Design, synthesis, and biological evaluation of novel asiatic acid derivatives as potential anticancer agents†
Bruno M. F. Gonçalvesab,
Jorge A. R. Salvador*ab,
Diana S. M. Santosa,
Silvia Maríncd and
Marta Cascante*cd
aLaboratory of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Coimbra, Portugal. E-mail: salvador@ci.uc.pt; Fax: +351 239 488 503; Tel: +351 239 488 479
bCenter for Neuroscience and Cell Biology, Coimbra, Portugal
cDepartment of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, Diagonal 643, 08028 Barcelona, Spain. E-mail: martacascante@ub.edu; Tel: +34 934021593
dInstitute of Biomedicine of University of Barcelona (IBUB) and Associated Unit to CSIC, Barcelona, Spain
First published on 13th April 2016
Abstract
A series of new asiatic acid derivatives modified in the A-ring and at C-28 were synthesized and their antiproliferative activity was evaluated against HT-29 and HeLa cell lines. Most of the derivatives tested here exhibited improved antiproliferative activity compared with asiatic acid. Among them, the best compounds, 7 and 8, were further evaluated against additional cancer cell lines (MCF-7, Jurkat, and PC-3 cells) and a nontumoral cell line (BJ). The most active compound, 7, exhibited IC50 values ranging from 1.62 μM in HeLa cells to 9.93 μM in MCF-7 cells. Further studies revealed that compound 7 arrested the cell cycle at the G0/G1 phase and induced caspase-dependent apoptosis in HeLa cells. Furthermore, this compound showed selectivity toward cancer cells, and a synergistic effect was observed after simultaneous treatment of HeLa cells with compound 7 and cisplatin. Collectively, our results suggest that compound 7 may be useful for the development of new anticancer therapies; thus, additional preclinical studies are warranted.
1. Introduction
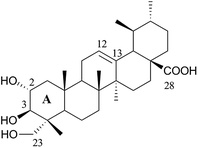
Natural triterpenes represent a structurally diverse group of organic compounds that display several pharmacological activities.1–5 Because of these activities, these compounds are very interesting leads in the field of drug discovery, particularly regarding the development of innovative anticancer drugs.4,6–11Asiatic acid 1 [AA (1), Fig. 1], is a naturally occurring pentacyclic triterpenoid that is found mainly in the traditional medicinal herb Centella asiatica. This compound has long been used in the field of dermatology as a wound-healing agent12–14 and is the major effective ingredient of the commercial drug Madecassol™.15 AA (1) also has other clinically useful therapeutic effects, including antioxidant, anti-inflammatory,16,17 neuro-protective,18,19 antidiabetic, hepato-protective,20 and, especially, antitumor activities.21–23 Because its antitumor activity, this compound has attracted a great interest and its anticancer effect has been studied by several research groups. AA (1) inhibits the proliferation of, and induces apoptosis in several cancer cell lines,21–26 and possesses a strong antiangiogenic potential against malignant gliomas.27 AA (1) also enhances the sensitivity of HT-29 cells to the anticancer drug irinotecan, with a synergistic effect when cells were first exposed to AA (1) and then to irinotecan.28 In addition, this compound proved to be relatively nontoxic in normal cells, which renders it a promising anticancer agent. However, the clinical utility of AA (1) in the treatment of cancer is limited by its modest anticancer activity and poor bioavailability. The chemical modification of the AA (1) backbone had a high impact on its biological activity and may represent the solution to improve not only the antitumor activity of AA (1), but also its pharmacokinetic properties.
Previous studies revealed that the introduction of amino moieties29–31 at C-28 of AA (1) significantly improved the anticancer activity of the compound against several cancer cell lines. The modification of the A-ring of AA (1) also increased its anticancer activity.11 In addition, in the last decades, the hydroxamic acid group was shown to be a potent moiety in the field of cancer therapy.32–35 Hydroxamic acid derivatives are well-known inhibitors of metal-containing enzymes, such as matrix metalloproteinases (MMPs)34 and histone deacetylases (HDACs), which are two enzyme families that are important for tumor development. However, the introduction of a hydroxamic acid moiety into the AA (1) structure has not been addressed. Therefore, to develop new AA (1) derivatives as potential anticancer drug candidates, here we designed and prepared a panel of new AA (1) derivatives bearing amino, amino acid, and hydroxamic acid moieties at C-28, combined with modifications at the A ring. The antiproliferative activities of the newly synthesized AA (1) derivatives against the HT-29 and HeLa cancer cell lines were evaluated and a structure–activity relationship (SAR) was established based on the IC50 obtained in HeLa cells. Additional studies were then conducted in HeLa cells to explore the mechanism of action of the most active compound, 7. Finally, the existence of synergism between compound 7 and cisplatin was investigated.
2. Results and discussion
2.1 Chemistry
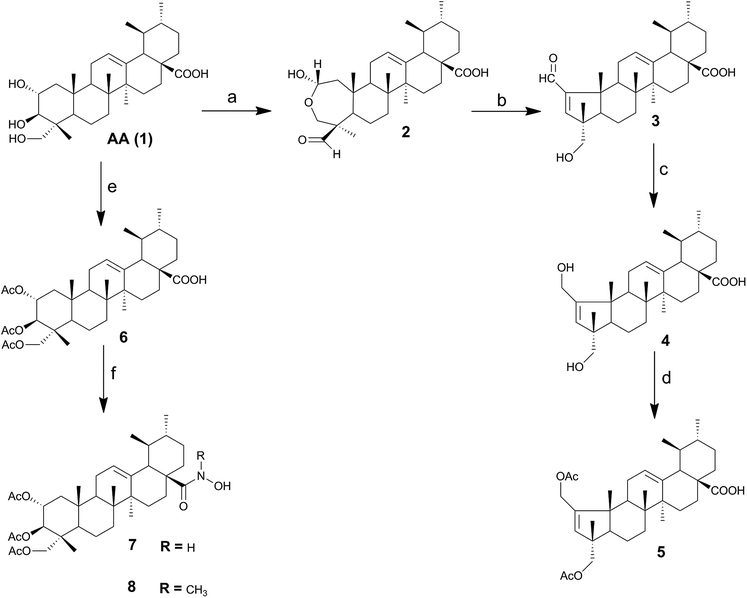
In this work, a series of novel AA (1) derivatives were successfully synthesized. As shown in Scheme 1, the synthesis started by the preparation of the intermediates 2, 3, 4, 5 and 6. The lactol derivative 2 was obtained by treatment of AA (1) with sodium periodate in methanol/water, by adapting a previously reported procedure.36 The treatment of derivative 2 with acetic acid and piperidine in dry benzene gave the α,β-unsaturated aldehyde 3. This compound was then reduced with sodium borohydride in anhydrous methanol, affording the diol derivative 4 in good yield (92%). The two hydroxyl groups of compound 4 were diacetylated with acetic anhydride and DMAP in THF, affording the intermediate 5. Commercially available asiatic acid was treated with acetic anhydride to give the triacetate derivative 6 in good yield. The intermediates 5 and 6 were then used as a starting point for the synthesis of new derivatives of AA (1).In the last decades, medicinal chemists have shown a renewed interest in hydroxamic acids and their derivatives; in the particular case of triterpenoid compounds, previous studies reported hydroxamic acid derivatives of glycyrrhetinic acid as being selective inhibitors of 11β-hydroxysteroid dehydrogenase 2.37,38 In this study, we prepared AA (1) hydroxamic acid derivatives from 1,1′-carbonyldiimidazole (CDI)-activated carboxylic acids and hydroxylamine hydrochloride. As CDI promoted the deprotonation of hydroxylamine hydrochloride, no additional base was necessary.39 As depicted in Scheme 1, the carboxylic acid derivative 6 was treated with CDI in THF to afford the respective N-acylimidazole intermediate, which was reacted with hydroxylamine hydrochloride or methylhydroxylamine hydrochloride to give the corresponding hydroxamic derivatives 7 and 8, in good yields. The hydroxamic acid derivative 9 was prepared from 5, according to the procedure described for compound 7 and using hydroxylamine hydrochloride. In the 1H NMR spectra of hydroxamic acid derivatives 7 and 9, the characteristic peak of the proton attached to the nitrogen atom was observed at around 6.25–6.27 ppm. In addition, on the 13C NMR spectrum, the signal of the hydroxamic acid carbonyl group was found at 178.4 ppm, a lower value than that observed for the corresponding carbonyl group of carboxylic acid (at around 183.1–183.6 ppm). The ester carbonyl carbons of compound 7 appeared in 13C NMR spectrum as signals at 170.8, 170.5 and 170.4 ppm. The signal at 125.5 ppm corresponded to the tertiary carbon C12, as this signal was present on DEPT 135.
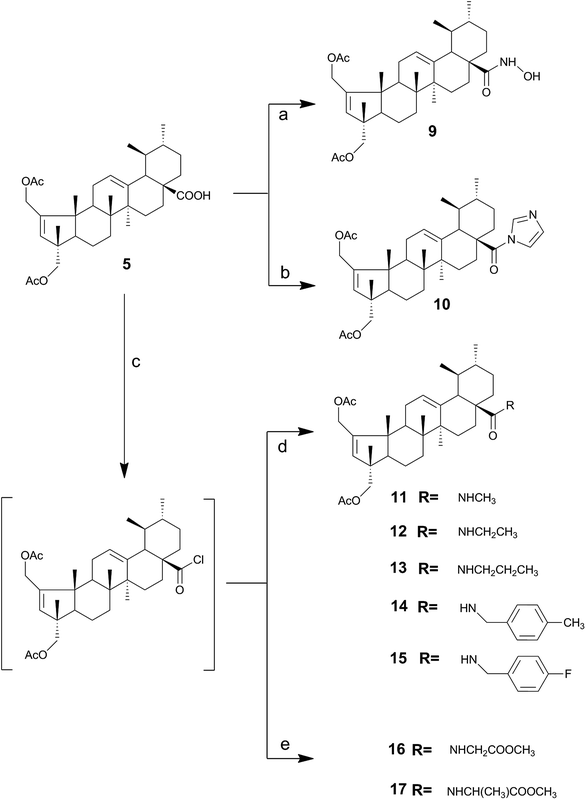
Previous studies performed by our group revealed that the introduction of an imidazole moiety at the C-28 position of triterpenoid compounds improved their cytotoxic activities.40–42 Thus, the N-acylimidazole derivative 10 was prepared in 63% yield after FCC via the reaction of compound 5 with CDI in THF at reflux (Scheme 2). The successful preparation of compound 10 was confirmed by the presence of three signals at 8.23, 7.52 and 7.03 ppm in the 1H NMR spectrum, which are typical of imidazole protons. A signal was also observed at δ 174.7 ppm in the 13C NMR spectrum corresponding to the C-28 carbon, which was different from the signal observed for the C-28 carboxylic acid carbonyl, at δ 183.6 ppm. The signals at 151.1 and 137.1 ppm were attributed to the quaternary carbons C2 and C13. The signals at 132.0 and 126.1 ppm were attributed to the tertiary carbons C3 and C12, respectively, as these signals appeared in the DEPT 135. The signals for the three tertiary carbons of the imidazole ring were found at 137.1, 129.7 and 117.4 ppm.
