ability of Oads toward the oxidation of CO molecules over Ti–graphene is investigated. It should be noted that the dissociative complex C is chosen as the final state in which the physisorbed N2 molecule can easily desorb, leaving Oads with an adsorption energy of −7.3 eV over the surface. The NBO analysis also reveals that the charge of the Oads atom on the surface of Ti–graphene (−0.73 e) is more negative than that of Al–graphene (−0.62 e), due to the large polarization of the Ti atom. This may provide a basis for the higher catalytic performance of Ti–graphene compared to that of Al–graphene. Upon desorption of N2 molecules, different co-adsorption configurations are examined from which the most favourable configuration is selected as the initial state (IS-3). In this complex, the CO molecule is placed far from Oads with the C–Oads and Ti–Oads bond distances of 2.71 and 1.87 Å, respectively (Fig. 3). The CO molecule reaches and reacts with Oads in TS-3 with the activation energy of 0.16 eV. This value is smaller than that of Pt–graphene (1.02 eV),76 P–graphene (1.1 eV) and N–graphene (0.53 eV),42 which suggests that Ti–graphene can be regarded as a promising candidate for the low-temperature oxidation of CO. The Ti–Oads bond is increased from 1.87 Å in IS-3 to 1.90 Å in TS-3 to facilitate the dissociation of Oads from the Ti atom. Also, the CO molecule gets closer (C–Oads = 1.96 Å) to the atomic Oads in order to react and form the CO2 molecule. Passing from TS-3, FS-3 is finally obtained and the physisorbed CO2 molecule is easily released from the surface with a small Eads of about −0.32 eV. It can be seen from Table 2 that the reaction step IS-3 → FS-3 has a negative ΔH298 and ΔG298 of −1.23 and −1.90 eV, respectively, which confirms the exothermic nature of this reaction and also the thermodynamically probable situation at ambient conditions.
Another reaction path which involves the reaction of the Oads moiety with a N2O molecule to produce N2 and O2 molecules is also studied. Fig. S2† shows the decomposition process of the second N2O molecule adsorbed on the Oads–Al–/Ti–graphene sheets. As is evident from Fig. S2,† the decomposition of N2O molecules over Oads–Al–graphene and Oads–Ti–graphene starts from the adsorption configurations IS-3 and IS-4, respectively. The calculated adsorption energy for the N2O molecule over Oads–Al–graphene and Oads–Ti–graphene is −0.26 and −0.33 eV, respectively. The NBO analysis results also indicate a small amount of charge transfer from N2O to the graphene surface. Meanwhile, the adsorption of the second N2O molecule tends to elongate the Al–Oads or Ti–Oads bond (Fig. S2†), which reveals the activation of the Oads moiety in the presence of the N2O molecule. In the transition state, the oxygen atom of N2O approaches the Oads moiety and the O–N bond of N2O is slightly elongated. The reaction is then continuously proceeded from the transition state to the final state where the O2 molecule is formed, leaving the N2 molecule physisorbed over the Al–/Ti–graphene system with the Eads value of −0.06 and −0.08 eV, respectively. The calculated activation energies for this reaction step are calculated to be 2.43 and 2.51 eV for Al– and Ti–graphene, respectively. These are consistent with the previous theoretical study reported by Song et al.,36 indicating that the N2O + Oads → N2 + O2 reaction is unlikely to take place over both Al– and Ti–graphene sheets under normal conditions.
Based on these results, we can predict the feasibility of using Al– and Ti–graphene in practical applications. According to our findings, it is found that both Al– and Ti–graphene have an outstanding catalytic activity toward the oxidation of CO by N2O molecules. Compared with Al–graphene, the decomposition of N2O over Ti–graphene can occur rapidly at room temperature due to its barrier-less reaction (C → Oads + N2). Although Eact of the second process of IS-2 → FS-2 in Al–graphene is smaller than that of IS-3 → FS-3 in Ti–graphene, the latter is preferred because the dissociative process of the N2O molecule takes place automatically via the negligible barrier energy. Besides, the relatively large activation energies obtained for the decomposition of N2O over Oads–Al–/Ti–graphene indicate the impossibility of this side reaction over these systems. Generally, these results can be helpful for expanding efficient noble metal-free catalysts toward the oxidation of CO based on graphene.
