 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of imidazolium-crosslinked chitosan aerogel and its prospect as a dye removing adsorbent†
Juho Antti
Sirviö
*,
Miikka
Visanko
and
Henrikki
Liimatainen
Fiber and Particle Engineering Research Unit, University of Oulu, P.O. Box 4300, FI-90014, Finland. E-mail: juho.sirvio@oulu.fi
First published on 8th June 2016
Abstract
The potential utility of Debus–Radziszewski imidazole synthesis in the fabrication of crosslinked chitosan was studied. Three-component crosslinking was achieved by using glyoxal and propionaldehyde to connect amine groups of chitosan via imidazolium crosslinking. A water-insoluble (at pH range of 2–10) chitosan was obtained at room temperature with a degree of substitution of 0.45 and aerogel was obtained after freeze-drying. The ability of the imidazolium-crosslinked chitosan (ICC) aerogel to absorb an anionic dye, Direct Yellow 27, from a model water was then studied. Based on the Langmuir isotherm, at a pH of 4, an adsorption maximum of 2340 mg g−1 (3.5 mmol g−1) was obtained. In addition, due to the permanent cationic charge of imidazolium group, ICC exhibited excellent adsorption capacity, even under alkaline conditions. Methylglyoxal and benzaldehyde were also used to obtain other types of ICC, demonstrating the versatility of Debus–Radziszewski imidazole synthesis for fabrication of modified chitosan.
Introduction
Chitosan is a semi-synthetic polymer obtained from naturally occurring chitin (the second most abundant polysaccharide after cellulose1) following alkaline hydrolysis (deacetylation).2,3 Chitosan is superior to chitin in a number of ways. For example, it has an abundance of reactive primary amino groups and solubility in water at a low pH. Due to the cationic nature of its amino groups, chitosan has been used in various applications for ion exchange and dye removal. In addition, its biocompatibility, biodegradability, and low toxicity mean that chitosan can be employed in many medical applications.4–7Chitosan can function as a solid absorbent of toxic and harmful dyes and of anions and heavy metals from waste waters.8–11 However, due to its high solubility under acidic solutions, chemical modification of chitosan is necessary to reduce its solubility and improve the recyclability of the solid adsorbent.12 The absorption capacity and recyclability of chitosan have been improved by grafting polymer side-chains onto chitosan.12 However, grafting chitosan with oil-based macromolecules decreases the bio-content of the absorbent and may induce problems related to unreacted toxic monomers. Acid-insoluble chitosan can be produced by crosslinking of chitosan chains with various reagents, such as epichlorohydrin and glutaraldehyde. However, these chemicals possess a number of disadvantages, such as toxicity.13 Chemical modifications can also decrease the cationicity (e.g. the conversion of amine groups to corresponding imines by glutaraldehyde) of chitosan, resulting in a decline in its absorption capacity.13
Debus–Radziszewski imidazole synthesis is a three-component chemical reaction where two amino groups react with vicinal dicarbonyl and carbonyl reagents to form a stable imidazole structure.14 The requirement of only an acid catalyst makes Debus–Radziszewski imidazole synthesis an environmentally friendly reaction, as water is the only by-product. Recently, this reaction was efficiently used to crosslink poly(L-lysine).15 The Debus–Radziszewski imidazole synthesis allows the alteration of reaction products by the varying of each components (amine, carbonyl and dicarbonyl), and consequently the reaction has been used to obtain various imidazole-based ionic liquids.16 The fact that dicarbonyl and carbonyl components can be obtained from natural resources enhances the sustainability of Debus–Radziszewski imidazole synthesis.
In this study, Debus–Radziszewski imidazole synthesis was used to obtain water-insoluble chitosan using glyoxal and propionaldehyde as dicarbonyl and carbonyl components, respectively. Crosslinked chitosan was characterized using elemental analysis and diffusion reflectance Fourier transform infrared spectroscopy and its performance as an anion exchange material was tested by examining the adsorption of an anionic dye from a model water solution. The effect of the solution pH, contact time, and initial dye concentration on the adsorption were studied. The versatility of Debus–Radziszewski imidazole synthesis in chitosan crosslinking was demonstrated using methylglyoxal and benzaldehyde as alternative dicarbonyl and carbonyl components, respectively.