As depicted in Scheme 2, the treatment of compound 5 with thionyl chloride in dry benzene, gave the acyl chloride derivative, followed by treatment with the respective amines in dry dichloromethane afforded the amide derivatives 11–15 in good yields. The formation of an amide bond at C-28 was confirmed by the presence of a strong N–H bending band at 1509–1527 cm−1 on the IR spectra. On the 13C NMR spectrum, the signal of the carbonyl group of the amide was observed at 177.6–178.7 ppm, which was a lower value than that observed for the carbonyl group of carboxylic acid (at 183.6 ppm).
The methyl ester amino acid derivatives 16 and 17 were obtained by treating 5 with thionyl chloride, to give the acyl chloride intermediate, which was further reacted with the corresponding amino acid methyl ester hydrochloride.
2.2 Biology
| Compound | Cell line/IC50b (μM) | |
|---|---|---|
| HT-29 | HeLa | |
| a HT-29 and HeLa cells were treated with increasing concentrations of each compound for 72 h. Viable cells were determined by MTT assay and IC50 values are expressed as the mean ± SD of three independent experiments. N.D. not determined.b IC50 is the concentration of compound that inhibits 50% of cell growth.c IC50 value obtained from literature, determined using the same experimental methodology and included here for comparison. | ||
| AA 1 | 64.33 ± 3.21 | 52.47 ± 0.06 |
| 2 | N.D. | 21.67 ± 1.04 |
| 3 | N.D. | 5.30 ± 0.20 |
| 4 | N.D. | 45.00 ± 1.55 |
| 5 | N.D. | 22.50 ± 0.75 |
| 6 | N.D. | 6.10 ± 0.28 |
| 7 | 4.12 ± 0.30 | 1.62 ± 0.10 |
| 8 | 7.03 ± 0.06 | 3.77 ± 0.23 |
| 9 | 19.00 ± 0.71 | 4.80 ± 0.28 |
| 10 | 13.15 ± 0.78 | 6.25 ± 0.07 |
| 11 | 16.15 ± 0.92 | 7.60 ± 0.42 |
| 12 | 14.53 ± 1.45 | 4.54 ± 0.84 |
| 13 | 14.10 ± 1.27 | 6.50 ± 0.5 |
| 14 | >60 | 6.25 ± 0.35 |
| 15 | >60 | 5.38 ± 0.530 |
| 16 | 22.00 ± 1.41 | 10.65 ± 0.35 |
| 17 | 13.75 ± 0.35 | 7.23 ± 0.39 |
| Cisplatin | 6.11c (ref. 43) | 2.28 ± 0.26 |
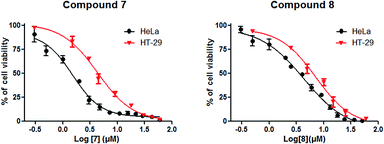 | ||
| Fig. 2 Dose-dependent effect of compounds 7 and 8 on HeLa and HT-29 cell viability. Results are presented as mean ± SD of three independent experiments. | ||
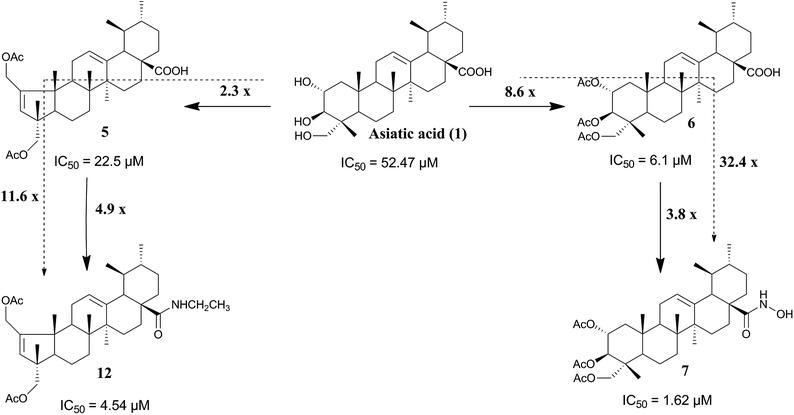
As shown in Table 1 and Fig. 2, the hydroxamic (7) and the methyl hydroxamic (8) derivatives, exhibited remarkable antiproliferative activities against HT-29 and HeLa cells, compared with AA (1). Compound 7 showed IC50 values of 4.12 and 1.62 μM against the HT-29 and HeLa cell lines, respectively. This compound was 32.4-fold more potent than AA (1) and 3.8-fold more potent than 6 against HeLa cells. Compound 7 also exhibited a stronger antiproliferative activity against HeLa cells than did the clinically used drug cisplatin. Derivative 8 was 14- and 1.6-fold more active against HeLa cells than AA (1) and compound 6, respectively. The conversion of the carboxylic acid group of compound 5 into a hydroxamic acid group afforded derivative 9, with an improvement of 4.7- and 10.9-fold in antiproliferative activity against HeLa cells compared with 5 and AA (1), respectively. These combined results suggest that the introduction of a hydroxamic acid group at C-28 has a positive impact on the anticancer activity of the compound.
The introduction of an imidazole ring at C-28 of compound 5, to afford compound 10, led to an increase in cell-growth-inhibition activity. The imidazole derivative 10 was 3.5- and 8.4-fold more active than 5 and AA (1), respectively, in HeLa cells (Table 1). These results are in good agreement with previous reports.42
Finally, the comparison of the IC50 values of compounds 11–17 revealed that the introduction of the amide functionality at C-28 of compound 5 resulted in a significant improvement of antiproliferative activity against HeLa cells. Compound 12 was 11.6-fold more potent than AA (1) and 5-fold more potent than compound 5. Derivatives 16 and 17, bearing a methyl ester amino acid residue at C-28, exhibited better antiproliferative activity in HeLa cells than did compound 5.
As compounds 7 and 8 were the most active compounds, they were selected, and their antiproliferative activity was further tested in breast (MCF-7), leukemia (Jurkat), and prostate (PC-3) cancer cell lines. As depicted in Table 2, both compounds exhibited a much higher antiproliferative activity against the tested cancer cell lines than did AA (1). Moreover, the selectivity of compound 7 was assessed on the nontumoral fibroblast cell line BJ. The analysis of the selectivity index (IC50 in the BJ cell line/IC50 in the cancer cell line) (Table 3) values revealed that compound 7 was 6 to 36 times more active in cancer cell lines than on nontumoral BJ cells. In contrast, this index ranged from 1.3 to 2.4 for AA (1). These results clearly suggest that 7 is more selective for cancer cells than AA (1). As compound 7 displayed the strongest antiproliferative effect, it was chosen for further studies in HeLa cells aimed at exploring its anticancer mechanism.
| Compounds | Cell line/IC50b (μM) | |||
|---|---|---|---|---|
| MCF-7 | Jurkat | PC-3 | BJ | |
| a The cell lines were treated with increasing concentrations of each compound for 72 h. Viable cells were determined by MTT assay and IC50 values are expressed as the mean ± SD of three independent experiments. N.D. not determined.b IC50 is the concentration of compound that inhibits 50% of cell growth.c IC50 values obtained from literature determined using the same experimental methodology and included here for comparison. | ||||
| AA (1) | 68.5 ± 2.5 | 37.17 ± 3.75 | 67.25 ± 0.35 | 88.7 ± 0.58 |
| 7 | 9.93 ± 1.01 | 2.47 ± 0.12 | 3.73 ± 0.46 | 59.0 ± 0.27 |
| 8 | 9.17 ± 0.58 | 2.43 ± 0.21 | 3.8 ± 0.8 | N.D. |
| Cisplatin | 19.0 ± 4.5 | 1.94c (ref. 44) | 4.6c (ref. 45) | 10.10 ± 2.00 |
| Compounds | Selectivity index (IC50 BJ cell line/IC50 cancer cell line) | ||||
|---|---|---|---|---|---|
| HT-29 | HeLa | MCF-7 | Jurkat | PC-3 | |
| AA (1) | 1.36 | 1.69 | 1.29 | 2.39 | 1.32 |
| 7 | 14.32 | 36.42 | 5.95 | 23.90 | 15.81 |
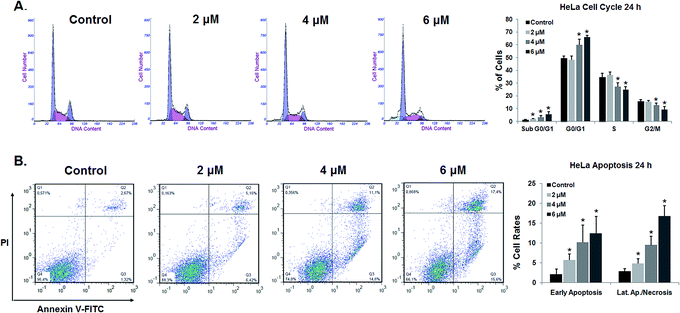
The percentage of cells in the G0/G1 phase rose from 49.45% in control cells to 65.97% in cells treated with 6 μM 7. Concomitantly, the percentage of cells in the S and G2/M phases was gradually reduced. An analysis of cell-cycle progression indicated that compound 7 induced cell-cycle arrest at the G1 phase.