4. Conclusions
In this study, the catalytic properties of Al– and Ti–graphene for the oxidation of CO by N2O molecules were investigated by means of DFT calculations. The equilibrium geometries of Al–/Ti–graphene sheets along with the N2O adsorption and dissociation configurations over them were studied in detail. Comparing the adsorption of N2O and CO molecules over Al–/Ti–graphene indicated that the N2O molecule is a stronger electron acceptor than the CO molecule. So, in the presence of both N2O and CO molecules as reaction gases, the Al or Ti site of the doped graphene is covered mainly by N2O molecules. Unlike Al–graphene, upon the adsorption of N2O molecules over Ti–graphene, it is quickly dissociated to N2 and Oads species via the barrier-less reaction pathway C → N2 + Oads. Then, the activated Oads goes through the second reaction (Oads + CO → CO2) in order to form CO2 molecules with the Eact of about 0.16 eV. It can be concluded that Ti–graphene has a greater catalytic activity than Al–graphene for CO oxidation by N2O molecules. Moreover, the decomposition of the second N2O molecule is unlikely to take place over Oads–Al–graphene and Oads–Ti–graphene sheets due to the calculated large activation energies. Overall, both Al– and Ti–graphene can be considered as promising non-noble metal catalysts for the low-temperature CO oxidation reaction by N2O molecules.References
- I. Blumenthal, J. R. Soc. Med., 2001, 94, 270–272 CAS.
- N. Lopez and J. K. Nørskov, J. Am. Chem. Soc., 2002, 124, 11262–11263 CrossRef CAS PubMed.
- L. D. Socaciu, J. Hagen, T. M. Bernhardt, L. Wöste, U. Heiz, H. Häkkinen and U. Landman, J. Am. Chem. Soc., 2003, 125, 10437–10445 CrossRef CAS PubMed.
- M. Chen, Y. Cai, Z. Yan, K. Gath, S. Axnanda and D. W. Goodman, Surf. Sci., 2007, 601, 5326–5331 CrossRef CAS.
- D.-J. Liu, J. Phys. Chem. C, 2007, 111, 14698–14706 CAS.
- L. Liu, F. Zhou, L. Wang, X. Qi, F. Shi and Y. Deng, J. Catal., 2010, 274, 1–10 CrossRef CAS.
- M. D. Esrafili and N. Saeidi, Superlattices Microstruct., 2015, 81, 7–15 CrossRef CAS.
- M. D. Esrafili and N. Saeidi, Phys. E, 2015, 74, 382–387 CrossRef CAS.
- M. D. Esrafili, P. Nematollahi and R. Nurazar, Superlattices Microstruct., 2016, 92, 60–67 CrossRef CAS.
- G. Walther, D. J. Mowbray, T. Jiang, G. Jones, S. Jensen, U. J. Quaade and S. Horch, J. Catal., 2008, 260, 86–92 CrossRef CAS.
- S. Wannakao, T. Nongnual, P. Khongpracha, T. Maihom and J. Limtrakul, J. Phys. Chem. C, 2012, 116, 16992–16998 CAS.
- M. D. Esrafili, N. Saeidi and P. Nematollahi, RSC Adv., 2015, 5, 100290–100298 RSC.
- H. Loirat, F. Caralp, M. Destriau and R. Lesclaux, J. Phys. Chem., 1987, 91, 6538–6542 CrossRef CAS.
- A. Gluhoi, M. Dekkers and B. Nieuwenhuys, J. Catal., 2003, 219, 197–205 CrossRef CAS.
- O. P. Balaj, I. Balteanu, T. T. Roßteuscher, M. K. Beyer and V. E. Bondybey, Angew. Chem., Int. Ed., 2004, 43, 6519–6522 CrossRef CAS PubMed.
- F. Rondinelli, N. Russo and M. Toscano, J. Chem. Theory Comput., 2008, 4, 1886–1890 CrossRef CAS PubMed.
- L. Zhao, Z. Liu, W. Guo, L. Zhang, F. Zhang, H. Zhu and H. Shan, Phys. Chem. Chem. Phys., 2009, 11, 4219–4229 RSC.
- T. Baidya, A. Marimuthu, M. Hegde, N. Ravishankar and G. Madras, J. Phys. Chem. C, 2007, 111, 830–839 CAS.
