AlCl3-mediated heteroarylation-cyclization strategy: one-pot synthesis of fused quinoxalines containing the central core of Lamellarin D†
K. Shiva Kumar*a,
Siddi Ramulu Meesaa,
Bandari Rajeshama,
Boyapally Bhaskera,
Mohd Ashraf Ashfaqb,
Aleem Ahmed Khanb,
Sagurthi Someswar Raoc and
Manojit Pal*d
aDepartment of Chemistry, Osmania University, Hyderabad-500 007, India. E-mail: shivakumarkota@yahoo.co.in; Tel: +91 40 27682337
bCentral Laboratory for Stem Cell Research and Translational Medicine, CLRD Deccan Colleges of Medical Sciences, Kanchanbagh, Hyderabad-500 058, India
cDepartment of Genetics & Biotechnology, Osmania University, Hyderabad, India 500 007
dDr Reddy's Institute of Life Sciences, Hyderabad Central University, Campus, Gachibowli, Hyderabad-500 046, India. E-mail: manojitpal@rediffmail.com; Tel: +91 40 6657 1500
First published on 10th May 2016
Abstract
An inexpensive, practical and one-pot method has been developed for the synthesis of quinoxalines fused with pyrano[3,4-b]indole, the central core of Lamellarin D. The methodology involved construction of the central pyranone ring via an AlCl3-mediated heteroarylation-cyclization method. A number of compounds were prepared using this methodology, some of which were converted to the corresponding indol-3-ylquinoxaline derivatives. Several of the pyrano[3,4-b]indole fused quinoxalines showed promising growth inhibition of cervical and lung cancer cells and good interactions with topoisomerase I in silico.
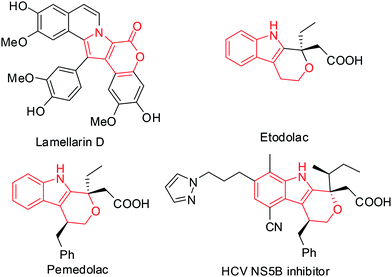
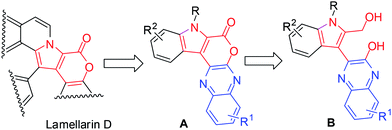
The pyrano[3,4-b]indole framework has been found to be an integral part of several bioactive compounds and drugs. For example, it is a central part of Lamellarin D (Fig. 1),1 a potent inhibitor of topoisomerase I that has shown strong cytotoxic activity against tumor cell lines.2 Indeed, compounds containing the pyrano[3,4-b]indole skeleton have also been developed as anti-inflammatory (e.g. Etodolac)3 and analgesic agents (e.g. Pemedolac)4 (Fig. 1). Very recently, pyrano[3,4-b]indole derivatives have been reported to be promising inhibitors of hepatitis C virus (HCV) NS5B polymerase5 (Fig. 1) and useful for the potential treatment of lymphoma.6 Quinoxalines, on the other hand are considered as privileged structures in the area of drug discovery and are known to be useful as anticancer drugs7 e.g. NSC 339004. Thus the combination of the structural features of both pyrano[3,4-b]indole (the central core of Lamellarin D) and quinoxaline in a single molecular entity represented by A (Fig. 2) and its modified form B (Fig. 2) was expected to provide new chemical space that might lead to the identification of novel molecules possessing noteworthy pharmacological properties. Prompted by this idea and due to our continuing interest in the identification of novel and potential cytotoxic agents,8 we decided to synthesize and evaluate the pyrano[3,4-b]indole fused quinoxaline derivatives for their anticancer properties. Notably, cancer is the second leading cause of death9 worldwide and therefore the search for new anticancer agents is desirable. We now report a one-step synthesis of pyrano[3,4-b]indole fused quinoxaline derivatives via an AlCl3 induced heteroarylation-cyclization method and evaluation of their potential anticancer properties.