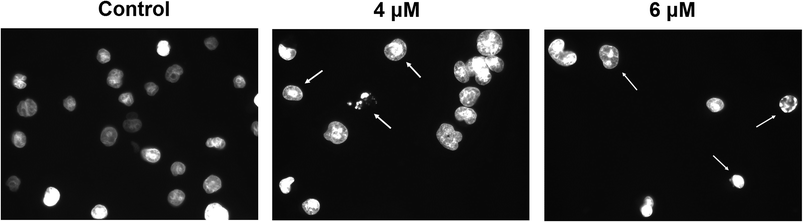
We also investigated if the growth-inhibitory activity of compound 7 was related to the induction of apoptosis. Apoptosis is a tightly regulated cell death program that plays a critical role in cell homeostasis by controlling cell proliferation.46 The dysregulation of the apoptotic process is related to the development of many types of cancer.47 In fact, the ability of cancer cells to evade apoptotic cell death is one of the hallmarks of cancer.48 Therefore, the induction of apoptosis in cancer cells is one of the recognized strategies that have been used for the development of more effective anticancer drugs.47,49,50
In the early stages of apoptosis, the cell membrane loses it asymmetry and phosphatidylserine (PS) translocates from the inner to the outer side of the cell membrane. The externalized PS can be detected using Annexin V-FITC, a fluorescent active dye that selectively binds to PS.51
Therefore, to explore whether compound 7 has the ability to induce apoptosis in HeLa cells, we performed an Annexin V-FITC/PI flow cytometry analysis. HeLa cells treated with different concentrations of compound 7 (2, 4, and 6 μM) for 24 h were analyzed by FACS after double staining with Annexin V/PI. The combined results of three independent experiments are depicted in Fig. 4B. HeLa cells treated with 7 at 2 μM for 24 h showed an increase in the percentage of Annexin-V-positive cells, from 4.94% in control cells to 10.53% in treated cells (5.70% of early apoptotic cells and 4.83% of late apoptotic cells). After increasing the concentration of the drug to 4 and 6 μM, the percentage of Annexin-V-positive cells rose to 19.55% and 29.18%, respectively. Our results suggest that compound 7 induces apoptosis in HeLa cells in a concentration-dependent manner.
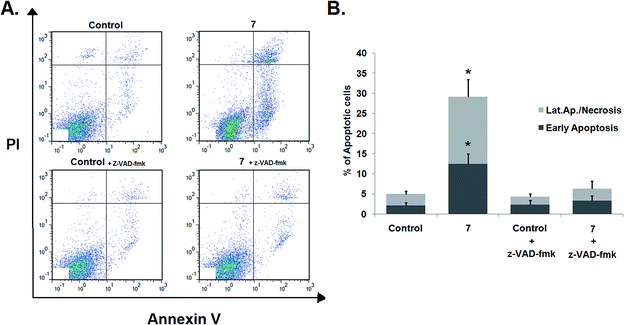
The effects of z-VAD-fmk on compound 7-induced apoptosis were evaluated by flow cytometry after staining with Annexin V-FITC/PI. Pretreatment with z-VAD-fmk did not affect the apoptosis levels in control cells (Fig. 6). However, the percentage of apoptotic cells among HeLa cells treated with compound 7 was significantly reduced when cells were pretreated with the caspase inhibitor (29.18% of total apoptotic cells in nontreated cells vs. 6.31% of apoptotic cells in cells pretreated with the caspase inhibitor), as shown in Fig. 6. These results suggest that compound 7 induces apoptosis via a caspase-dependent mechanism.
To confirm our hypothesis, HeLa cells were treated simultaneously with cisplatin and compound 7 at different concentration combinations (maintaining the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the concentrations of cisplatin and compound 7, respectively) for 72 h, and the combination index (CI) values were calculated based on the Chou–Talalay method55 using the CompuSyn software. As shown in Table 4, the CI values obtained were lower than 1 for all the combinations tested. These data suggest the existence of a synergistic effect between cisplatin and compound 7 in the inhibition of HeLa cells viability.
1 between the concentrations of cisplatin and compound 7, respectively) for 72 h, and the combination index (CI) values were calculated based on the Chou–Talalay method55 using the CompuSyn software. As shown in Table 4, the CI values obtained were lower than 1 for all the combinations tested. These data suggest the existence of a synergistic effect between cisplatin and compound 7 in the inhibition of HeLa cells viability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in HeLa cell line. CI values were calculated using the Chou–Talalay method and the CompuSyn software. CI values <1 indicate the existence of synergisma
1, in HeLa cell line. CI values were calculated using the Chou–Talalay method and the CompuSyn software. CI values <1 indicate the existence of synergisma
| Cisplatin (μM) | 7 (μM) | 1 – Viability | CI |
|---|---|---|---|
| a HeLa cells were treated with compound 7 and cisplatin simultaneously. | |||
| 0.42 | 0.21 | 0.30 | 0.57 |
| 1.02 | 0.51 | 0.46 | 0.84 |
| 1.80 | 0.90 | 0.71 | 0.69 |
| 3.00 | 1.50 | 0.77 | 0.91 |
| 4.20 | 2.10 | 0.88 | 0.73 |
| 6.00 | 3.00 | 0.89 | 0.92 |
| 10.20 | 5.10 | 0.97 | 0.64 |
| 18.00 | 9.00 | 0.99 | 0.46 |
3. Conclusions
In the present study, we developed and synthesized a panel of new derivatives of AA (1) modified in the A-ring and at C-28. The new derivatives showed improved antiproliferative activities against several cancer cell lines compared with AA (1). Compound 7, bearing a hydroxamic acid moiety at C-28, displayed the best antiproliferative activity among all the derivatives tested and was more selective toward cancer cell lines than AA (1). Further studies revealed that compound 7 led to cell-cycle arrest at the G0/G1 phase and induced apoptosis mediated by caspases in the HeLa cell line. Moreover, we observed a synergistic inhibitory effect on the proliferation of HeLa cells between compound 7 and cisplatin. These findings led us to conclude that compound 7 may be a valuable candidate for further studies aimed at the development of effective anticancer drugs.4. Experimental
4.1 Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)], NaIO4 (131.30 mg, 0.61 mmol, 1.5 eq.) was added. The reaction mixture was stirred at room temperature. After 2 h the reaction mixture was evaporated under reduced pressure to remove the organic phase. The obtained crude was dispersed by water (40 mL) and extracted with ethyl acetate (3 × 40 mL). The resulting organic phase was washed with water (4 × 40 mL) and brine (40 mL), dried over Na2SO4, filtered, and concentrated under vacuum to afford 2 as a white powder (quantitative). Mp: 198.5–201.4 °C. νmax/cm−1 (KBr): 3421.1, 2948.6, 2927.4, 2871.5, 2732.6, 2630.4, 1716.3, 1695.1, 1457.0, 1378.9, 1037.5. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.29 (t, J = 3.3 Hz, 1H, H-12), 5.14–5.11 (m, 1H, H-2), 3.94 (d, J = 13.4 Hz, 1H), 3.75 (d, J = 13.2 Hz, 1H), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.95 (d, J = 6.0 Hz, 3H), 0.86 (s, 3H), 0.85 (d, J = 5.4 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 206.1 (CHO), 182.9 (C28), 138.1 (C13), 126.0 (C12), 93.7, 65.4, 61.2, 53.4, 62.7, 48.1, 45.2, 43.7, 42.6, 40.1, 40.0, 38.9, 38.8, 38.6, 33.6, 30.6, 27.8, 24.6, 24.1, 23.2, 21.1, 20.6, 20.4, 17.9, 16.9, 14.6 ppm. DI-ESI-MS m/z: 487.15 ([M + H]+). Calcd for C30H46O5·H2O: C, 71.39; H, 9.59. Found: C, 71.49; H, 9.85%.
1)], NaIO4 (131.30 mg, 0.61 mmol, 1.5 eq.) was added. The reaction mixture was stirred at room temperature. After 2 h the reaction mixture was evaporated under reduced pressure to remove the organic phase. The obtained crude was dispersed by water (40 mL) and extracted with ethyl acetate (3 × 40 mL). The resulting organic phase was washed with water (4 × 40 mL) and brine (40 mL), dried over Na2SO4, filtered, and concentrated under vacuum to afford 2 as a white powder (quantitative). Mp: 198.5–201.4 °C. νmax/cm−1 (KBr): 3421.1, 2948.6, 2927.4, 2871.5, 2732.6, 2630.4, 1716.3, 1695.1, 1457.0, 1378.9, 1037.5. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.29 (t, J = 3.3 Hz, 1H, H-12), 5.14–5.11 (m, 1H, H-2), 3.94 (d, J = 13.4 Hz, 1H), 3.75 (d, J = 13.2 Hz, 1H), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.95 (d, J = 6.0 Hz, 3H), 0.86 (s, 3H), 0.85 (d, J = 5.4 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 206.1 (CHO), 182.9 (C28), 138.1 (C13), 126.0 (C12), 93.7, 65.4, 61.2, 53.4, 62.7, 48.1, 45.2, 43.7, 42.6, 40.1, 40.0, 38.9, 38.8, 38.6, 33.6, 30.6, 27.8, 24.6, 24.1, 23.2, 21.1, 20.6, 20.4, 17.9, 16.9, 14.6 ppm. DI-ESI-MS m/z: 487.15 ([M + H]+). Calcd for C30H46O5·H2O: C, 71.39; H, 9.59. Found: C, 71.49; H, 9.85%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 3 as a white solid (353.77 mg, 73%). Mp: 183.5–186.1 °C. νmax/cm−1 (KBr): 3428.8, 2946.7, 2925.5, 2869.6, 2726.9, 2632.4, 1689.3, 1581.3, 1454.1, 1380.8, 1041.4. 1H NMR (400 MHz, CDCl3): δ = 9.72 (s, 1H, CHO), 6.66 (s, 1H, H-3), 5.28 (t, J = 3.1 Hz, 1H, H-12), 3.62 (d, J = 10.7 Hz, 1H, H-23), 3.45 (d, J = 10.7 Hz, 1H, H-23), 1.25 (s, 3H), 1.10 (s, 3H), 1.01 (s, 3H), 0.93 (d, J = 6.2 Hz, 3H), 0.88 (s, 3H), 0.84 (d, J = 6.3 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.9 (CHO), 183.3 (C28), 159.3 (C3), 158.9 (C2), 137.5 (C13), 126.6 (C12), 69.4, 56.3, 52.6, 50.9, 49.4, 47.9, 44.1, 42.4, 41.4, 38.8 (2C), 36.6, 33.5, 30.6, 28.2, 27.1, 24.0 (2C), 21.2, 19.0, 18.7, 17.4, 17.0, 15.9 ppm; DI-ESI-MS m/z: 469.03 ([M + H]+). Calcd for C30H44O4·H2O: C, 74.04; H, 9.53. Found: C, 73.68; H, 9.75%.