Materials and methods
Chitosan (medium molecular weight), propionaldehyde, glyoxal, methylglyoxal, and benzaldehyde were obtained from Sigma Aldrich (Germany) and were used without further purifications. Acetic acid and 0.1 M hydrochloride acid were purchased from Fluka (Germany). The acetic acid was diluted with deionized water to obtain a 10% solution. Direct Yellow 27 for the adsorption experiments was obtained from Sigma Aldrich (Germany). Deionized water was used throughout the experiments.Crosslinking of chitosan
One gram (6.1 mmol) of chitosan was first dissolved in 100 mL of 10% acetic acid and then mixed with 0.35 mL (3.1 mmol) of glyoxal (40% in water) and 0.22 mL (3.1 mmol) of propionaldehyde. The reaction was started by adjusting the pH to 5 by adding 10% NaOH solution under vigorous stirring. The reaction was allowed to proceed for 2 days at room temperature. The chitosan gel that formed was transferred to a beaker containing 600 mL of water and mixed for 1 h, after which mixture was filtrated and the product washed with 2 L of water. The obtained hydrogel was then freeze-dried to obtain an imidazole-crosslinked chitosan (ICC1) aerogel.A similar procedure was employed to obtain ICC2 and ICC3, using 0.35 mL (3.1 mmol) of glyoxal (40% in water) and 3.1 mmol of benzaldehyde and 0.497 mL (3.1 mmol) of methylglyoxal (40% in water) and 0.22 mL (3.1 mmol) of propionaldehyde, respectively. These products (ICC2 and ICC3) were only used to demonstrate the feasibility of the synthesis for alternative reactants.
The degree of substitution was calculated according to eqn (1) (ref. 17) after elemental analysis (PerkinElmer CHNS/O 2400 Series II, USA).
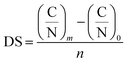 | (1) |
| Sample | Carbon (%) | Nitrogen (%) |
|---|---|---|
| Chitosan | 41.3 | 7.23 |
| ICC1 | 39.0 | 5.53 |
| ICC2 | 39.2 | 3.33 |
| ICC3 | 34.4 | 2.84 |
In addition, the deacetylation degree (XD) of the original chitosan was calculated using eqn (2):18
| XD = 100 × (4 − 0.583093 × wC/N) | (2) |
Diffusion reflectance Fourier transform infrared spectroscopy
Diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy was used to characterize the ICC. The spectra of chitosan and freeze-dried ICC were collected with a Bruker Vertex 80v spectrometer (USA). The spectra were obtained at a range of 600–4000 cm−1. For each sample, 40 scans were taken at a resolution of 2 cm−1.Field-emission scanning electron microscopy
Field-Emission Scanning Electron Microscopy (FESEM) image of the ICC1 was obtained using Sigma HD VP FESEM (Zeiss, Germany). Sample was placed on carbon tape and sputter-coating with platinum (Pt). The accelerating voltage during imaging was 3 kV.Adsorption of anionic dye
Adsorption experiments were carried out in batches. Dye stock solution of 1 g L−1 was prepared by dissolving the anionic dye (Direct Yellow 27) in deionized water, and dilutions of desired concentrations were made from the stock. NaOH and HCl were used to adjust the pH of the anionic dye solutions to 2–10. After dilution and pH adjustment, 100 mg L−1 of ICC were added, and the solution was shaken for 5 min to 24 h using a Flask Shaker (GWP, UK). Then, approximately 10 mL of the solution were collected with a syringe and filtrated using a 0.45 μm cellulose acetate membrane (VWR, USA). The absorbance of the sample was measured spectrophotometrically at 393 nm using Shimadzu UV-Vis spectroscopy (Japan). The concentration of the dye in the sample and the quantity of the adsorbed dye were calculated using a calibration curve based on the absorbance results obtained with different dye solutions at different concentrations.Adsorption kinetics
Pseudo-first-order and pseudo-second-order kinetics were employed to describe the kinetics of the adsorption of the dye by ICC1. The pseudo-first-order kinetic model is shown in eqn (3),19,20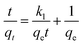 | (3) |
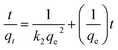 | (4) |
Adsorption isotherms
The adsorption mechanisms were studied using Langmuir and Freundlich isotherm models. A linear form of the Langmuir isotherm is shown in eqn (6),20,22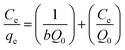 | (5) |
The logarithmic form of the Freundlich isotherm is shown in eqn (6),20
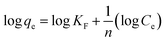 | (6) |
Results and discussion
