Catalytic property of poly(ethylene terephthalate-co-isophthalate) synthesized with a novel Sb/Al bimetallic compound catalyst
Fuchen Zhang ,
Hongjun Kang,
Yongping Bai*,
Bo Jiang,
Yudong Huang and
Li Liu
,
Hongjun Kang,
Yongping Bai*,
Bo Jiang,
Yudong Huang and
Li Liu
School of Chemical Engineering and Technology, Harbin Institute of Technology, Harbin 150001, P. R. China. E-mail: baifengbai@hit.edu.cn; Fax: +86-0451-86414086; Tel: +86-0451-86413711
First published on 6th July 2016
Abstract
In this paper, the hydrolysis process of aluminum subacetate (AlSA) was studied by Fourier transform infrared (FT-IR), 27Al nuclear magnetic resonance (NMR), X-ray diffraction (XRD), and particle size distribution analysis, and poly(ethylene terephthalate-co-isophthalate) (PETI) was synthesized by using a compound catalyst made of its hydrolysate, γ-AlOOH, and ethylene glycol stibium (EGSb). Meanwhile, the effects of the γ-AlOOH addition stage on the catalytic effect and the relative properties of the polyester were investigated. The results showed that the Sb and Al compound catalyst had obvious synergistic effects on the process of the polyester polycondensation. With the same dosage of catalyst, the usage of EGSb and the polycondensation time were reduced by 60% and 18.6%, respectively, and the diethylene glycol content in the polyester also decreased by using the compound catalyst, compared with that obtained when only using the EGSb catalyst. The use of γ-AlOOH could play a catalytic role and also shows a unique crosslinking center, which could improve the melting point and glass transition temperature.
1. Introduction
Currently, poly(ethylene terephthalate-co-isophthalate) (PETI), a commercial copolyester, has a low melting point, good crystal and optical performances, and excellent air tightness,1,2 compared with poly(ethylene terephthalate) (PET). Therefore, PETI has been widely applied in the fields of bottle blow moulding and biaxial oriented films. To date, researchers have carried out much exploration in the field of polyester synthesis catalysts, such as stibium, titanium, and zirconium series catalysts, and it has been validated that these catalysts have good catalytic effects. However, there are many disadvantages in using these catalysts such as easy hydrolysis, poor thermal stability, and the chroma of the synthesized polyester.3–5 However, it is easy for stibium series catalysts to be released out of some of the polyester final products, such as food packaging or soft-drink bottles, and stibium is a potential human carcinogen, which poses a threat to human health and environmental safety.6,7 Thus far, ethylene glycol stibium (EGSb) has been used as a catalyst in most polyester manufacturing industries due to its good catalytic efficiency and thermal stability;8 however, the synthesized polyester presents a gray chroma and poor environmental friendliness.9,10 Since the awareness of green chemistry is progressively increasing, new types of catalysts that can substitute or reduce the dosage of EDSb have gained considerable attention as promising methods for green processes. Therefore, it is necessary to search for new types of catalysts that can substitute or reduce the dosage of EDSb. New series of catalysts, such as titanium dioxide/silicon dioxide sols, chelated complexes of titanium, attapulgite-supported aluminum oxide hydroxide, etc., have already been proven to have excellent condensation catalytic performances.11–13 One very effective approach to reduce the usage of stibium series catalysts is to combine them with other catalysts.14Hydrated alumina (Al2O3·nH2O) is a large family, which can be divided into Al(OH)3, AlOOH, and gel (ρ-Al2O3·nH2O) according to the number of water molecules in the chemical and crystal phase structures. With changes in the preparation formulation and process, a complicated reciprocal transformation is generated among these crystal phase structures.15 Among the family of hydrate alumina, AlOOH, which can be used as a catalyst and a catalyst support in many reactions, has been researched and applied the most.16,17 AlOOH can be prepared by the hydrolysis of an aluminum inorganic salt, carboxylic acid salt, and aluminum alkoxide.18–20 Aluminum subacetate (AlSA), which possesses definite antibacterial and antifungal properties, can be used as a disinfectant in the field of medical treatment. Otitis media and otitis externa are treated using Burow's solution, which is prepared from acetic acid and AlSA.21 There are no detailed reports about the preparation of AlOOH via the hydrolysis of AlSA.