1) to afford 3 as a white solid (353.77 mg, 73%). Mp: 183.5–186.1 °C. νmax/cm−1 (KBr): 3428.8, 2946.7, 2925.5, 2869.6, 2726.9, 2632.4, 1689.3, 1581.3, 1454.1, 1380.8, 1041.4. 1H NMR (400 MHz, CDCl3): δ = 9.72 (s, 1H, CHO), 6.66 (s, 1H, H-3), 5.28 (t, J = 3.1 Hz, 1H, H-12), 3.62 (d, J = 10.7 Hz, 1H, H-23), 3.45 (d, J = 10.7 Hz, 1H, H-23), 1.25 (s, 3H), 1.10 (s, 3H), 1.01 (s, 3H), 0.93 (d, J = 6.2 Hz, 3H), 0.88 (s, 3H), 0.84 (d, J = 6.3 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.9 (CHO), 183.3 (C28), 159.3 (C3), 158.9 (C2), 137.5 (C13), 126.6 (C12), 69.4, 56.3, 52.6, 50.9, 49.4, 47.9, 44.1, 42.4, 41.4, 38.8 (2C), 36.6, 33.5, 30.6, 28.2, 27.1, 24.0 (2C), 21.2, 19.0, 18.7, 17.4, 17.0, 15.9 ppm; DI-ESI-MS m/z: 469.03 ([M + H]+). Calcd for C30H44O4·H2O: C, 74.04; H, 9.53. Found: C, 73.68; H, 9.75%.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.06 (s, 3H, OCOC
3), 2.06 (s, 3H, OCOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.17 (s, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 5.6 Hz), 0.85 (d, J = 6.6 Hz), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 183.6 (C28), 171.3 (OCO), 170.8 (OCO), 151.3 (C2), 138.7 (C13), 131.8 (C3), 125.3 (C12), 72.3, 62.6, 58.0, 52.6, 50.7, 47.9, 46.3, 43.1, 42.3, 41.1, 38.8, 38.8, 36.6, 33.7, 30.5, 28.2, 26.2, 24.0, 23.8, 21.2, 21.0 (2C), 19.1, 18.6, 17.8, 17.0, 16.6 ppm. DI-ESI-MS m/z: 555.04 ([M + H]+).
3), 1.17 (s, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 5.6 Hz), 0.85 (d, J = 6.6 Hz), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 183.6 (C28), 171.3 (OCO), 170.8 (OCO), 151.3 (C2), 138.7 (C13), 131.8 (C3), 125.3 (C12), 72.3, 62.6, 58.0, 52.6, 50.7, 47.9, 46.3, 43.1, 42.3, 41.1, 38.8, 38.8, 36.6, 33.7, 30.5, 28.2, 26.2, 24.0, 23.8, 21.2, 21.0 (2C), 19.1, 18.6, 17.8, 17.0, 16.6 ppm. DI-ESI-MS m/z: 555.04 ([M + H]+).![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) –OH), 5.28 (t, J = 3.0 Hz, 1H, H-12), 5.19–5.13 (m, 1H, H-2), 5.08 (d, J = 10.3 Hz, 1H, H3), 3.85 (d, J = 11.9 Hz, 1H, H-23), 3.57 (d, J = 11.8 Hz, 1H, H-23), 2.08 (s, 3H, OCOCH3), 2.02 (s, 3H, OCOCH3), 1.97 (s, 3H, OCOCH3), 1.10 (s, 3H), 1.07 (s, 3H), 0.94 (d, J = 5.8 Hz, 3H), 0.88 (s, 3H), 0.85 (d, J = 6.2 Hz, 3H), 0.75 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.4 (C28), 170.8 (OCO), 170.5 (OCO), 170.4 (OCO), 138.0 (C13), 125.5 (C12), 74.8, 69.9, 65.3, 52.6, 48.0, 47.6, 47.5, 43.7, 41.9, 41.9, 39.5, 38.9, 38.7, 37.8, 36.6, 32.4, 30.5, 27.9, 24.0, 23.5, 23.3, 21.1 (2C), 20.9, 20.8, 17.9, 17.0, 16.9 (2C), 13.9 ppm. DI-ESI-MS m/z: 630.12 ([M + H]+), 652.46 ([M + Na]+). Calcd for C36H55NO8: C, 68.65; H, 8.80; N, 2.22. Found: C, 69.01; H, 9.20; N, 1.84.
–OH), 5.28 (t, J = 3.0 Hz, 1H, H-12), 5.19–5.13 (m, 1H, H-2), 5.08 (d, J = 10.3 Hz, 1H, H3), 3.85 (d, J = 11.9 Hz, 1H, H-23), 3.57 (d, J = 11.8 Hz, 1H, H-23), 2.08 (s, 3H, OCOCH3), 2.02 (s, 3H, OCOCH3), 1.97 (s, 3H, OCOCH3), 1.10 (s, 3H), 1.07 (s, 3H), 0.94 (d, J = 5.8 Hz, 3H), 0.88 (s, 3H), 0.85 (d, J = 6.2 Hz, 3H), 0.75 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.4 (C28), 170.8 (OCO), 170.5 (OCO), 170.4 (OCO), 138.0 (C13), 125.5 (C12), 74.8, 69.9, 65.3, 52.6, 48.0, 47.6, 47.5, 43.7, 41.9, 41.9, 39.5, 38.9, 38.7, 37.8, 36.6, 32.4, 30.5, 27.9, 24.0, 23.5, 23.3, 21.1 (2C), 20.9, 20.8, 17.9, 17.0, 16.9 (2C), 13.9 ppm. DI-ESI-MS m/z: 630.12 ([M + H]+), 652.46 ([M + Na]+). Calcd for C36H55NO8: C, 68.65; H, 8.80; N, 2.22. Found: C, 69.01; H, 9.20; N, 1.84.![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.08 (s, 3H, OCOCH3), 2.02 (s, 3H, OCOCH3), 1.97 (s, 3H, OCOCH3), 1.09 (s, 3H), 1.08 (s, 3H), 0.94 (d, J = 6.1 Hz, 3H), 0.88 (s, 3H), 0.85 (d, J = 6.3 Hz, 3H), 0.78 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.6 (C28), 170.9 (OCO), 170.4 (OCO), 170.4 (OCO), 137.9 (C13), 125.5 (C12), 74.8, 69.9, 65.3, 52.6, 47.7, 47.6, 47.5, 43.8, 42.1, 41.9, 39.7, 39.6, 39.0, 38.7, 37.8, 36.7, 32.5, 30.5, 27.8, 23.9, 23.4, 23.3, 21.1, 21.1, 20.9, 20.8, 17.9, 17.2, 17.0, 16.9, 13.9 ppm. DI-ESI-MS m/z: 644.21 ([M + H]+), 666.45 ([M + Na]+). Calcd for C37H57NO8: C, 69.02; H, 8.92; N, 2.18. Found: C, 68.62; H, 9.30; N, 2.21.
3), 2.08 (s, 3H, OCOCH3), 2.02 (s, 3H, OCOCH3), 1.97 (s, 3H, OCOCH3), 1.09 (s, 3H), 1.08 (s, 3H), 0.94 (d, J = 6.1 Hz, 3H), 0.88 (s, 3H), 0.85 (d, J = 6.3 Hz, 3H), 0.78 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.6 (C28), 170.9 (OCO), 170.4 (OCO), 170.4 (OCO), 137.9 (C13), 125.5 (C12), 74.8, 69.9, 65.3, 52.6, 47.7, 47.6, 47.5, 43.8, 42.1, 41.9, 39.7, 39.6, 39.0, 38.7, 37.8, 36.7, 32.5, 30.5, 27.8, 23.9, 23.4, 23.3, 21.1, 21.1, 20.9, 20.8, 17.9, 17.2, 17.0, 16.9, 13.9 ppm. DI-ESI-MS m/z: 644.21 ([M + H]+), 666.45 ([M + Na]+). Calcd for C37H57NO8: C, 69.02; H, 8.92; N, 2.18. Found: C, 68.62; H, 9.30; N, 2.21.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 9 as a white solid (46.6 mg, 30%). Mp: 86.1–88.8 °C. νmax/cm−1 (ATR): 3250.5, 2926.0, 2.871.5, 1741.0, 1380.5, 1233.0, 1029.5. 1H NMR (400 MHz, CDCl3): δ = 6.27 (s, 1H, N
1) to afford 9 as a white solid (46.6 mg, 30%). Mp: 86.1–88.8 °C. νmax/cm−1 (ATR): 3250.5, 2926.0, 2.871.5, 1741.0, 1380.5, 1233.0, 1029.5. 1H NMR (400 MHz, CDCl3): δ = 6.27 (s, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) OH), 5.44 (s, 1H, H-3), 5.26 (s, 1H), 4.70 (d, J = 14.2 Hz, 1H), 4.57 (d, J = 14.2 Hz, 1H), 3.93 (d, J = 10.5 Hz, 1H, H-23), 3.84 (d, J = 10.5 Hz, 1H, H-23), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.17 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.95 (s, 3H), 0.86–0.81 (3, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.4 (C28), 171.3 (OCO), 170.8 (OCO), 151.2 (C2), 138.6 (C13), 131.8 (C3), 125.5 (C12), 72.3, 62.6, 58.1, 52.7, 50.6, 48.1, 46.3, 43.1, 42.3, 41.1, 38.7, 38.7, 36.6, 33.7, 30.5, 28.2, 26.1, 24.0, 23.8, 21.1, 21.0 (2C), 19.1, 18.5, 17.8, 17.0, 16.6 ppm. DI-ESI-MS m/z: 570.09 ([M + H]+).
OH), 5.44 (s, 1H, H-3), 5.26 (s, 1H), 4.70 (d, J = 14.2 Hz, 1H), 4.57 (d, J = 14.2 Hz, 1H), 3.93 (d, J = 10.5 Hz, 1H, H-23), 3.84 (d, J = 10.5 Hz, 1H, H-23), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.17 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.95 (s, 3H), 0.86–0.81 (3, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.4 (C28), 171.3 (OCO), 170.8 (OCO), 151.2 (C2), 138.6 (C13), 131.8 (C3), 125.5 (C12), 72.3, 62.6, 58.1, 52.7, 50.6, 48.1, 46.3, 43.1, 42.3, 41.1, 38.7, 38.7, 36.6, 33.7, 30.5, 28.2, 26.1, 24.0, 23.8, 21.1, 21.0 (2C), 19.1, 18.5, 17.8, 17.0, 16.6 ppm. DI-ESI-MS m/z: 570.09 ([M + H]+).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 10 as a white solid (136.3 mg, 63%). Mp: 85.4–88.0 °C. νmax/cm−1 (ATR): 3135.0, 2927.0, 2872.5, 1737.5, 1458.5, 1367.5, 1226.5, 1034.0. 1H NMR (400 MHz, CDCl3): δ = 8.23 (s, 1H, H2′), 7.52 (s, 1H, H5′), 7.03 (s, 1H, H4′), 5.43 (s, 1H, H-3), 5.19 (t, J = 3.0 Hz, 1H, H-12), 4.67 (d, J = 14.2 Hz, 1H), 4.54 (d, J = 14.2 Hz, 1H), 3.91 (d, J = 10.6 Hz, 1H, H-23), 3.82 (d, J = 10.6 Hz, 1H, H-23), 2.07 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.14 (s, 3H), 1.12 (s, 3H), 1.00–0.98 (m, 6H), 0.90 (d, J = 6.1 Hz, 3H), 0.74 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 174.7 (C28), 171.2 (OCO), 170.7 (OCO), 151.1 (C2), 137.7, 137.1, 132.0 (C3), 129.7, 126.1 (C12), 117.4, 72.2, 62.5, 58.1, 54.2, 50.9, 50.4, 46.3, 43.1, 42.4, 41.0, 39.0, 38.7, 35.5, 33.5, 30.3, 27.9, 26.1, 25.0, 23.8, 21.0, 20.9 (2C), 19.1, 18.3, 17.7, 17.1, 16.5 ppm. DI-ESI-MS m/z: 604.89 ([M + H]+). Calcd for C37H52N2O5·0.5H2O: C, 72.40; H, 8.70; N, 4.56. Found: C, 72.66; H, 8.77; N, 4.48.