Debus–Radziszewski imidazole crosslinking of chitosan was performed in a 10% acetic acid solution using a stoichiometric amount (1 mol of carbonyl components per 2 mol of chitosan) of glyoxal and propionaldehyde at room temperature. Without adjusting the pH (the initial pH was around 3), the solution remained a free-flowing liquid, even after a prolonged reaction time (3 days). However, after adjusting the pH to 5, the solution almost immediately started to form a gel-like material. The reaction was allowed to proceed for 2 days to ensure complete crosslinking. The chitosan gel turned to light yellow after 1 h and to red after 2 days. The crosslinked product did not dissolve in water, and a near quantitate yield was obtained after washing with water. According to the elemental analysis, the degree of substitution was 0.39.A possible reaction mechanism for the formation of the ICC is presented in Scheme 1. In the proposed reaction, glyoxal first reacts with amines of chitosan to form imine crosslinks between the chitosan molecules. Due to the aqueous instability of the imine bond and lack of a cationic charge, glyoxal is not an ideal choice to crosslink chitosan, especially when water purification applications are concerned. The crosslinking with glyoxal is similar to that of glutaraldehyde widely used in the literature.12 The addition of aldehyde to glyoxal crosslinked chitosan leads to the formation of charged imidazole structure between the chitosan molecules. Imidazoles are known to be highly stable. Thus, the driving force underlying the crosslinking of chitosan is assumed to be the formation of imidazole moieties.
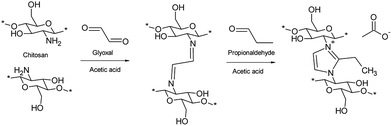 | ||
| Scheme 1 Reaction mechanism illustrating the crosslinking of chitosan with Debus–Radziszewski imidazole synthesis. | ||
The DRIFT spectrum of chitosan shows typical stretching vibrations of OH-groups and N–H around 3500 cm−1 (Fig. 1). The bands at wavenumbers of 1665 and 1600 cm−1 are associated with the vibrations of the carbonyl bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the amide group and the vibrations of the amine group, respectively.23 In the spectrum of ICC1, aromatic C
O) of the amide group and the vibrations of the amine group, respectively.23 In the spectrum of ICC1, aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and C
C stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N ring stretching vibrations at 1581 and 1416 cm−1, respectively, are due to the formation of an imidazole group.24 The absence of a C
N ring stretching vibrations at 1581 and 1416 cm−1, respectively, are due to the formation of an imidazole group.24 The absence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peak at 1635 cm−1 points to the absence of a significant level of glyoxal crosslinks25 and effective crosslinking of chitosan by Debus–Radziszewski imidazole synthesis.
N peak at 1635 cm−1 points to the absence of a significant level of glyoxal crosslinks25 and effective crosslinking of chitosan by Debus–Radziszewski imidazole synthesis.
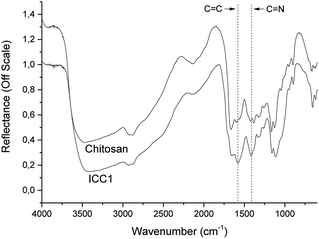 | ||
Fig. 1 DRIFT spectra of chitosan and ICC1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and C C stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N ring stretching vibrations of the imidazolium moiety are marked with a dashed line. N ring stretching vibrations of the imidazolium moiety are marked with a dashed line. | ||
After freeze-drying, a brownish aerogel was obtained. Despite the insolubility of ICC1, the freeze-dried aerogel was highly hydrophilic, likely due to the presence of charged groups. The aerogel absorbed up to 21 times water compared to its own mass. Based on FESEM images (Fig. S2†), the aerogel exhibited irregularly shaped macrostructure with very smooth surfaces. The ion exchange capability of the crosslinked chitosan aerogel was demonstrated by adsorbing anionic dye (Direct Yellow 27) (see Fig. S1† for the structure of the dye) with ICC1 from a model water. According to Fig. 2, the ICC1 had a high adsorption capacity (481–351 mg g−1) in the whole studied pH range (2–10), and the adsorption maximum was observed at pH 4 (96% retention of initial 2.5 mg of dye by 5 mg of ICC1). Chitosan-based materials were reported to have high retention of anionic dyes at a low pH.12 However, studies also reported sharp decreases in the adsorption capacity of chitosan-based adsorbents with alkaline solutions.12,26,27 Here, the high capacity was retained at a high pH. This was likely due to the presence of imidazolium moieties, which have a permanent cationic charge and being more stable within a wider pH range than primary amines of chitosan. Other adsorption mechanisms, such as an aromatic interaction between the imidazolium groups of ICC, may also exist.