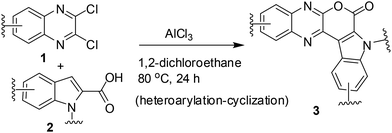
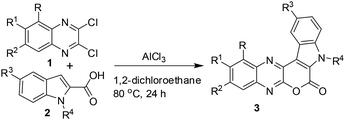
A number of methods have been reported for the synthesis of pyrano[3,4-b]indole derivatives1,10 that include (i) Pd-mediated construction of the chromeno[3,4-b]indole ring (ii) two-step procedure involving Sonogashira coupling of dihydropyran propargyl ether with iodoanilines followed by intramolecular Friedel–Crafts cyclization in the presence of Sc(OTf)3 and (iii) saponification of ethyl-3-(2-bromophenyl)-1H-pyrrole-2-carboxylate followed by a Cu-mediated Ullmann-type ring closure onto a bromide. All these approaches however require 2–3 step synthesis or harsh reaction conditions or the use of special reagents/expensive transition metal catalysts. We therefore decided to develop an inexpensive, practical and one-pot method for the synthesis of pyrano[3,4-b]indole fused quinoxaline derivatives. Recently, we have observed that derivatives of 2,3-dichloroquinoxaline can react smoothly with indole-2-carboxylic acids in the presence of AlCl3 to give pyrano[3,4-b]indole fused quinoxalines in good yields (Scheme 1). The reaction seems to proceed via AlCl3 induced C–C bond (heteroarylation) followed by C–O bond formation (cyclization) in a single pot. To the best of our knowledge the present heteroarylation-cyclization strategy and its use for the synthesis of pyrano[3,4-b]indole fused quinoxalines is not known in the literature. The preliminary results of this study are presented.
The AlCl3-induced C–C bond forming reaction between heteroaryl chlorides containing –C(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– moiety and various arenes or heteroarenes has been explored by us earlier.11 Recently we reported a one pot synthesis of benzofuran fused N-heterocycles via C–C followed by C–O bond formation strategy.11d We envisaged that a similar type of reaction between 2,3-dichloroquinoxaline that contains a –N
N– moiety and various arenes or heteroarenes has been explored by us earlier.11 Recently we reported a one pot synthesis of benzofuran fused N-heterocycles via C–C followed by C–O bond formation strategy.11d We envisaged that a similar type of reaction between 2,3-dichloroquinoxaline that contains a –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Cl)–C(Cl)
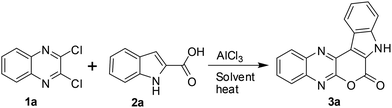
C(Cl)–C(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– moiety and indole-2-carboxylic acids may proceed via initial heteroarylation followed by subsequent cyclization in the same pot. Accordingly, 2,3-dichloroquinoxaline12 (1a, 1.0 equiv.) was reacted with indole-2-carboxylic acids (2a, 1.0 equiv.) in 1,2-dichloroethane (5 mL) in the presence of 2.25 equiv. of AlCl3 at 80 °C for 8 h, when the product 3a was isolated in 33% yield (entry 1, Table 1). The yield of 3a was increased to 85% when the reaction time was increased to 24 h (entry 2, Table 1). We were delighted with this observation that prompted us to proceed for further study. The increase of reaction time did not improve the yield of 3a (entry 3, Table 1) whereas the use of lower quantity of AlCl3 (ref. 13) decreased the yield significantly (entry 4, Table 1). We also examined the use of other solvents such as CHCl3, EtOAc, MeCN, and toluene. However, none of these solvents were found to be effective in terms of product yield (entries 5–8, Table 1).
N– moiety and indole-2-carboxylic acids may proceed via initial heteroarylation followed by subsequent cyclization in the same pot. Accordingly, 2,3-dichloroquinoxaline12 (1a, 1.0 equiv.) was reacted with indole-2-carboxylic acids (2a, 1.0 equiv.) in 1,2-dichloroethane (5 mL) in the presence of 2.25 equiv. of AlCl3 at 80 °C for 8 h, when the product 3a was isolated in 33% yield (entry 1, Table 1). The yield of 3a was increased to 85% when the reaction time was increased to 24 h (entry 2, Table 1). We were delighted with this observation that prompted us to proceed for further study. The increase of reaction time did not improve the yield of 3a (entry 3, Table 1) whereas the use of lower quantity of AlCl3 (ref. 13) decreased the yield significantly (entry 4, Table 1). We also examined the use of other solvents such as CHCl3, EtOAc, MeCN, and toluene. However, none of these solvents were found to be effective in terms of product yield (entries 5–8, Table 1).