In this paper, the coordination state of the AlSA ionization products and the composition of the AlSA hydrolysate are investigated and PETI is synthesized by using a compound catalyst made of the AlSA hydrolysate, (γ-AlOOH), and EGSb. The influence of the addition ratio and addition time of the compound catalyst on the catalytic effect and thermal performances of the polyester (PETI) are studied and the catalytic mechanism of γ-AlOOH has been analyzed in the reaction process.
2. Experimental section
2.1 Materials
Pure terephthalic acid (PTA, 99.9%) was provided by Yizheng Ge Lin Man Chemical Co., Ltd. Isophthalic acid (IPA, 99.9%) was purchased from AGIC International Chemical Company (Japan). EGSb (Sb content of 56–57%) was supplied by Shanghai Qi Zhi Chemical Co., Ltd. Ethylene glycol (EG, 99.8%) and chloroform (CHCl3) were obtained from Jiangsu Yong Feng Chemical Co., Ltd. AlSA, trimethyl phosphate (TEP), phenol, and acetylene tetrachloride were purchased from Aladdin Industrial Corporation (Shanghai, China). The chemicals without marked purity were of analytical grade.2.2 General methods
When the temperature at the top of the esterification process tower was below 100 °C, the actual water yield of the esterification process reached 92–96% of the theoretical water yield and the reaction would undergo the polycondensation stage. In this stage, the TEP stabilizer, which accounted for 0.005 wt% of the diacid, was added and the reactor was evacuated to low pressure (30 kPa) within 1 h while maintaining the temperature at 270 °C. The prepolycondensation and polycondensation reactions were carried out at 270 °C. When the power of the mixing motor reached 62 W, the rotation speed was decreased to 25 Hz and the final polycondensation was conducted under a vacuum degree of less than 20 Pa. The reaction endpoint was judged by a motor power of 68 W. The synthesized polyester (PETI) was cut into grains and stored in a cool dry place.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) dissolving a PETI sample using a 0.05 mol L−1 KOH–EtOH solution in an automatic potentiometric titrator (916 Ti-Touch, Switzerland) equipped with a photometric electrode. The liquid product of PETI with methanol alcoholysis was analyzed using a gas chromatograph equipped with a mass selective detector (GC-MS 6800, China) to determine the content of diethylene glycol (DEG). Characteristic viscosity was obtained by dissolving a PETI sample in a phenol/acetylene tetrachloride solution (mass ratio, 50
2) dissolving a PETI sample using a 0.05 mol L−1 KOH–EtOH solution in an automatic potentiometric titrator (916 Ti-Touch, Switzerland) equipped with a photometric electrode. The liquid product of PETI with methanol alcoholysis was analyzed using a gas chromatograph equipped with a mass selective detector (GC-MS 6800, China) to determine the content of diethylene glycol (DEG). Characteristic viscosity was obtained by dissolving a PETI sample in a phenol/acetylene tetrachloride solution (mass ratio, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) in a Ubbelohde viscometer at room temperature.
50) in a Ubbelohde viscometer at room temperature.3. Results and discussion
3.1 Analysis of AlSA hydrolysis to form γ-AlOOH
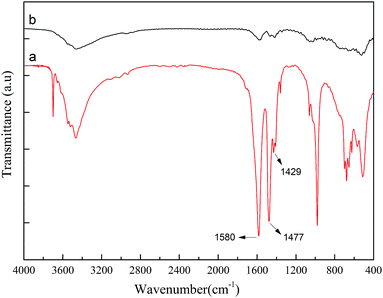
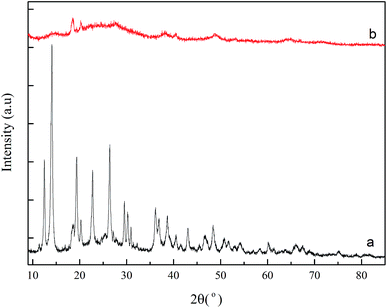
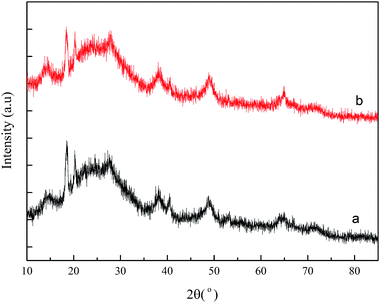
Fig. 1 shows the FT-IR spectra before and after AlSA hydrolysis. The FT-IR spectrum of AlSA (Fig. 1a) exhibited the characteristic bands of νOH (3699–3000 cm−1), νCOO (1580, 1477 cm−1), νCOO (1427, 1408 cm−1), δOH (1062, 978 cm−1), δAl–OH (697, 571 cm−1), and νAl–O (508 cm−1), respectively.22 As shown in Fig. 1b, it was observed that the main characteristic bands of AlSA disappeared after hydrolysis and additional peaks appeared. The peaks at 1382 and 638 cm−1 were the stretching vibration of the –OH groups in AlOOH and the peak at 1083 cm−1 was the symmetrical deformation vibration of AlO–H in AlOOH, which indicated that γ-AlOOH was generated.23Fig. 2 presents the XRD patterns of AlSA (a) and its hydrolysate (b). As can be seen in Fig. 2, it was indicated that γ-AlOOH (JCPDS #74-1895) was generated after AlSA (JCPDS #74-0319) hydrolysis. In addition, some polymorphous Al(OH)3 with bayerite (JCPDS #20-0011) and doyleite (JCPDS #89-4333) was also generated. Therefore, it was necessary to remove the Al(OH)3 precipitate after the hydrolysis reaction.