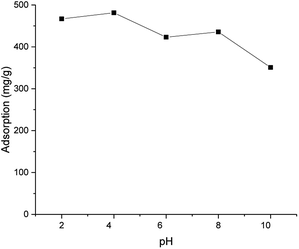 | ||
| Fig. 2 Adsorption capacity of ICC1 as a function of the pH (conditions: 50 mg L−1 of dye; 100 mg L−1 of ICC1; 24 h shaking time; volume of 50 mL; room temperature). | ||
The results of the kinetic studies are presented in Fig. 3. The adsorption plateau was obtained after 360 min, as only a minimal increase was observed when the shaking time was increased to 24 h. This finding is in line with that of previous studies of chitosan-based adsorbents, where the adsorption maximum was observed after a few hours.27,28 The best fit was found using the pseudo-second order kinetic model (R2 = 0.99946 compared to R2 = 0.97242 for the pseudo-first order kinetic model). The pseudo-second order kinetic constant was 4.35 × 10−5. The calculated adsorption maximum was 495 mg g−1, which is in line with the experimental value (481 mm g−1).
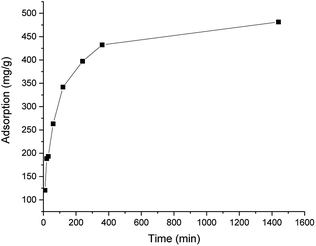 | ||
| Fig. 3 Adsorption capacity of ICC1 as a function of the shaking time (conditions: 50 mg L−1 of dye; 100 mg L−1 of ICC1; total volume of 100 mL; room temperature). | ||
The maximum adsorption capacity was highly dependent on the initial concentration of the dye (Fig. 4). The adsorption capacity increased almost linearly from 210 to 2430 mg g−1 when the initial dye concentration was increased from 10 to 300 mg g−1. A plateau was observed after the dye concentration increased to 300 mg L−1. The adsorption followed the Langmuir adsorption isotherm (R2 = 0.97352 for the Langmuir isotherm vs. R2 = 0.76452 for the Freundlich isotherm). Based on the Langmuir equation (eqn (4)), the calculated adsorption maximum was 2340 mg g−1, which is consistent with the experimental adsorption maximum (2430 mg g−1).
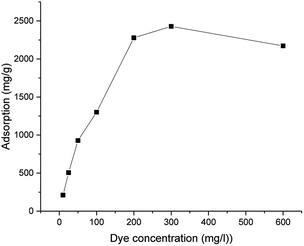 | ||
| Fig. 4 Adsorption capacity of ICC1 as a function of the dye concentration (conditions: 50 mg L−1 of ICC1; 24 h shaking time; volume of 50 mL; room temperature). | ||
The maximum adsorption capacity of ICC1 was very high compared to that of many bio-based and inorganic adsorbents. For example, the adsorption maximums of glutaraldehyde crosslinked chitosan were between 108 and 775 mg g−1 for 11 different anionic dyes (of note, in most cases, the adsorption capacity of glutaraldehyde-crosslinked chitosan was still higher than that of active carbon).29 Poly(acrylamide) grafted chitosan powder had adsorption maximum of 1211 mg g−1 towards anionic dye (Remazol Yellow Gelb 3RS).30 Of other biomacromolecule-based adsorbents, the maximum adsorption capacity of a nanocellulose-amphoteric polyvinylamine microgel was 1469.7 mg g−1 for an anionic dye, Acid Red GR,31 and as high as 2296 mg g−1 for C.I. Acid Blue 324 using tertiary amine starch ether.32 An adsorption maximum of 1800 mg g−1 was reported for methyl orange using inorganic Mg–Al layered double hydroxide.33 Due to the high variety of studied dyes and adsorption experimental setups found in the literature, a direct comparison between the different adsorbents might not be meaningful (e.g., no correlation was found between the amount of charged groups in dyes and the adsorption maximum of glutaraldehyde crosslinked chitosan).29 However, it can be concluded that the anionic dye adsorption capacity of ICC1 is among the highest reported for organic and inorganic materials.