1) to afford 10 as a white solid (136.3 mg, 63%). Mp: 85.4–88.0 °C. νmax/cm−1 (ATR): 3135.0, 2927.0, 2872.5, 1737.5, 1458.5, 1367.5, 1226.5, 1034.0. 1H NMR (400 MHz, CDCl3): δ = 8.23 (s, 1H, H2′), 7.52 (s, 1H, H5′), 7.03 (s, 1H, H4′), 5.43 (s, 1H, H-3), 5.19 (t, J = 3.0 Hz, 1H, H-12), 4.67 (d, J = 14.2 Hz, 1H), 4.54 (d, J = 14.2 Hz, 1H), 3.91 (d, J = 10.6 Hz, 1H, H-23), 3.82 (d, J = 10.6 Hz, 1H, H-23), 2.07 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.14 (s, 3H), 1.12 (s, 3H), 1.00–0.98 (m, 6H), 0.90 (d, J = 6.1 Hz, 3H), 0.74 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 174.7 (C28), 171.2 (OCO), 170.7 (OCO), 151.1 (C2), 137.7, 137.1, 132.0 (C3), 129.7, 126.1 (C12), 117.4, 72.2, 62.5, 58.1, 54.2, 50.9, 50.4, 46.3, 43.1, 42.4, 41.0, 39.0, 38.7, 35.5, 33.5, 30.3, 27.9, 26.1, 25.0, 23.8, 21.0, 20.9 (2C), 19.1, 18.3, 17.7, 17.1, 16.5 ppm. DI-ESI-MS m/z: 604.89 ([M + H]+). Calcd for C37H52N2O5·0.5H2O: C, 72.40; H, 8.70; N, 4.56. Found: C, 72.66; H, 8.77; N, 4.48.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 11 as a white solid (187.2 mg, 91%). Mp: 94.8–97.0 °C. νmax/cm−1 (ATR): 3418.5, 2927.0, 2870.5, 1739.5, 1635.0, 1527.0, 1454.5, 1376.5, 1235.5, 1033.5. 1H NMR (400 MHz, CDCl3): δ = 5.91 (d, J = 4.4 Hz, 1H, N
1) to afford 11 as a white solid (187.2 mg, 91%). Mp: 94.8–97.0 °C. νmax/cm−1 (ATR): 3418.5, 2927.0, 2870.5, 1739.5, 1635.0, 1527.0, 1454.5, 1376.5, 1235.5, 1033.5. 1H NMR (400 MHz, CDCl3): δ = 5.91 (d, J = 4.4 Hz, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH3), 5.45 (s, 1H, H-3), 5.28 (t, J = 3.0 Hz, 1H, H-12), 4.69 (d, J = 14.2 Hz, 1H), 4.57 (d, J = 14.2 Hz, 1H), 3.93 (d, J = 10.6 Hz, 1H, H-23), 3.84 (d, J = 10.6 Hz, 1H, H-23), 2.72 (d, J = 4.6 Hz, 3H, NHC
CH3), 5.45 (s, 1H, H-3), 5.28 (t, J = 3.0 Hz, 1H, H-12), 4.69 (d, J = 14.2 Hz, 1H), 4.57 (d, J = 14.2 Hz, 1H), 3.93 (d, J = 10.6 Hz, 1H, H-23), 3.84 (d, J = 10.6 Hz, 1H, H-23), 2.72 (d, J = 4.6 Hz, 3H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.17 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.94 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.82 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 178.7 (C28), 171.2 (OCO), 170.8 (OCO), 150.8 (C2), 141.1 (C13), 132.1 (C3), 124.9 (C12), 72.3, 62.5, 58.0, 53.9, 50.5, 47.6, 46.3, 43.1, 42.7, 41.0, 39.4, 39.1, 36.8, 33.3, 30.8, 28.0, 26.2, 26.2, 24.9, 23.6, 21.2, 21.0, 20.9, 19.0, 18.2, 17.2, 17.3, 16.6 ppm. DI-ESI-MS m/z: 568.52 ([M + H]+), 590.49 ([M + Na]+).
3), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.17 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.94 (s, 3H), 0.86 (d, J = 6.4 Hz, 3H), 0.82 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 178.7 (C28), 171.2 (OCO), 170.8 (OCO), 150.8 (C2), 141.1 (C13), 132.1 (C3), 124.9 (C12), 72.3, 62.5, 58.0, 53.9, 50.5, 47.6, 46.3, 43.1, 42.7, 41.0, 39.4, 39.1, 36.8, 33.3, 30.8, 28.0, 26.2, 26.2, 24.9, 23.6, 21.2, 21.0, 20.9, 19.0, 18.2, 17.2, 17.3, 16.6 ppm. DI-ESI-MS m/z: 568.52 ([M + H]+), 590.49 ([M + Na]+).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 12 as a white solid (113 mg, 72%). Mp: 83.5–86.0 °C. νmax/cm−1 (ATR): 3404.5, 2929.5, 2871.5, 1739.0, 1635.5, 1522.5, 1450.5, 1360.0, 1234.0, 1028.0. 1H NMR (400 MHz, CDCl3): δ = 5.81 (t, J = 4.4 Hz, 1H, N
1) to afford 12 as a white solid (113 mg, 72%). Mp: 83.5–86.0 °C. νmax/cm−1 (ATR): 3404.5, 2929.5, 2871.5, 1739.0, 1635.5, 1522.5, 1450.5, 1360.0, 1234.0, 1028.0. 1H NMR (400 MHz, CDCl3): δ = 5.81 (t, J = 4.4 Hz, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH2CH3), 5.45 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 4.69 (d, J = 14.3 Hz, 1H), 4.56 (d, J = 14.3 Hz, 1H), 3.93 (d, J = 10.6 Hz, 1H, H-23), 3.83 (d, J = 10.6 Hz, 1H, H-23), 3.34–3.24 (m, 1H, NHC
CH2CH3), 5.45 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 4.69 (d, J = 14.3 Hz, 1H), 4.56 (d, J = 14.3 Hz, 1H), 3.93 (d, J = 10.6 Hz, 1H, H-23), 3.83 (d, J = 10.6 Hz, 1H, H-23), 3.34–3.24 (m, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3), 3.15–3.05 (m, 1H, NHC
2CH3), 3.15–3.05 (m, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3), 2.08 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.17 (s, 3H), 1.11 (s, 3H), 1.08 (t, J = 7.4 Hz, 3H, NHCH2C
2CH3), 2.08 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.17 (s, 3H), 1.11 (s, 3H), 1.08 (t, J = 7.4 Hz, 3H, NHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 0.99 (s, 3H), 0.94 (s, 3H), 0.86–0.85 (m, 6H) ppm. 13C (100 MHz, CDCl3): δ = 177.8 (C28), 171.2 (OCO), 170.1 (OCO), 150.9 (C2), 140.9 (C13), 132.1 (C3), 124.9 (C12), 72.8, 62.5, 58.0, 54.0, 50.5, 47.4, 46.3, 43.1, 42.2, 41.1, 39.4, 39.0, 37.0, 34.3, 33.5, 30.8, 28.0, 26.2, 24.7, 23.4, 21.2, 21.0, 20.9, 19.0, 18.5, 17.8, 17.3, 16.6, 14.5 ppm. DI-ESI-MS m/z: 582.50 ([M + H]+), 604.51 ([M + Na]+). Calcd for C36H55NO5·0.25H2O: C, 73.74; H, 9.54; N, 2.39. Found: C, 73.65; H, 9.93; N, 2.43.