- C.-K. Siu, S. Reitmeier, I. Balteanu, V. Bondybey and M. Beyer, Eur. Phys. J. D, 2007, 43, 189–192 CrossRef CAS.
- X. L. Xu, E. Yang, J. Q. Li, Y. Li and W. K. Chen, ChemCatChem, 2009, 1, 384–392 CrossRef CAS.
- K. Kartha, M. Pai, A. Banerjee, R. Pai, S. Meena and S. Bharadwaj, J. Mol. Catal. A: Chem., 2011, 335, 158–168 CrossRef CAS.
- Y. Chen, B. Gao, J.-X. Zhao, Q.-H. Cai and H.-G. Fu, J. Mol. Model., 2012, 18, 2043–2054 CrossRef CAS PubMed.
- J.-X. Zhao, Y. Chen and H.-G. Fu, Theor. Chem. Acc., 2012, 131, 1242 CrossRef.
- S. Lin, X. Ye and J. Huang, Phys. Chem. Chem. Phys., 2015, 17, 888–895 RSC.
- E. Kan, Z. Li and J. Yang, Nano, 2008, 3, 433–442 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- W. Choi, I. Lahiri, R. Seelaboyina and Y. S. Kang, Crit. Rev. Solid State Mater. Sci., 2010, 35, 52–71 CrossRef CAS.
- L. L. Zhang, R. Zhou and X. Zhao, J. Mater. Chem., 2010, 20, 5983–5992 RSC.
- K. R. Ratinac, W. Yang, S. P. Ringer and F. Braet, Environ. Sci. Technol., 2010, 44, 1167–1176 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2009, 110, 132–145 CrossRef PubMed.
- B. Guo, L. Fang, B. Zhang and J. R. Gong, Insci. J., 2011, 1, 80–89 CrossRef CAS.
- T. Wehling, K. Novoselov, S. Morozov, E. Vdovin, M. Katsnelson, A. Geim and A. Lichtenstein, Nano Lett., 2008, 8, 173–177 CrossRef CAS PubMed.
- M. Zhou, Y. H. Lu, Y. Q. Cai, C. Zhang and Y. P. Feng, Nanotechnology, 2011, 22, 385502 CrossRef PubMed.
- Y. Li, Z. Zhou, G. Yu, W. Chen and Z. Chen, J. Phys. Chem. C, 2010, 114, 6250–6254 CAS.
- H.-P. Zhang, X.-G. Luo, H.-T. Song, X.-Y. Lin, X. Lu and Y. Tang, Appl. Surf. Sci., 2014, 317, 511–516 CrossRef CAS.
- E. H. Song, J. M. Yan, J. S. Lian and Q. Jiang, J. Phys. Chem. C, 2012, 116, 20342–20348 CAS.
- M. Wu, C. Cao and J. Jiang, New J. Phys., 2010, 12, 063020 CrossRef.
- E. H. Song, Z. Wen and Q. Jiang, J. Phys. Chem. C, 2011, 115, 3678–3683 CAS.
- L. Ma, J.-M. Zhang, K.-W. Xu and V. Ji, Appl. Surf. Sci., 2015, 343, 121–127 CrossRef CAS.
- L. Zhang and Z. Xia, J. Phys. Chem. C, 2011, 115, 11170–11176 CAS.
- L. Zhang, J. Niu, L. Dai and Z. Xia, Langmuir, 2012, 28, 7542–7550 CrossRef CAS PubMed.
- M. D. Esrafili, R. Mohammad-Valipour, S. M. Mousavi-Khoshdel and P. Nematollahi, ChemPhysChem, 2015, 16, 3719–3727 CrossRef CAS PubMed.
- Y. Tang, Z. Liu, X. Dai, Z. Yang, W. Chen, D. Ma and Z. Lu, Appl. Surf. Sci., 2014, 308, 402–407 CrossRef CAS.
- M. D. Esrafili, R. Nurazar and E. Vessally, Int. J. Quantum Chem., 2015, 115, 1153–1160 CrossRef CAS.
- M. D. Esrafili, N. Saeidi and P. Nematollahi, Chem. Phys. Lett., 2016, 649, 37–43 CrossRef CAS.
- L. Wang, Q. Luo, W. Zhang and J. Yang, Int. J. Hydrogen Energy, 2014, 39, 20190–20196 CrossRef CAS.