In addition to ion exchange properties, imidazole-modified chitosan can potentially be used in various other applications, such as gene delivery34 and selective separation of CO2 from CH4.35 With these and other possible applications in mind, it would be beneficial to be able to adjust the properties of imidazole cross-linked chitosan. The feasibility of Debus–Radziszewski imidazole synthesis in chitosan crosslinking was further demonstrated by using methylglyoxal instead of glyoxal, together with propionaldehyde (ICC2). Aryl aldehyde (benzaldehyde) was also studied instead of propionaldehyde, together with glyoxal (ICC3). Methylglyoxal was also studied instead of glyoxal, together with propionaldehyde (ICC3). Both reactions led to the formation of ICC (DRIFT spectra and schematic illustrations of the synthesis of ICC2 and ICC3 are presented as ESI in Fig. S3 and Scheme S1,† respectively). The spectrum of ICC3 was similar to that of ICC. However, the wavenumber of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and C
C stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N ring stretching vibrations were higher: 1651 and 1454 cm−1, respectively. C
N ring stretching vibrations were higher: 1651 and 1454 cm−1, respectively. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and C
C stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N ring stretching of ICC2 occurred at 1561 and 1432 cm−1, respectively, whereas an aromatic vibration band of ICC2 was observed at a wavelength of 1615 cm−1, strongly overlapping with the amide and amine bands of chitosan. The DS of ICC2 and ICC3 was 1.51 and 1.17, respectively. The results obtained using benzaldehyde and methylglyoxal indicate that various reagents can significantly alter the reaction efficiency of chitosan crosslinking. In addition, it is possible to fabricate disparate types of ICC using divergent reagents. Thus, the properties of ICC can be adjusted in accordance with the particular application.
N ring stretching of ICC2 occurred at 1561 and 1432 cm−1, respectively, whereas an aromatic vibration band of ICC2 was observed at a wavelength of 1615 cm−1, strongly overlapping with the amide and amine bands of chitosan. The DS of ICC2 and ICC3 was 1.51 and 1.17, respectively. The results obtained using benzaldehyde and methylglyoxal indicate that various reagents can significantly alter the reaction efficiency of chitosan crosslinking. In addition, it is possible to fabricate disparate types of ICC using divergent reagents. Thus, the properties of ICC can be adjusted in accordance with the particular application.
Conclusions
This study demonstrated that Debus–Radziszewski imidazole synthesis was an efficient method to obtain crosslinked chitosan with permanent cationic charge. A water-insoluble aerogel was obtained by a straightforward method using stoichiometric amounts of reagents. Compared to other bio-based and inorganic adsorbents, ICC showed excellent adsorption capacity of anionic dyes, indicating that the fabrication route was suitable for obtaining sustainable ion exchange material. As demonstrated here, various dicarbonyl and carbonyl compounds can be used in crosslinking chitosan with Debus–Radziszewski imidazole synthesis, thereby providing novel ways to produce chitosan-based materials with adjustable properties.Acknowledgements
Ms Jaana Larionova is gratefully acknowledged for her contribution to the adsorption experiments. Dr Ilkka Miinalainen acknowledged for his help with the FESEM images.Notes and references
- A. K. Singla and M. Chawla, J. Pharm. Pharmacol., 2001, 53, 1047–1067 CrossRef CAS PubMed.
- M. N. V. Ravi Kumar, React. Funct. Polym., 2000, 46, 1–27 CrossRef.
- S. Kumar, J. d. A. e Silva, M. Y. Wani, C. M. F. Dias and A. J. F. N. Sobral, J. Dispersion Sci. Technol., 2016, 37, 155–158 CrossRef CAS.
- R. C. F. Cheung, T. B. Ng, J. H. Wong and W. Y. Chan, Mar. Drugs, 2015, 13, 5156–5186 CrossRef CAS PubMed.
- P. Garg, S. Kumar, S. Pandey, H. Seonwoo, P.-H. Choung, J. Koh and J. H. Chung, J. Mater. Chem. B, 2013, 1, 6053–6065 RSC.
- S. Kumar, J. Dutta and P. K. Dutta, Int. J. Biol. Macromol., 2009, 45, 330–337 CrossRef CAS PubMed.
- S. Kumar and J. Koh, Int. J. Mol. Sci., 2012, 13, 6102–6116 CrossRef CAS PubMed.
- S. Babel and T. A. Kurniawan, J. Hazard. Mater., 2003, 97, 219–243 CrossRef CAS PubMed.
- T. Feng and L. Xu, RSC Adv., 2013, 3, 21685–21690 RSC.
- A. H. Gedam and R. S. Dongre, RSC Adv., 2015, 5, 54188–54201 RSC.