| Entry | Solvent | Timeb (h) | % yieldc |
|---|---|---|---|
| a All the reactions were carried out using compound 1a (1.0 equiv.), 2a (1.0 equiv.) and AlCl3 (2.25 equiv.) in a solvent (5 mL) at 80 °C.b Total time taken to yield the product 3a.c Isolated yield.d The reaction was carried out 1.25 equiv. of AlCl3. | |||
| 1 | ClCH2CH2Cl | 8 | 33 |
| 2 | ClCH2CH2Cl | 24 | 85 |
| 3 | ClCH2CH2Cl | 36 | 80 |
| 4 | ClCH2CH2Cl | 24 | 35d |
| 5 | CHCl3 | 24 | 55 |
| 6 | EtOAc | 24 | 15 |
| 7 | CH3CN | 24 | 26 |
| 8 | Toluene | 24 | 42 |
Thus, a combination of AlCl3 in 1,2-dichloroethane at 80 °C (entry 2, Table 1) was found to be optimal for the preparation of 3a and was used to prepare its other analogues for in vitro pharmacological studies.
A small library of desired compounds (3) were prepared (Table 2) by reacting a number of 2,3-dichloroquinoxalines (1) with various indole-2-carboxylic acids (2a–f) using the optimized reaction conditions. The reaction proceeded well in all these cases irrespective of presence or absence of a substituent on the indole nitrogen of reactant 2. Generally, the products were isolated in good to acceptable yields and were characterized by spectral data. The compound 3a was characterized by the appearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal at 1708 cm−1 in IR and at 153.5 ppm in 13C NMR spectra. Notably, the starting indole-2-carboxylic acid (2a), showed O–H stretching frequency at 2995 cm−1 in the IR and a C
O signal at 1708 cm−1 in IR and at 153.5 ppm in 13C NMR spectra. Notably, the starting indole-2-carboxylic acid (2a), showed O–H stretching frequency at 2995 cm−1 in the IR and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal at 161.7 ppm in the 13C NMR spectra.
O signal at 161.7 ppm in the 13C NMR spectra.
| Entry | Quinoxaline 1; R, R1, R2 | Indole acids 2; R3 R4 | Product 3; % yieldb |
|---|---|---|---|
| a All the reactions were carried out using compound 1 (1.0 equiv.), 2 (1.0 equiv.) and AlCl3 (2.25 equiv.) in a DCE (5 mL) at 80 °C for 24 h.b Isolated yield. | |||
| 1 | 1a; H, H, H | 2a; H, H | 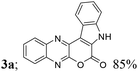 |
| 2 | 1a | 2b; H, Me | 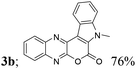 |
| 3 | 1a | 2c; H, Et | 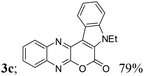 |
| 4 | 1a | 2d; H, CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
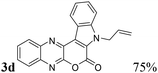 |
| 5 | 1a | 2e; F, H | 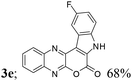 |
| 6 | 1a | 2f; Me, H | 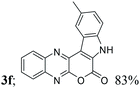 |
| 7 | 1b; H, Me, H | 2a | 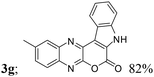 |
| 8 | 1b | 2b | 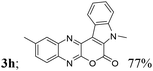 |
| 9 | 1c; H, Me, Me | 2a | 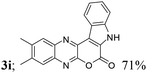 |
| 10 | 1c | 2c | 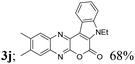 |
| 11 | 1d; Me, H, Me | 2a | 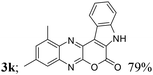 |
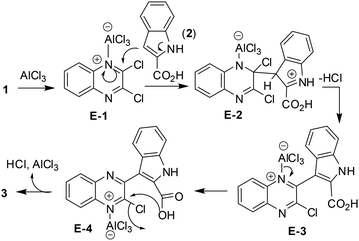
Mechanistically, the reaction seems to proceed (Scheme 2) via (i) complexation of one of the azomethine nitrogen [–C(Cl)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–] of 1 with AlCl3 to give E-1, (ii) then nucleophilic attack at the adjacent chlorine bearing carbon by indole C-3 of 2 to give E-2, (iii) release of HCl to afford E-3, (iv) which on release of AlCl3 followed by intramolecular cyclization via E-4 aided by AlCl3 gave 3.