3), 0.99 (s, 3H), 0.94 (s, 3H), 0.86–0.85 (m, 6H) ppm. 13C (100 MHz, CDCl3): δ = 177.8 (C28), 171.2 (OCO), 170.1 (OCO), 150.9 (C2), 140.9 (C13), 132.1 (C3), 124.9 (C12), 72.8, 62.5, 58.0, 54.0, 50.5, 47.4, 46.3, 43.1, 42.2, 41.1, 39.4, 39.0, 37.0, 34.3, 33.5, 30.8, 28.0, 26.2, 24.7, 23.4, 21.2, 21.0, 20.9, 19.0, 18.5, 17.8, 17.3, 16.6, 14.5 ppm. DI-ESI-MS m/z: 582.50 ([M + H]+), 604.51 ([M + Na]+). Calcd for C36H55NO5·0.25H2O: C, 73.74; H, 9.54; N, 2.39. Found: C, 73.65; H, 9.93; N, 2.43.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 3
1 → 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 13 as a white solid (122 mg, 57%). Mp: 77.0–79.5 °C. νmax/cm−1 (ATR): 3368.5, 2959.0, 2921.5, 2872.5, 1739.5, 1635.0, 1525.0, 1455.5, 1369.0, 1234.5, 1033.5. 1H NMR (400 MHz, CDCl3): δ = 5.87 (t, J = 4.3 Hz, 1H, N
1) to afford 13 as a white solid (122 mg, 57%). Mp: 77.0–79.5 °C. νmax/cm−1 (ATR): 3368.5, 2959.0, 2921.5, 2872.5, 1739.5, 1635.0, 1525.0, 1455.5, 1369.0, 1234.5, 1033.5. 1H NMR (400 MHz, CDCl3): δ = 5.87 (t, J = 4.3 Hz, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH2CH2CH3), 5.44 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 4.68 (d, J = 14.2 Hz, 1H), 4.56 (d, J = 14.2 Hz, 1H), 3.92 (d, J = 10.6 Hz, 1H, H-23), 3.83 (d, J = 10.6 Hz, 1H, H-23), 3.32–3.24 (m, 1H, NHC
CH2CH2CH3), 5.44 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 4.68 (d, J = 14.2 Hz, 1H), 4.56 (d, J = 14.2 Hz, 1H), 3.92 (d, J = 10.6 Hz, 1H, H-23), 3.83 (d, J = 10.6 Hz, 1H, H-23), 3.32–3.24 (m, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2CH3), 2.99–2.92 (m, 1H, NHC
2CH2CH3), 2.99–2.92 (m, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2CH3), 2.07 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.16 (s, 3H), 1.11 (s, 3H), 0.99 (s, 3H), 0.94 (s, 3H), 0.89 (t, J = 7.5 Hz, 7.49 Hz, 3H, NHCH2CH2C
2CH2CH3), 2.07 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.16 (s, 3H), 1.11 (s, 3H), 0.99 (s, 3H), 0.94 (s, 3H), 0.89 (t, J = 7.5 Hz, 7.49 Hz, 3H, NHCH2CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 0.86 (d, J = 6.6 Hz, 3H), 0.84 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 177.8 (C28), 171.2 (OCO), 170.7 (OCO), 150.9 (C2), 140.9 (C13), 132.1 (C3), 124.9 (C12), 72.3, 62.5, 58.0, 54.0, 50.5, 47.6, 46.3, 43.1, 42.8, 41.1, 41.1, 39.4, 39.0, 37.1, 33.5, 30.8, 28.0, 26.2, 24.7, 23.5, 22.5, 21.2, 20.9, 20.9, 19.0, 18.5, 17.8, 17.3, 16.6, 11.5 ppm. DI-ESI-MS m/z: 596.54 ([M + H]+), 618.53 ([M + Na]+).
3), 0.86 (d, J = 6.6 Hz, 3H), 0.84 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 177.8 (C28), 171.2 (OCO), 170.7 (OCO), 150.9 (C2), 140.9 (C13), 132.1 (C3), 124.9 (C12), 72.3, 62.5, 58.0, 54.0, 50.5, 47.6, 46.3, 43.1, 42.8, 41.1, 41.1, 39.4, 39.0, 37.1, 33.5, 30.8, 28.0, 26.2, 24.7, 23.5, 22.5, 21.2, 20.9, 20.9, 19.0, 18.5, 17.8, 17.3, 16.6, 11.5 ppm. DI-ESI-MS m/z: 596.54 ([M + H]+), 618.53 ([M + Na]+).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 14 as a white solid (140.3 mg, 79%). Mp: 82.1–84.9 °C. νmax/cm−1 (ATR): 3367.0, 2923.0, 2870.5, 1739.5, 1635.5, 1516.5, 1455.5, 1376.0, 1235.0, 1031.0. 1H NMR (400 MHz, CDCl3): δ = 7.13 (s, 4H, Ar-H), 6.07 (t, J = 4.2 Hz, 1H, N
1) to afford 14 as a white solid (140.3 mg, 79%). Mp: 82.1–84.9 °C. νmax/cm−1 (ATR): 3367.0, 2923.0, 2870.5, 1739.5, 1635.5, 1516.5, 1455.5, 1376.0, 1235.0, 1031.0. 1H NMR (400 MHz, CDCl3): δ = 7.13 (s, 4H, Ar-H), 6.07 (t, J = 4.2 Hz, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH2ArCH3), 5.45 (s, 1H, H-3), 5.18 (t, J = 2.6 Hz, 1H, H-12), 4.67 (d, J = 14.2 Hz, 1H), 4.56 (d, J = 14.3 Hz, 1H), 4.49 (dd, J1 = 14.4 Hz, J2 = 5.9 Hz, 1H, NHC
CH2ArCH3), 5.45 (s, 1H, H-3), 5.18 (t, J = 2.6 Hz, 1H, H-12), 4.67 (d, J = 14.2 Hz, 1H), 4.56 (d, J = 14.3 Hz, 1H), 4.49 (dd, J1 = 14.4 Hz, J2 = 5.9 Hz, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2ArCH3), 4.13 (dd, J1 = 14.5 Hz, J2 = 4.3 Hz, 1H, NHC
2ArCH3), 4.13 (dd, J1 = 14.5 Hz, J2 = 4.3 Hz, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2ArCH3), 3.93 (d, J = 10.6 Hz, 1H, H-23), 3.84 (d, J = 10.6 Hz, 1H, H-23), 2.34 (s, 3H, NHCH2ArC
2ArCH3), 3.93 (d, J = 10.6 Hz, 1H, H-23), 3.84 (d, J = 10.6 Hz, 1H, H-23), 2.34 (s, 3H, NHCH2ArC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.14 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.94 (s, 3H), 0.84 (d, J = 6.6 Hz, 3H), 0.75 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.6 (C28), 171.2 (OCO), 170.8 (OCO), 150.9 (C2), 140.7, 137.1, 135.3 (Ar-C), 132.0 (C3), 129.3 (2C, Ar-C), 128.1 (2C, Ar-C), 125.1 (C12), 77.3, 62.5, 58.0, 54.0, 50.3, 47.7, 46.3, 43.4, 43.1, 42.8, 41.1, 39.4, 39.0, 37.1, 33.5, 30.8, 28.0, 26.1, 24.7, 23.5, 21.2, 21.1, 20.9 (2C), 19.0, 18.6, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 658.29 ([M + H]+), 680.40 ([M + Na]+). Calcd for C42H59NO5: C, 76.67; H, 9.04; N, 2.13. Found: C, 76.32; H, 9.14; N, 2.23.
3), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.14 (s, 3H), 1.11 (s, 3H), 1.00 (s, 3H), 0.94 (s, 3H), 0.84 (d, J = 6.6 Hz, 3H), 0.75 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.6 (C28), 171.2 (OCO), 170.8 (OCO), 150.9 (C2), 140.7, 137.1, 135.3 (Ar-C), 132.0 (C3), 129.3 (2C, Ar-C), 128.1 (2C, Ar-C), 125.1 (C12), 77.3, 62.5, 58.0, 54.0, 50.3, 47.7, 46.3, 43.4, 43.1, 42.8, 41.1, 39.4, 39.0, 37.1, 33.5, 30.8, 28.0, 26.1, 24.7, 23.5, 21.2, 21.1, 20.9 (2C), 19.0, 18.6, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 658.29 ([M + H]+), 680.40 ([M + Na]+). Calcd for C42H59NO5: C, 76.67; H, 9.04; N, 2.13. Found: C, 76.32; H, 9.14; N, 2.23.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 15 as a white solid (108.8 mg, 61%). Mp: 82.9–85.3 °C. νmax/cm−1 (ATR): 3413.0, 2928.0, 2870.5, 1739.0, 1635.5, 1509.5, 1455.0, 1381.0, 1220.5, 1033.5. 1H NMR (400 MHz, CDCl3): δ = 7.22–7.19 (m, 2H), 7.00 (t, J = 8.61 Hz, 2H), 6.13 (t, J = 5.1 Hz, 1H, N
1) to afford 15 as a white solid (108.8 mg, 61%). Mp: 82.9–85.3 °C. νmax/cm−1 (ATR): 3413.0, 2928.0, 2870.5, 1739.0, 1635.5, 1509.5, 1455.0, 1381.0, 1220.5, 1033.5. 1H NMR (400 MHz, CDCl3): δ = 7.22–7.19 (m, 2H), 7.00 (t, J = 8.61 Hz, 2H), 6.13 (t, J = 5.1 Hz, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH2ArF), 5.44 (s, 1H, H-3), 5.19 (t, J = 2.5 Hz, 1H, H-12), 4.67 (d, J = 14.3 Hz, 1H), 4.55 (d, J = 14.3 Hz, 1H), 4.49 (dd, J1 = 14.5 Hz, J2 = 5.9 Hz, 1H, NHC
CH2ArF), 5.44 (s, 1H, H-3), 5.19 (t, J = 2.5 Hz, 1H, H-12), 4.67 (d, J = 14.3 Hz, 1H), 4.55 (d, J = 14.3 Hz, 1H), 4.49 (dd, J1 = 14.5 Hz, J2 = 5.9 Hz, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2ArF), 4.14 (dd, J1 = 14.9 Hz, J2 = 4.7 Hz, 14.6 Hz, 1H, NHC
2ArF), 4.14 (dd, J1 = 14.9 Hz, J2 = 4.7 Hz, 14.6 Hz, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2ArF), 3.92 (d, J = 10.6 Hz, 1H, H-23), 3.83 (d, J = 10.7 Hz, 1H, H-23), 2.07 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.13 (s, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.94 (s, 3H), 0.84 (d, J = 6.2 Hz, 3H), 0.72 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.8 (C28), 171.2 (OCO), 170.7 (OCO), 162.1 (d, J = 245.2 Hz, C4′′), 150.9 (C2), 140.7 (C13), 134.2 (C1′′), 132.1 (C3), 129.6, 129.5, 125.2 (C12), 115.5, 115.3, 72.2, 62.5, 58.0, 54.0, 50.5, 47.7, 46.3, 43.1, 42.9, 42.7, 41.1, 39.4, 39.0, 37.2, 33.5, 30.8, 28.0, 26.1, 24.7, 23.4, 21.1, 20.9 (2C), 19.0, 18.5, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 662.40 ([M + H]+). Calcd for C41H56FNO5·0.25H2O: C, 73.90; H, 8.55; N, 2.10. Found: C, 73.95; H, 8.60; N, 2.23.