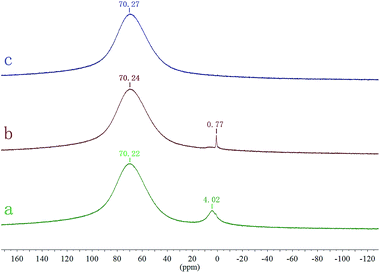
27Al NMR spectroscopy was used to obtain information about the Al species in liquid media. With the chemical shift of Al(H2O)63+ ionized from Al(NO3)3 for reference, the chemical shifts of [Al(H2O)6]3+, [Al2(OH)2(H2O)8]4+, and Al30([Al30O8(OH)56(H2O)24]18+) in six-fold coordinated Al species were 0–0.4 ppm, 0–0.77 ppm, and 70 ppm, respectively.24,25 Fig. 3a shows that AlSA could slowly hydrolyze two coordination structures at the normal temperature of the water. The chemical shift at 4.02 ppm shows that a small amount of Al(H2O)63+ was generated from AlSA hydrolysis and the chemical shift at 70.22 ppm indicates that mostly the Al30([Al30O8(OH)56(H2O)24]18+) coordination ion was produced. Fig. 3b shows that the chemical shift of [Al(H2O)6]3+ disappeared after 3 h hydrolysis at 100 °C and the chemical shift at 0.77 ppm indicates that [(H2O)4Al(μ-OH)(H2O)4]4+ was formed. After displacement of water in the solution by EG, only the Al30([Al30O8(OH)56(H2O)24]18+) coordination ion existed in the solution, as Fig. 3c shows.
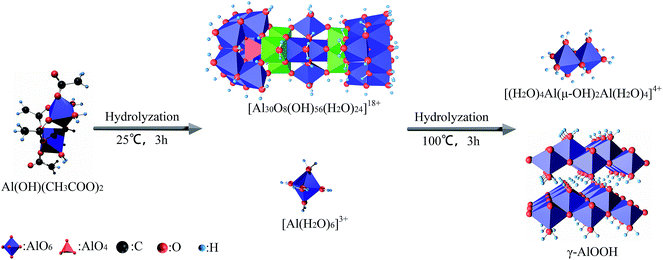
The coordination structure of Al and the carboxylic acid could be distinguished by infrared spectroscopy. The absorption peaks of νCOO in the single coordination structure appeared at 1370 cm−1 and 1650 cm−1, while the absorption peaks of νCOO in the dual coordination structure appeared at 1450 cm−1 and 1580 cm−1.26,27 It could be speculated that the coordination structure of the single and dual coordination structures simultaneously existed in the coordination structures of AlSA from its FT-IR spectrum. Therefore, the change in the hypothetical coordination structure model in the process of AlSA hydrolysis is shown in Fig. 4. The hydrolyzate γ-AlOOH was made up of AlO6 layered octahedrons, which were linked by hydrogen bonds.28
As seen in Fig. 5, AlSA could not dissolve in water and glycol acetate and the particle size distribution of the untreated AlSA was very large, with an average particle diameter d(0.5) of 8.829 μm. The average particle diameter of the γ-AlOOH sol generated from AlSA hydrolysis could reduce to 0.478 μm. After displacement of water with EG, its average particle diameter could reach up to 1.342 μm. This was conducive to improving its surface area by reducing the particle size of the catalyst, which could improve the reaction activity and catalytic property of the catalyst.