N–] of 1 with AlCl3 to give E-1, (ii) then nucleophilic attack at the adjacent chlorine bearing carbon by indole C-3 of 2 to give E-2, (iii) release of HCl to afford E-3, (iv) which on release of AlCl3 followed by intramolecular cyclization via E-4 aided by AlCl3 gave 3.
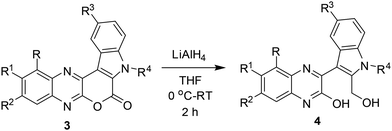
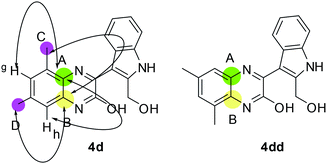
Having synthesized a number of pyrano[3,4-b]indole fused quinoxalines (3) we then focused on the preparation of the next set of target molecules via chemical modifications of 3. Accordingly, some of the synthesized compounds e.g. 3a, 3c, 3f, 3k were treated with LiAlH4 to afford the 3-(2-(hydroxymethyl)-1H-indol-3-yl)quinoxalin-2-ol derivatives (4) in good yields (Table 3). The formation of 4 was indicated by the appearance of (i) a broad singlet or triplet near 5.56–5.33 δ due to the alcoholic proton, (ii) a broad singlet near 11.57–11.45 δ due to the phenolic OH proton and (iii) the CH2 protons near 4.77–4.85 δ in their corresponding 1HNMR spectra (see ESI†). A Heteronuclear Multiple Bond Correlation (HMBC) study performed by using compound 4d indicated two 4-bond correlations for the ring junction carbon B [with methyl C (2.38 δ, s) and methyl D (2.41 δ, s) separately], two 4-bond (7.15 δ, s) separately] and one 3-bond correlation for the ring junction carbon A [with methyl C (2.38 δ, s)] (Fig. 3, see also ESI†). Since an opposite HMBC result was expected for the regioisomer 4dd, hence 4d appeared to be the structure of isolated product.
| Entry | Compound 3 R, R1, R2, R3, R4 | Product 4 | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: compound 3 (1.0 mmol), LiAlH4 (1.2 mmol) in dry THF (15 mL) at 0 °C for 15 min and then at room temp for 2 h under a nitrogen atmosphere.b Isolated yield. | |||
| 1 | H, H, H, H, H (3a) | 4a | 82 |
| 2 | H, H, H, H, Et (3c) | 4b | 79 |
| 3 | H, H, H, Me, H (3f) | 4c | 84 |
| 4 | Me, H, Me, H, H (3k) | 4d | 81 |
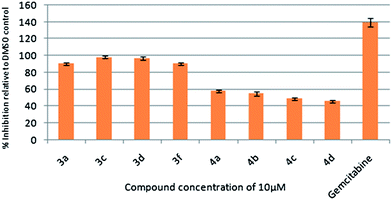
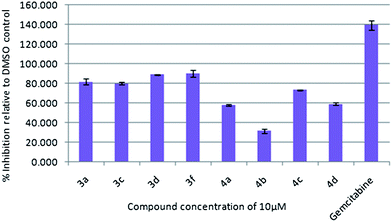
To assess their anticancer properties some of the compounds (3 and 4) synthesized were tested in vitro at 10 μM against TZM-BL (human cervical carcinoma cells) and A549 (human lung carcinoma cells) cell lines using a sulphorhodamine B (SRB) assay with gemcitabine as a reference compound. In general, compound 3 showed better activities than 4. For example, compounds 3a, 3c, 3d and 3f showed 90–98% inhibition against TZM-BL (Fig. 4) and 79–90% against A549 cell lines (Fig. 5) whereas compound 4 showed relatively lower growth inhibition (57–31%) against both the cell lines, except 4c against A549 cell line (Fig. 5). It appeared from this study that the presence of a pyran ring might be important for superior anti-cancer activities showed by 3 over 4.