2ArF), 3.92 (d, J = 10.6 Hz, 1H, H-23), 3.83 (d, J = 10.7 Hz, 1H, H-23), 2.07 (s, 3H, OCOCH3), 2.05 (s, 3H, OCOCH3), 1.13 (s, 3H), 1.10 (s, 3H), 0.99 (s, 3H), 0.94 (s, 3H), 0.84 (d, J = 6.2 Hz, 3H), 0.72 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.8 (C28), 171.2 (OCO), 170.7 (OCO), 162.1 (d, J = 245.2 Hz, C4′′), 150.9 (C2), 140.7 (C13), 134.2 (C1′′), 132.1 (C3), 129.6, 129.5, 125.2 (C12), 115.5, 115.3, 72.2, 62.5, 58.0, 54.0, 50.5, 47.7, 46.3, 43.1, 42.9, 42.7, 41.1, 39.4, 39.0, 37.2, 33.5, 30.8, 28.0, 26.1, 24.7, 23.4, 21.1, 20.9 (2C), 19.0, 18.5, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 662.40 ([M + H]+). Calcd for C41H56FNO5·0.25H2O: C, 73.90; H, 8.55; N, 2.10. Found: C, 73.95; H, 8.60; N, 2.23.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 16 as a white solid (179 mg, 79%). Mp: 74.5–76.6 °C. νmax/cm−1 (ATR): 3395.0, 2923.5, 2871.5, 1739.0, 1650.0, 1520.5, 1451.0, 1368.0, 1234.0, 1031.0. 1H NMR (400 MHz, CDCl3): δ = 6.50 (t, J = 4.1 Hz, 1H, –N
1) to afford 16 as a white solid (179 mg, 79%). Mp: 74.5–76.6 °C. νmax/cm−1 (ATR): 3395.0, 2923.5, 2871.5, 1739.0, 1650.0, 1520.5, 1451.0, 1368.0, 1234.0, 1031.0. 1H NMR (400 MHz, CDCl3): δ = 6.50 (t, J = 4.1 Hz, 1H, –N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH2COOCH3), 5.44 (s, 1H, H-3), 5.38 (t, J = 3.1 Hz, 1H, H-12), 4.69 (d, J = 14.3 Hz, 1H), 4.57 (d, J = 14.2 Hz, 1H), 4.09 (dd, J1 = 18.5 Hz, J2 = 5.3 Hz and, 1H, –NHC
CH2COOCH3), 5.44 (s, 1H, H-3), 5.38 (t, J = 3.1 Hz, 1H, H-12), 4.69 (d, J = 14.3 Hz, 1H), 4.57 (d, J = 14.2 Hz, 1H), 4.09 (dd, J1 = 18.5 Hz, J2 = 5.3 Hz and, 1H, –NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2COOCH3), 3.93 (d, J = 10.5 Hz, 1H, H-23), 3.84 (dd, J1 = 18.5 Hz, J2 = 3.9 Hz, 1H, –NHC
2COOCH3), 3.93 (d, J = 10.5 Hz, 1H, H-23), 3.84 (dd, J1 = 18.5 Hz, J2 = 3.9 Hz, 1H, –NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2COOCH3), 3.83 (d, J = 10.7 Hz, 1H, H-23), 3.75 (s, 3H, –NHCH2COOC
2COOCH3), 3.83 (d, J = 10.7 Hz, 1H, H-23), 3.75 (s, 3H, –NHCH2COOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.15 (s, 3H), 1.12 (s, 3H), 0.99 (s, 3H), 0.95 (s, 3H), 0.88 (d, J = 6.3 Hz, 3H), 0.77 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.1, 171.2, 170.7 (OCO), 170.6 (OCO), 151.0 (C2), 140.0 (C13), 131.9 (C3), 125.7 (C12), 72.3, 62.5, 58.0, 53.7, 52.3, 50.5, 47.7, 46.3, 43.2, 42.6, 41.5, 41.1, 39.4, 39.0, 36.9, 33.5, 30.8, 28.0, 26.3, 24.8, 23.6, 21.2, 21.0, 21.0, 19.0, 18.0, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 626.27 ([M + H]+), 648.42 ([M + Na]+).
3), 2.08 (s, 3H, OCOCH3), 2.06 (s, 3H, OCOCH3), 1.15 (s, 3H), 1.12 (s, 3H), 0.99 (s, 3H), 0.95 (s, 3H), 0.88 (d, J = 6.3 Hz, 3H), 0.77 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.1, 171.2, 170.7 (OCO), 170.6 (OCO), 151.0 (C2), 140.0 (C13), 131.9 (C3), 125.7 (C12), 72.3, 62.5, 58.0, 53.7, 52.3, 50.5, 47.7, 46.3, 43.2, 42.6, 41.5, 41.1, 39.4, 39.0, 36.9, 33.5, 30.8, 28.0, 26.3, 24.8, 23.6, 21.2, 21.0, 21.0, 19.0, 18.0, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 626.27 ([M + H]+), 648.42 ([M + Na]+).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 3
1 → 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 17 as a white solid (140 mg, 61%). Mp: 68.9–71.6 °C. νmax/cm−1 (ATR): 3405.0, 2925.0, 2872.0, 1738.0, 1655.5, 1507.5, 1448.5, 1371.5, 1234.5, 1028.0. 1H NMR (400 MHz, CDCl3): δ = 6.59 (d, J = 5.8 Hz, 1H, N
1) to afford 17 as a white solid (140 mg, 61%). Mp: 68.9–71.6 °C. νmax/cm−1 (ATR): 3405.0, 2925.0, 2872.0, 1738.0, 1655.5, 1507.5, 1448.5, 1371.5, 1234.5, 1028.0. 1H NMR (400 MHz, CDCl3): δ = 6.59 (d, J = 5.8 Hz, 1H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH(CH3)COOH), 5.43 (s, 1H, H-3), 5.36 (t, J = 3.0 Hz, 1H, H-12), 4.69 (d, J = 14.4 Hz, 1H), 4.57 (d, J = 14.4 Hz, 1H), 4.50–4.42 (m, 1H, NHC
CH(CH3)COOH), 5.43 (s, 1H, H-3), 5.36 (t, J = 3.0 Hz, 1H, H-12), 4.69 (d, J = 14.4 Hz, 1H), 4.57 (d, J = 14.4 Hz, 1H), 4.50–4.42 (m, 1H, NHC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)COOCH3), 3.93 (d, J = 10.7 Hz, 1H, H-23), 3.83 (d, J = 10.6 Hz, 1H, H-23), 3.73 (s, 3H, NHCH(CH3)COOC
(CH3)COOCH3), 3.93 (d, J = 10.7 Hz, 1H, H-23), 3.83 (d, J = 10.6 Hz, 1H, H-23), 3.73 (s, 3H, NHCH(CH3)COOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.08 (s, 3H, OCOC
3), 2.08 (s, 3H, OCOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.05 (s, 3H, OCOC
3), 2.05 (s, 3H, OCOC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.36 (d, J = 7.0 Hz, 3H, NHCH(C
3), 1.36 (d, J = 7.0 Hz, 3H, NHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)COOCH3), 1.15 (s, 3H), 1.11 (s, 3H), 0.98 (s, 3H), 0.95 (s, 3H), 0.88 (d, J = 6.4 Hz, 3H), 0.75 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 177.3, 173.7, 171.2 (OCO), 170.2 (OCO), 151.1 (C2), 139.3 (C13), 131.7 (C3), 125.8 (C12), 77.3, 62.5, 58.0, 53.6, 52.4, 50.6, 48.2, 47.6, 46.3, 43.2, 42.6, 41.2, 39.3, 39.0, 37.2, 33.6, 30.8, 28.0, 26.3, 24.6, 23.5, 21.2, 21.0 (2C), 19.0, 18.7, 18.1, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 640.20 ([M + H]+).
3)COOCH3), 1.15 (s, 3H), 1.11 (s, 3H), 0.98 (s, 3H), 0.95 (s, 3H), 0.88 (d, J = 6.4 Hz, 3H), 0.75 (s, 3H) ppm. 13C (100 MHz, CDCl3): δ = 177.3, 173.7, 171.2 (OCO), 170.2 (OCO), 151.1 (C2), 139.3 (C13), 131.7 (C3), 125.8 (C12), 77.3, 62.5, 58.0, 53.6, 52.4, 50.6, 48.2, 47.6, 46.3, 43.2, 42.6, 41.2, 39.3, 39.0, 37.2, 33.6, 30.8, 28.0, 26.3, 24.6, 23.5, 21.2, 21.0 (2C), 19.0, 18.7, 18.1, 17.8, 17.2, 16.6 ppm. DI-ESI-MS m/z: 640.20 ([M + H]+).4.2 Biology
Asiatic acid and its derivatives were suspended in dimethyl sulfoxide (DMSO) to prepare 20 mM stock solutions that were stored at −80 °C. To obtain final assay concentrations, the stock solutions were diluted in culture medium. The final concentration of DMSO in working solutions was always equal or lower than 0.5%.
For Jurkat cells, the cell viability was determined by XTT assay. Briefly, 4 × 103 cells per well were seeded in 96 well plates with 100 μL of medium. After 24 h of incubation, 100 μL of new medium containing the tested compounds at different concentrations was added. After an incubation period of 72 hours, 100 μL of XTT solution were added to each well and the plates were incubated for 4 hours at 37 °C. The relative cell viability, compared with the viability of non treated cells, was analyzed by measuring the absorbance at 450 nm on an ELISA plate reader (Tecan Sunrise MR20-301, TECAN, Salzburg, Austria).
Acknowledgements
Jorge A. R. Salvador thanks Universidade de Coimbra for financial support. Bruno M. F. Goncalves thanks Fundação para a Ciência e a Tecnologia for financial support (SFRH/BD/69193/2010). MC and SM thanks MICINN of Spain and FEDER Funds (grant number SAF2011-25726) and Agència Catalana d'Ajuts Universitaris I de Recerca (AGAUR) (2014SGR1017). MC thanks also ICREA Foundation (Icrea Academia Award programme, Generalitat de Catalunya). The authors would like to acknowledge UC-NMR facility, which is supported in part by FEDER – European Regional Development Fund through the COMPETE Programme (Operational Programme for Competitiveness) and by National Funds through FCT – Fundação para a Ciência e a Tecnologia (Portuguese Foundation for Science and Technology) through grants REEQ/481/QUI/2006, RECI/QEQ-QFI/0168/2012, CENTRO-07-CT62-FEDER-002012, and Rede Nacional de Ressonância Magnética Nuclear (RNRMN), for NMR data. The authors are grateful to Laboratory of Mass Spectrometry (LEM) of the Node CEF/UC integrated in the National Mass Spectrometry Network (RNEM) of Portugal, for the MS analyses. We acknowledge Fundação para a Ciência e Tecnologia (FCT) for the project REDE/1501/REM/2005 and Dr Carlos Cordeiro for providing data from the FTICR-MS at the Faculdade de Ciências da Universidade de Lisboa, Portugal. The authors would like to acknowledge Centro de Apoio Científico e Tecnolóxico á Investigación (C.A.C.T.I.), Universidade de Vigo, Campos Lagoas – Marcosende, 15, 36310 Vigo, for elemental analysis and CCIT-scientific and technological centers UB for flow cytometry analysis support.Notes and references
- P. Dzubak, M. Hajduch, D. Vydra, A. Hustova, M. Kvasnica, D. Biedermann, L. Markova, M. Urban and J. Sarek, Nat. Prod. Rep., 2006, 23, 394–411 RSC.