- Z. Ao, S. Li and Q. Jiang, Solid State Commun., 2010, 150, 680–683 CrossRef CAS.
- M. Chi and Y.-P. Zhao, Comput. Mater. Sci., 2009, 46, 1085–1090 CrossRef CAS.
- Y. Sun, L. Chen, F. Zhang, D. Li, H. Pan and J. Ye, Solid State Commun., 2010, 150, 1906–1910 CrossRef CAS.
- W. Zhao and Q. Y. Meng, Adv. Mater. Res., 2013, 602, 870–873 Search PubMed.
- Q. Jiang, Z. Ao, S. Li and Z. Wen, RSC Adv., 2014, 4, 20290–20296 RSC.
- J. Dai, J. Yuan and P. Giannozzi, Appl. Phys. Lett., 2009, 95, 232105 CrossRef.
- H.-P. Zhang, X.-G. Luo, X.-Y. Lin, X. Lu, Y. Leng and H.-T. Song, Appl. Surf. Sci., 2013, 283, 559–565 CrossRef CAS.
- M. Rojas and E. Leiva, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 155415 CrossRef.
- R. Gholizadeh and Y.-X. Yu, Appl. Surf. Sci., 2015, 357, 1187–1195 CrossRef CAS.
- R. R. Sadhankar and D. T. Lynch, J. Catal., 1994, 149, 278–291 CrossRef CAS.
- J. Arenas-Alatorre, A. Gómez-Cortés, M. Avalos-Borja and G. Díaz, J. Phys. Chem. B, 2005, 109, 2371–2376 CrossRef CAS PubMed.
- P. Giese, H. Kirsch, M. Wolf and C. Frischkorn, J. Phys. Chem. C, 2011, 115, 10012–10018 CAS.
- M. D. Esrafili, P. Nematollahi and H. Abdollahpour, Appl. Surf. Sci., 2016, 378, 418–425 CrossRef CAS.
- Q. G. Jiang, Z. M. Ao and Q. Jiang, Phys. Chem. Chem. Phys., 2013, 15, 10859–10865 RSC.
- S. Chu, L. Hu, X. Hu, M. Yang and J. Deng, Int. J. Hydrogen Energy, 2011, 36, 12324–12328 CrossRef CAS.
- L. Hu, X. Hu, X. Wu, C. Du, Y. Dai and J. Deng, Phys. B, 2010, 405, 3337–3341 CrossRef CAS.
- Y. Okamoto, Chem. Phys. Lett., 2006, 420, 382–386 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc, Wallingford CT, 2009.
- Y. Zhao and D. G. Truhlar, J. Am. Chem. Soc., 2007, 129, 8440–8442 CrossRef CAS PubMed.
- M. D. Esrafili and R. Nurazar, Comput. Mater. Sci., 2014, 92, 172–177 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- E. Glendening, J. Badenhoop, A. Reed, J. Carpenter, J. Bohmann, C. Morales and F. Weinhold, NBO 5.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, 2004 Search PubMed.
- Z. Ao, J. Yang, S. Li and Q. Jiang, Chem. Phys. Lett., 2008, 461, 276–279 CrossRef CAS.
- H.-P. Zhang, X.-G. Luo, X.-Y. Lin, X. Lu and Y. Leng, Int. J. Hydrogen Energy, 2013, 38, 14269–14275 CrossRef CAS.
- Y.-A. Lv, G.-L. Zhuang, J.-G. Wang, Y.-B. Jia and Q. Xie, Phys. Chem. Chem. Phys., 2011, 13, 12472–12477 RSC.
- V. Tzitzios and V. Georgakilas, Chemosphere, 2005, 59, 887–891 CrossRef CAS PubMed.
- E. Song, J. Yan, J. Lian and Q. Jiang, J. Phys. Chem. C, 2012, 116, 20342–20348 CAS.
- Y.-H. Lu, M. Zhou, C. Zhang and Y.-P. Feng, J. Phys. Chem. C, 2009, 113, 20156–20160 CAS.
- E. Song, Z. Wen and Q. Jiang, J. Phys. Chem. C, 2011, 115, 3678–3683 CAS.
- Y. Tang, Z. Yang and X. Dai, Phys. Chem. Chem. Phys., 2012, 14, 16566–16572 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04326g |
| This journal is © The Royal Society of Chemistry 2016 |