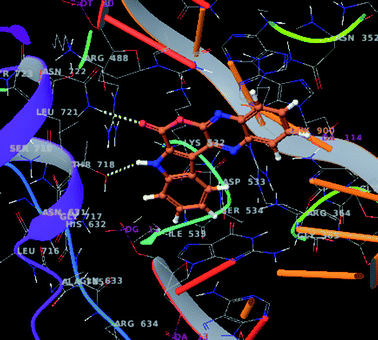
In view of topoisomerase I inhibitory properties of Lamellarin D (Fig. 1) the compounds 3a, 3d and 3f were assessed in silico against human DNA topoisomerase I (1k4t) protein. The Glide score (−5.81 for 3a, −6.62 for 3d and −5.51 for 3f vs. −6.08 for Lamellarin D) indicated these molecules interacted well with the protein. For example interaction of compound 3a (Fig. 6) was mainly contributed by (i) a H-bonding between the carbonyl oxygen of 3a and Asn-722, (ii) a H-bonding between the indole –NH of 3a and Thr-718 and (iii) π–π stacking interactions between the indole benzene ring and His234 of the 1k4t protein. Similarly, the interaction of 3d was contributed by H-bonding between the quinoxaline nitrogen of 3d and Arg-364 of the 1k4t protein and that of 3f was contributed by a H-bonding between indole –NH of 3f and Glu-356 (see ESI†). Overall, these compounds showing promising cancer cell growth inhibitory properties in vitro and good interactions with topoisomerase I in silico are of further interest.
In conclusion, an inexpensive, practical and one-pot method has been developed for the synthesis of pyrano[3,4-b]indole fused quinoxalines. The methodology involved construction of the central pyranone ring via an AlCl3-mediated heteroarylation-cyclization method. The methodology is operationally simple, does not require the use of expensive reagents or catalysts, and is amenable for scale-up preparation. A number of compounds were prepared by using this methodology some of which were converted to the corresponding indol-3-yl quinoxaline derivatives. Several of pyrano[3,4-b]indole fused quinoxalines showed promising growth inhibition of cervical and lung cancer cells and good interactions with topoisomerase I in silico. Our study suggests that pyrano[3,4-b]indole fused with quinoxaline framework could be an attractive template for the identification of novel anticancer agents and the synthetic methodology developed could be useful for building diversity based library of compounds related to this framework.
Acknowledgements
KSK thanks University Grants Commission (UGC), New Delhi, for the award of Assistant Professorship under its FRP and DST, India, for the financial support (SR/FTP/CS-017/2012). B. R. and B. B. thanks UGC and CSIR, New Delhi, respectively, for a Junior Research Fellowship.Notes and references
- C. Neagoie, E. Vedrenne, F. Buron, J. Y. Mérour, S. Rosca, S. Bourg, O. Lozach, L. Meijer, B. Baldeyrou, A. Lansiaux and S. Routier, Eur. J. Med. Chem., 2012, 49, 379 CrossRef CAS PubMed.
- (a) E. Marco, W. Laine, C. Tardy, A. Lansiaux, M. Iwao, F. Ishibashi, C. Bailly and F. Gago, J. Med. Chem., 2005, 48, 3796 CrossRef CAS PubMed; (b) C. Bailly, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 363 CrossRef CAS PubMed.
- C. A. Demerson, L. G. Humber, A. H. Philipp and R. R. Martel, J. Med. Chem., 1976, 19, 391 CrossRef CAS.
- A. H. Katz, C. A. Demerson, C.-C. Shaw, A. A. Asselin, L. G. Humber, K. M. Conway, G. Gavin, C. Guinosso, N. P. Jensen, D. Mobilio, R. Noureldin, J. Schmid, U. Shah, D. Van Engen, T. T. Chau and B. M. Weichman, J. Med. Chem., 1988, 31, 1244 CrossRef CAS PubMed.
- (a) A. Gopalsamy, K. Lim, G. Ciszewski, K. Park, J. B. Ellingboe, S. Insaf, J. Upeslacis, T. S. Mansour, G. Krishnamurthy, M. Damarla, Y. Pyatski, D. Ho, A. Y. M. Howe, M. Orlowski, B. Feld and J. O'Connell, J. Med. Chem., 2004, 47, 6603 CrossRef CAS PubMed; (b) M. G. LaPorte, T. L. Draper, L. E. Miller, C. W. Blackledge, L. K. Leister, E. Amparo, A. R. Hussey, D. C. Young, S. K. Chunduru, C. A. Benetatos, G. Rhodes, A. Gopalsamy, T. Herbertz, C. J. Burns and S. M. Condon, Bioorg. Med. Chem. Lett., 2010, 20, 2968 CrossRef CAS PubMed.