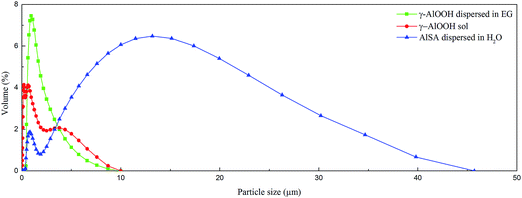 | ||
| Fig. 5 The particle size distributions of AlSA dispersed in H2O, γ-AlOOH dispersed in H2O, and γ-AlOOH dispersed in EG. | ||
3.2 The catalytic effect of the AlSA/EGSb compound catalyst
The balance of the chain growth and thermal degradation reaction existed in the process of polyester polycondensation. According to the catalytic activity theory of metal catalysts proposed by Tomita,29 the rate parameters p of the chain extension reaction of Al and Sb metal catalysts in the process of the catalytic condensation reaction were 47 and 76, respectively, and the rate parameters of the thermal degradation reaction were 5.0 and 5.5, respectively, which indicated that the catalytic activity of the metal Al catalyst was lower than that of the metal Sb catalyst and thus the thermal degradation ability of the metal Al catalyst was weaker than that of the metal Sb catalyst. Fig. 6 shows that the polycondensation time decreased firstly with the improvement of the γ-AlOOH content and then increased with a γ-AlOOH content of more than 60 wt% in the compound catalyst when the γ-AlOOH was added in the esterification stage, which indicated that an obvious synergistic effect existed between the two types of catalysts. Ramadugu, Ilgen, et al.30,31 proved through theoretical calculations and experimental results that Al and Sb catalysts could form complexes. The complexes could enhance the catalytic activity of the compound catalyst and also might adsorb Sb compounds to enhance its catalytic activity due to the good adsorption property of γ-AlOOH.32 However, when γ-AlOOH was added in the prepolycondensation stage, the synergistic effect of the Al and Sb catalysts became quite weak. Therefore, the improvement in the polycondensation time was much lower than that of adding γ-AlOOH in the esterification stage, which might be because γ-AlOOH dehydration proceeded at high temperatures of 265–270 °C to produce Al(OH)3 and Al2O3 in the prepolycondensation stage and its catalytic activity was lost.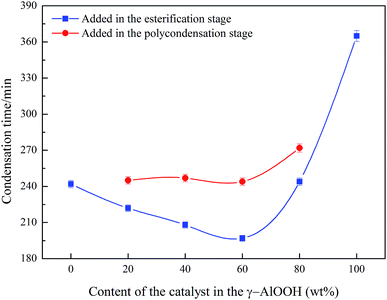 | ||
| Fig. 6 The effect of the additive amount of γ-AlOOH on polycondensation time in the compound catalyst. | ||
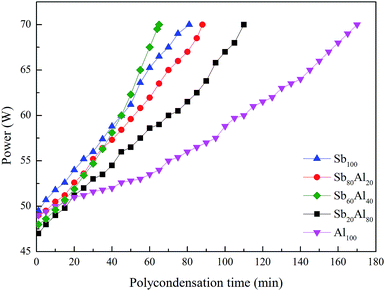
As the viscosity of the melt polyester is proportional to the stirring power, it will be possible to determine the viscosity of the melt polyester by the stirring power change, which could characterize the catalytic efficiency of the catalyst. Fig. 7 shows that when γ-AlOOH was added in the stage of polycondensation, the maximum rate of the polycondensation reaction was observed when the mass proportion of EDSb and γ-AlOOH was 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40. According to the results, the catalytic efficiency of the compound catalyst is higher than that obtained from using EDSb alone. In addition, its catalytic efficiency is lowest when using γ-AlOOH alone. This may prove that EDSb and γ-AlOOH can indeed produce a synergistic catalytic effect.
40. According to the results, the catalytic efficiency of the compound catalyst is higher than that obtained from using EDSb alone. In addition, its catalytic efficiency is lowest when using γ-AlOOH alone. This may prove that EDSb and γ-AlOOH can indeed produce a synergistic catalytic effect.