- R.-Y. Kuo, K. Qian, S. L. Morris-Natschke and K.-H. Lee, Nat. Prod. Rep., 2009, 26, 1321–1344 RSC.
- H. Sheng and H. Sun, Nat. Prod. Rep., 2011, 28, 543–593 RSC.
- J. A. R. Salvador, V. M. Moreira, B. M. F. Gonçalves, A. S. Leal and Y. Jing, Nat. Prod. Rep., 2012, 29, 1463–1479 RSC.
- J. A. R. Salvador, A. S. Leal, D. P. S. Alho, B. M. F. Gonçalves, A. S. Valdeira, V. I. S. Mendes and Y. Jing, Stud. Nat. Prod. Chem., 2014, 41, 33–73 CAS.
- M. K. Shanmugam, A. H. Nguyen, A. P. Kumar, B. K. H. Tan and G. Sethi, Cancer Lett., 2012, 320, 158–170 CrossRef PubMed.
- S. M. Kamble, S. N. Goyal and C. R. Patil, RSC Adv., 2014, 4, 33370 RSC.
- M. K. Shanmugam, X. Dai, A. P. Kumar, B. K. H. Tan, G. Sethi and A. Bishayee, Cancer Lett., 2014, 346, 206–216 CrossRef CAS PubMed.
- J. Fu, Y. Zou, Z. Huang, C. Yan, Q. Zhou, H. Zhang, Y. Lai, S. Peng and Y. Zhang, RSC Adv., 2015, 5, 19445–19454 RSC.
- K. Tang, J. Huang, J. Pan, X. Zhang and W. Lu, RSC Adv., 2015, 5, 19620–19623 RSC.
- B. M. F. Gonçalves, J. A. R. Salvador, S. Marín and M. Cascante, RSC Adv., 2016, 6, 3967–3985 RSC.
- A. D. Widgerow, L. A. Chait, R. Stals and P. J. Stals, Aesthetic Plast. Surg., 2000, 24, 227–234 CrossRef CAS PubMed.
- B.-S. Jeong, Arch. Pharmacal Res., 2006, 29, 556–562 CrossRef CAS.
- A. de Fátima, L. V. Modolo, A. C. C. Sanches and R. R. Porto, Mini-Rev. Med. Chem., 2008, 8, 879–888 CrossRef.
- J. Liu, T. He, Q. Lu, J. Shang, H. Sun and L. Zhang, Diabetes/Metab. Res. Rev., 2010, 26, 448–454 CrossRef PubMed.
- K.-J. Yun, J.-Y. Kim, J.-B. Kim, K.-W. Lee, S.-Y. Jeong, H.-J. Park, H.-J. Jung, Y.-W. Cho, K. Yun and K.-T. Lee, Int. Immunopharmacol., 2008, 8, 431–441 CrossRef CAS PubMed.
- S.-S. Huang, C.-S. Chiu, H.-J. Chen, W.-C. Hou, M.-J. Sheu, Y.-C. Lin, P.-H. Shie and G.-J. Huang, Evid. Base. Compl. Alternative Med., 2011, 2011, 895857 Search PubMed.
- R. G. Krishnamurthy, M.-C. Senut, D. Zemke, J. Min, M. B. Frenkel, E. J. Greenberg, S.-W. Yu, N. Ahn, J. Goudreau, M. Kassab, K. S. Panickar and A. Majid, J. Neurosci. Res., 2009, 87, 2541–2550 CrossRef CAS PubMed.
- Y. Xiong, H. Ding, M. Xu and J. Gao, Neurochem. Res., 2009, 34, 746–754 CrossRef CAS PubMed.
- K. Ma, Y. Zhang, D. Zhu and Y. Lou, Eur. J. Pharmacol., 2009, 603, 98–107 CrossRef CAS PubMed.
- Y. S. Lee, D.-Q. Jin, E. J. Kwon, S. H. Park, E.-S. Lee, T. C. Jeong, D. H. Nam, K. Huh and J.-A. Kim, Cancer Lett., 2002, 186, 83–91 CrossRef CAS PubMed.
- B. C. Park, K. O. Bosire, E.-S. Lee, Y. S. Lee and J.-A. Kim, Cancer Lett., 2005, 218, 81–90 CrossRef CAS PubMed.
- D. M. Gurfinkel, S. Chow, R. Hurren, M. Gronda, C. Henderson, C. Berube, D. W. Hedley and A. D. Schimmer, Apoptosis, 2006, 11, 1463–1471 CrossRef CAS PubMed.
- Y. Hsu, P. Kuo, L. Lin and C. Lin, J. Pharmacol. Exp. Ther., 2005, 313, 333–344 CrossRef CAS PubMed.
- C. W. Cho, D. S. Choi, M. H. Cardone, C. W. Kim, A. J. Sinskey and C. Rha, Cell Biol. Toxicol., 2006, 22, 393–408 CrossRef CAS PubMed.
- X.-L. Tang, X.-Y. Yang, H.-J. Jung, S.-Y. Kim, S.-Y. Jung, D.-Y. Choi, W.-C. Park and H. Park, Biol. Pharm. Bull., 2009, 32, 1399–1405 CAS.
- C. V. Kavitha, C. Agarwal, R. Agarwal and G. Deep, PLoS One, 2011, 6, e22745 CAS.
- P. Bunpo, K. Kataoka, H. Arimochi, H. Nakayama, T. Kuwahara, U. Vinitketkumnuen and Y. Ohnishi, J. Exp. Med., 2005, 52, 65–73 Search PubMed.
- Y. Meng, Y. Li, F. Li, Y. Song, H. Wang, H. Chen and B. Cao, J. Asian Nat. Prod. Res., 2012, 14, 844–855 CrossRef CAS PubMed.
- J.-F. Li, R.-Z. Huang, G.-Y. Yao, M.-Y. Ye, H.-S. Wang, Y.-M. Pan and J.-T. Xiao, Eur. J. Med. Chem., 2014, 86, 175–188 CrossRef CAS PubMed.
- Y. Jing, G. Wang, Y. Ge, M. Xu and Z. Gong, Molecules, 2015, 20, 7309–7324 CrossRef CAS PubMed.
- D. Pal and S. Saha, J. Adv. Pharm. Technol. Res., 2012, 3, 92–99 CrossRef CAS PubMed.
- N. Saban and M. Bujak, Cancer Chemother. Pharmacol., 2009, 64, 213–221 CrossRef CAS PubMed.
- J. Jiang, A. Thyagarajan-Sahu, V. Krchňák, A. Jedinak, G. E. Sandusky and D. Sliva, PLoS One, 2012, 7, e34283 CAS.
- Y. Zhang, J. Feng, Y. Jia, X. Wang, L. Zhang, C. Liu, H. Fang and W. Xu, J. Med. Chem., 2011, 54, 2823–2838 CrossRef CAS PubMed.
- B. Singh and R. P. Rastogi, Phytochemistry, 1969, 8, 917–921 CrossRef CAS.
- C. Stanetty, L. Czollner, I. Koller, P. Shah, R. Gaware, T. Da Cunha, A. Odermatt, U. Jordis, P. Kosma and D. Classen-Houben, Bioorg. Med. Chem., 2010, 18, 7522–7541 CrossRef CAS PubMed.
- R. Gaware, R. Khunt, L. Czollner, C. Stanetty, T. Da Cunha, D. V. Kratschmar, A. Odermatt, P. Kosma, U. Jordis and D. Claßen-Houben, Bioorg. Med. Chem., 2011, 19, 1866–1880 CrossRef CAS PubMed.
- N. Usachova, G. Leitis, A. Jirgensons and I. Kalvinsh, Synth. Commun., 2010, 40, 927–935 CrossRef CAS.
- R. C. Santos, J. A. R. Salvador, S. Marín and M. Cascante, Bioorg. Med. Chem., 2009, 17, 6241–6250 CrossRef CAS PubMed.
- A. S. Leal, R. Wang, J. A. R. Salvador and Y. Jing, Bioorg. Med. Chem., 2012, 20, 5774–5786 CrossRef CAS PubMed.
- A. S. Leal, R. Wang, J. A. R. Salvador and Y. Jing, Org. Biomol. Chem., 2013, 11, 1726–1738 CAS.
- F. Chu, X. Xu, G. Li, S. Gu, K. Xu, Y. Gong, B. Xu, M. Wang, H. Zhang, Y. Zhang, P. Wang and H. Lei, Molecules, 2014, 19, 18215–18231 CrossRef PubMed.
- M. Antunovic, B. Kriznik, E. Ulukaya, V. T. Yilmaz, K. C. Mihalic, J. Madunic and I. Marijanovic, Anticancer Drugs, 2015, 26, 180–186 CrossRef CAS PubMed.
- S. Dhar, W. L. Daniel, D. A. Giljohann, C. A. Mirkin and S. J. Lippard, J. Am. Chem. Soc., 2009, 131, 14652–14653 CrossRef CAS PubMed.
- J. F. R. Kerr, A. H. Wyllie and A. R. Currie, Br. J. Cancer, 1972, 26, 239–257 CrossRef CAS PubMed.
- R. S. Wong, J. Exp. Clin. Cancer Res., 2011, 30, 87 CrossRef CAS PubMed.
- D. Hanahan, Cell, 2000, 100, 57–70 CrossRef CAS PubMed.
- S. Kasibhatla and B. Tseng, Mol. Cancer Ther., 2003, 2, 573–580 CAS.
- S. Fulda and K.-M. Debatin, Oncogene, 2006, 25, 4798–4811 CrossRef CAS PubMed.
- I. Vermes, C. Haanen, H. Steffens-Nakken and C. Reutelingsperger, J. Immunol. Methods, 1995, 184, 39–51 CrossRef CAS PubMed.
- T. Y. K. Takara, Y. Obata, E. Yoshikawa, N. Kitada, T. Sakaeda and N. Ohnishi, Cancer Chemother. Pharmacol., 2006, 58, 785–793 CrossRef PubMed.
- R. Cortés, M. Tarrado-Castellarnau, D. Talancón, C. López, W. Link, D. Ruiz, J. J. Centelles, J. Quirante and M. Cascante, Metallomics, 2014, 622–633 RSC.
- T.-C. Chou, Cancer Res., 2010, 70, 440–446 CrossRef CAS PubMed.
- T. C. Chou and P. Talalay, Adv. Enzyme Regul., 1984, 22, 27–55 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04597a |
| This journal is © The Royal Society of Chemistry 2016 |