- F. A. Nardella, US Pat., 6573292, 2003.
- H. Gao, E. F. Yamasaki, K. K. Chan, L. L. Shen and R. M. Snapka, Mol. Pharmacol., 2003, 63, 1382 CrossRef CAS PubMed.
- For our earlier effort, see: (a) N. K. Swamy, L. K. Tatini, J. M. Babu, P. Annamalai and M. Pal, Chem. Commun., 2007, 1035 RSC; (b) C. Rajitha, P. K. Dubey, V. Sunku, F. J. Piedrafita, V. R. Veeramaneni and M. Pal, Eur. J. Med. Chem., 2011, 46, 4887 CrossRef CAS PubMed; (c) N. Mulakayala, D. Rambabu, M. R. Raja, M. Chaitanya, C. S. Kumar, A. M. Kalle, R. Krishna, M. Reddy, M. V. B. Rao and M. Pal, Bioorg. Med. Chem., 2012, 20, 759 CrossRef CAS PubMed; (d) U. R. Chamakura, E. Sailaja, B. Dulla, A. M. Kalle, S. Bhavani, D. Rambabu, R. Kapavarapu, M. V. B. Rao and M. Pal, Bioorg. Med. Chem. Lett., 2014, 24, 1366 CrossRef PubMed; (e) B. Dulla, E. Sailaja, U. R. Chamakura, M. Aeluri, A. M. Kalle, S. Bhavani, D. Rambabu, M. V. B. Rao and M. Pal, Tetrahedron Lett., 2014, 55, 921 CrossRef CAS; (f) A. Nakhi, R. Adepu, D. Rambabu, R. Kishore, G. R. Vanaja, A. M. Kalle and M. Pal, Bioorg. Med. Chem. Lett., 2012, 22, 4418 CrossRef CAS PubMed.
- D. M. Parkin, F. Bray, J. Ferlay and P. Pisani, Ca-Cancer J. Clin., 2005, 55, 74 CrossRef PubMed.
- (a) C. Dialer, D. Imbri, S. P. Hansen and T. Opatz, J. Org. Chem., 2015, 80, 11605 CrossRef CAS PubMed; (b) R. M. Medeiros, S. E. Schaus and J. A. Porco Jr, Org. Lett., 2011, 13, 4012 CrossRef PubMed; (c) P. Nealmongkol, K. Tangdenpaisal, S. Sitthimonchai, S. Ruchirawat and N. Thasana, Tetrahedron, 2013, 69, 9277 CrossRef CAS.
- For leading references, see: (a) M. Pal, V. R. Batchu, K. Parasuraman and K. R. Yeleswarapu, J. Org. Chem., 2003, 68, 6806 CrossRef CAS PubMed; (b) M. Pal, V. R. Batchu, I. Dager, N. K. Swamy and S. Padakanti, J. Org. Chem., 2005, 70, 2376 CrossRef CAS PubMed; (c) K. S. Kumar, S. Chamakuri, P. Vishweshwar, J. Iqbal and M. Pal, Tetrahedron Lett., 2010, 51, 3269 CrossRef CAS; (d) K. S. Kumar, R. Adepu, R. Kapavarapu, D. Rambabu, G. R. Krishna, C. M. Reddy, K. K. Priya, K. V. L. Parsa and M. Pal, Tetrahedron Lett., 2012, 53, 1134 CrossRef CAS.
- L. Mao, H. Sakurai and T. Hirao, Synthesis, 2004, 2535 CAS.
- We also examined the use of some other Lewis acids in place of AlCl3 such as FeCl3, BF3 and BiCl3. However, the reaction did not proceed in these cases. We thank one of the reviewers for his relevant suggestion.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and copies of spectra. See DOI: 10.1039/c6ra07507j |
| This journal is © The Royal Society of Chemistry 2016 |