The PETI polymerization process includes three stages: esterification, precondensation, and condensation. Without the addition of other acids, the esterification reaction could be conducted spontaneously with the catalysis of H+ generated from the reaction system under high temperature and high pressure. In the esterification and prepolycondensation stages, excessive amounts of EG existed in the reaction system. With the reaction of H+, it was easy to produce ether due to the occurring hydroxyl dehydration condensation reaction; meanwhile, the polyester chains containing hydroxyls would also conduct the etherification reaction, which increases the flexibility of the polyester chain segment and reduces the thermal stability of the polyester. Therefore, it is usual to reduce the formation of ether bond structure units with sodium acetate and metal acetate.33,34 These ether bond structure units could be separated into DEG through alcoholysis of PETI, the content of which could indirectly show the occurrence degree of side reactions in the esterification and prepolycondensation stages. Fig. 8 shows that the increase of the γ-AlOOH content in the compound catalyst could significantly reduce the DEG content of PETI, and it was also seen that the inhibition effect of the DEG formation with the addition of γ-AlOOH in the prepolycondensation stage was better than that with the addition of γ-AlOOH in the esterification stage, which might be because Al(OH)3 produced from parts of the dehydration of γ-AlOOH at high temperatures could capture H+ in the reaction system, and thus the generation probability of the ether bond structure unit decreased. The DEG could be produced in the esterification and prepolycondensation stages, so adding γ-AlOOH in the esterification stage could continue to inhibit the generation of DEG in the two stages.
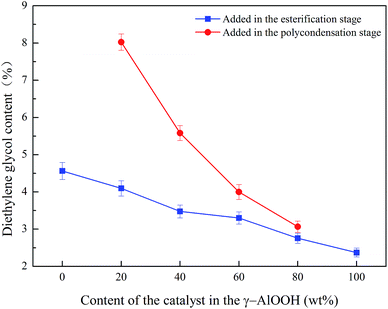 | ||
| Fig. 8 The effect of the content of γ-AlOOH in the compound catalyst on the content of diethylene glycol. | ||
In order to study the chemical composition changes of γ-AlOOH in the catalytic reaction process, γ-AlOOH dispersed in EG was heated at 198 °C for 3 h and the FT-IR spectrum of the obtained product was contrasted with that of ethylene glycol aluminum (EGAl) prepared by the method described elsewhere.4 As shown in Fig. 9, the FT-IR spectrum showed that the methylene absorption peaks appeared at 2947 cm−1 and 2866 cm−1, respectively, indicating that EGAl was produced. In addition, the gas chromatography spectra show that 12.4% of γ-AlOOH was converted to EGAl. Fig. 10 shows that the formation of EGAl prepared by the two methods was accompanied by the generation of Al(OH)3, which could be due to the hydrolysis of EGAl. When Al(OH)3 and EGAl were heated at 198 °C for 3 h, the reaction product was EGAl, as shown in Fig. 9c. These results indicate that part of γ-AlOOH was associated with EGSb in the catalytic reaction process to produce a synergistic catalytic effect, and the rest of γ-AlOOH could react with EG to form EGAl, which could participate in the condensation reaction. Under the same experimental conditions, we compared the polycondensation time of EGSb and EGAl. The results showed that their polycondensation times are 315 min and 240 min, respectively, suggesting that the polycondensation catalytic efficiency of EGAl is lower than that of EGSb. Thus, it is further proven that the synergistic effect of γ-AlOOH and EGSb played an important role in increasing the catalyst efficiency.
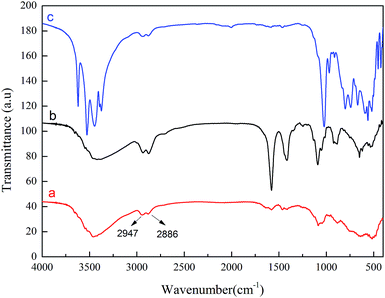 | ||
| Fig. 9 The FT-IR spectrum of EG reacting with γ-AlOOH (a), aluminium isopropoxide (b) and Al(OH)3 (c) at 198 °C, respectively. | ||
 | ||
| Fig. 10 The XRD patterns of the product obtained from EG reacting with γ-AlOOH (a) and aluminium isopropoxide (b) at 198 °C, respectively. | ||
3.3 Properties
In the field of polyester synthesis, besides the polycondensation time, other properties were used to measure the catalytic activity of the catalyst such as thermal performance, characteristic viscosity, and chromaticity. The experiments showed that change in the γ-AlOOH content in the compound catalyst had little impact on the characteristic viscosity of the polyester, which was maintained at about 0.75 dL g−1. However, its content has an obvious impact on the carboxyl end group value and chromaticity of PETI, as shown in Table 1, which could be used to measure the occurrence degree of side reactions in the process of the polyester synthesis. Usually, the more side reactions that take place, the greater the values of the carboxyl end group and chromaticity will be. As the thermal degradation ability and catalytic activity of the Al catalyst were lower than those of the Sb catalyst, the polycondensation time was prolonged and the side effects increased at a certain proportion of the compound catalyst. However, when γ-AlOOH was added in the prepolycondensation stage and the additive amount of the Sb and Al compound catalyst was 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, the values of the carboxyl end group and chromaticity were better than those obtained when only using the EGSb catalyst.