- H. Liu and C. Wang, RSC Adv., 2013, 4, 3864–3872 RSC.
- M. Vakili, M. Rafatullah, B. Salamatinia, A. Z. Abdullah, M. H. Ibrahim, K. B. Tan, Z. Gholami and P. Amouzgar, Carbohydr. Polym., 2014, 113, 115–130 CrossRef CAS PubMed.
- X. Shen, J. L. Shamshina, P. Berton, G. Gurau and R. D. Rogers, Green Chem., 2015, 18, 53–75 RSC.
- H. Debus, Ann. Chem. Pharm., 1858, 107, 199–208 CrossRef.
- K.-S. Krannig, D. Esposito and M. Antonietti, Macromolecules, 2014, 47, 2350–2353 CrossRef CAS.
- D. Esposito, S. Kirchhecker and M. Antonietti, Chem.–Eur. J., 2013, 19, 15097–15100 CrossRef CAS PubMed.
- M. Jiang, K. Wang, J. F. Kennedy, J. Nie, Q. Yu and G. Ma, Int. J. Biol. Macromol., 2010, 47, 696–699 CrossRef CAS PubMed.
- Z. M. dos Santos, A. L. P. F. Caroni, M. R. Pereira, D. R. da Silva and J. L. C. Fonseca, Carbohydr. Res., 2009, 344, 2591–2595 CrossRef CAS PubMed.
- S. Y. Lagergren, Zur Theorie der sogenannten Adsorption gelöster Stoffe, 1898 Search PubMed.
- C. Namasivayam and D. Sangeetha, Adsorption, 2006, 12, 103–117 CrossRef CAS.
- Y. S. Ho and G. McKay, Chem. Eng. J., 1998, 70, 115–124 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- S. M. L. Silva, C. R. C. Braga, M. V. L. Fook, C. M. O. Raposo, L. H. Carvalho and E. L. Canedo, in Infrared Spectroscopy – Materials Science, Engineering and Technology, ed. T. Theophile, InTech, 2012 Search PubMed.
- R. B. Sarker and A. H. Bhuiyan, Thin Solid Films, 2011, 519, 5912–5916 CrossRef CAS.
- X. Chang, D. Chen and X. Jiao, J. Phys. Chem. B, 2008, 112, 7721–7725 CrossRef CAS PubMed.
- M. M. El-Zawahry, F. Abdelghaffar, R. A. Abdelghaffar and A. G. Hassabo, Carbohydr. Polym., 2016, 136, 507–515 CrossRef CAS PubMed.
- L. Cui, Z. Xiong, Y. Guo, Y. Liu, J. Zhao, C. Zhang and P. Zhu, Carbohydr. Polym., 2015, 132, 330–337 CrossRef CAS PubMed.
- S. Chatterjee, T. Chatterjee, S.-R. Lim and S. H. Woo, Bioresour. Technol., 2011, 102, 4402–4409 CrossRef CAS PubMed.
- E. Guibal, P. McCarrick and J. M. Tobin, Sep. Sci. Technol., 2003, 38, 3049–3073 CrossRef CAS.
- G. Z. Kyzas and N. K. Lazaridis, J. Colloid Interface Sci., 2009, 331, 32–39 CrossRef CAS PubMed.
- L. Jin, Q. Sun, Q. Xu and Y. Xu, Bioresour. Technol., 2015, 197, 348–355 CrossRef CAS PubMed.
- Y. Shi, B. Ju and S. Zhang, Carbohydr. Polym., 2012, 88, 132–138 CrossRef CAS.
- G. Darmograi, B. Prelot, G. Layrac, D. Tichit, G. Martin-Gassin, F. Salles and J. Zajac, J. Phys. Chem. C, 2015, 119, 23388–23397 CAS.
- B. Shi, H. Zhang, Z. Shen, J. Bi and S. Dai, Polym. Chem., 2013, 4, 840–850 RSC.
- S. Saedi, B. Nikravesh, F. Seidi, L. Moradi, A. A. Shamsabadi, M. B. Salarabadi and H. Salimi, RSC Adv., 2015, 5, 67299–67307 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical structure of Direct Yellow 27; FESEM images of ICC1 aerogel; schematic illustration of the synthesis of imidazolium crosslinked chitosan using methylglyoxal and benzaldehyde. See DOI: 10.1039/c6ra08301c |
| This journal is © The Royal Society of Chemistry 2016 |