40, the values of the carboxyl end group and chromaticity were better than those obtained when only using the EGSb catalyst.
| Proportionsa (Sb/Al) | Adding stage of γ-AlOOH | IV (dL g−1) | Mnb | –COOH (mol t−1) | Chromaticity | ||
|---|---|---|---|---|---|---|---|
| L | a | b | |||||
| a Sb and Al stand for ethylene glycol stibium (EGSb) and γ-AlOOH, respectively.b The Mn value could be approximately calculated according to the formula [η] = KMα, where K = 2.1 × 10−4 and α = 0.82. | |||||||
| 100/0 | Esterification | 0.79 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772 772 |
13.63 | 71.65 | −0.91 | 5.79 |
| 80/20 | Esterification | 0.75 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 525 525 |
16.07 | 72.64 | −1.02 | 6.17 |
| 60/40 | Esterification | 0.74 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 832 832 |
13.16 | 74.12 | −0.78 | 6.96 |
| 40/60 | Esterification | 0.75 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
16.07 | 70.07 | −0.46 | 11.81 |
| 20/80 | Esterification | 0.75 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 647 647 |
19.54 | 72.03 | −0.20 | 11.08 |
| 80/20 | Prepolycondensation | 0.75 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 456 456 |
14.63 | 71.14 | −1.28 | 8.03 |
| 60/40 | Prepolycondensation | 0.75 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 435 435 |
13.13 | 69.73 | −0.63 | 4.65 |
| 40/60 | Prepolycondensation | 0.75 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508 508 |
16.35 | 67.63 | −0.80 | 10.64 |
| 20/80 | Prepolycondensation | 0.76 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 989 989 |
18.29 | 70.91 | −0.87 | 7.07 |
| 0/100 | Esterification | 0.69 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 432 432 |
20.70 | 72.07 | −1.01 | 9.94 |
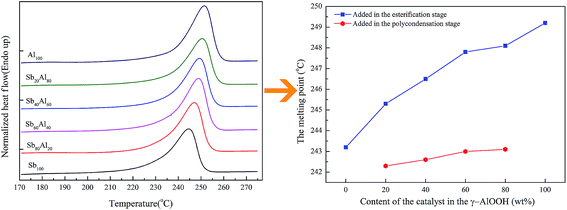
Although the content of γ-AlOOH in the compound catalyst had little impact on the characteristic viscosity of PETI, the melting point (Mm) and the glass transition temperature (Tg) increased with the increase in γ-AlOOH content, as shown in Fig. 11 and 12. When γ-AlOOH was added in the esterification stage, the Mm increased from 243.2 °C to 249.2 °C and the Tg increased from 74.6 °C to 78.6 °C with the increase in the γ-AlOOH addition amount, which might be due to the formation of macromolecular aluminum alkoxide from the catalytic reaction residues reacting with the end hydroxy of PETI or the formation of the coordination structure from the reaction product and end carboxyl of PETI, affecting the thermal motion of the chain segment. The aluminium alkoxide or coordination structure had been verified through experimental results by McMahon and Li et al.35,36 The hypothesis design model of the polymer is shown in Fig. 13. However, when γ-AlOOH was added in the prepolycondensation stage, the Mm of PETI rose only 0.8 °C, which might be due to the deactivation of γ-AlOOH at high temperatures.
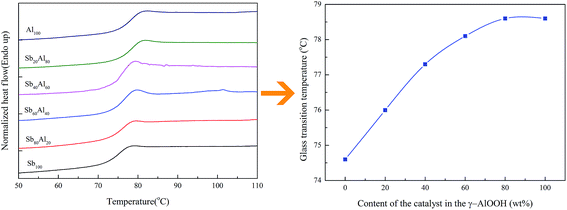 | ||
| Fig. 11 The change plots of the glass transition temperature (Tg) of PETI with the addition of γ-AlOOH in the esterification stage. | ||
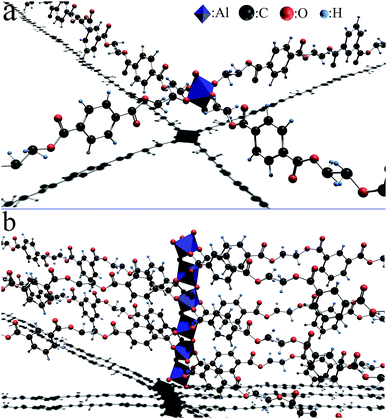 | ||
| Fig. 13 The structural model of the crosslink center from the catalytic reaction residue of γ-AlOOH reacting with the end hydroxyl (a) and end carboxyl (b) of PETI. | ||
Fig. 14 shows the TG curve of the γ-AlOOH added in the esterification and prepolycondensation stages, respectively. It can be seen that the addition stage and the proportion of the compound catalyst had no obvious effects on the thermal decomposition temperature of PETI and there was only a small difference in the residual mass, indicating that the usage of compound catalyst did not affect the thermal stability of PETI.
 | ||
| Fig. 14 The TG curve of the γ-AlOOH added in the esterification stage (a) and the prepolycondensation stage (b). | ||
4. Conclusions
AlSA could ionize the Al(H2O)63+ and Al30([Al30O8(OH)56(H2O)24]18+) coordination ions in water slowly and its main hydrolysates were γ-AlOOH and some polymorphous Al(OH)3 with bayerite and doyleite. The compound catalyst made of γ-AlOOH and EGSb showed obvious synergetic effects in the process of the catalytic polycondensation of PETI. When the γ-AlOOH and EGSb were added in the esterification stage and the content of γ-AlOOH in the compound catalyst was less than 60 wt%, the polycondensation time decreased with increasing γ-AlOOH content. However, when its content was more than 60 wt%, the polycondensation time was prolonged because of the synergetic effect dropping off. When the mass ratio of γ-AlOOH and EGSb was 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, polycondensation time decreased by 18.6% compared with that obtained only using the EGSb catalyst. Meanwhile, the use of γ-AlOOH could play a catalytic role and also reduced the DEG content in the polyester effectively.
40, polycondensation time decreased by 18.6% compared with that obtained only using the EGSb catalyst. Meanwhile, the use of γ-AlOOH could play a catalytic role and also reduced the DEG content in the polyester effectively.
The improvement of the melting point and glass transition temperature show that the catalytic reaction residues of γ-AlOOH could play a crosslink center role in the PETI structure. The catalytic effect of the added γ-AlOOH in the esterification stage for the polyester synthesis was better than that of the added γ-AlOOH in the prepolycondensation stage and EGAl from the γ-AlOOH reacting with EG participated in the polycondensation reaction in the process of the catalytic reaction.
The above results showed that the use of a compound catalyst made of γ-AlOOH and EGSb could improve the catalytic efficiency and thermal properties of PETI. It has industrial application prospects in reducing the usage of Sb series catalysts for polyester synthesis.
References
- G. P. Karayannidis, D. N. Bikiaris, G. Z. Papageorgiou and S. V. Pastras, J. Appl. Polym. Sci., 2002, 86, 1931–1941 CrossRef CAS.
- R. Liu, Y. Hu, M. Hibbs, D. Collard, D. Schiraldi, A. Hiltner and E. Baer, J. Appl. Polym. Sci., 2005, 98, 1615–1628 CrossRef CAS.
- M. Yin, C. Li, G. Guan, X. Yuan, D. Zhang and Y. Xiao, Polym. Eng. Sci., 2009, 49, 1562–1572 CAS.
- B. Xiao, L. Wang, R. Mei and G. Wang, J. Polym. Res., 2011, 18, 2221–2227 CrossRef CAS.
- Y.-K. Yang, S. B. Bae and Y.-T. Hwang, Tetrahedron Lett., 2013, 54, 1239–1242 CrossRef CAS.
- S. Rungchang, S. Numthuam, X. Qiu, Y. Li and T. Satake, J. Food Eng., 2013, 115, 322–329 CrossRef CAS.
- M. Haldimann, A. Alt, A. Blanc, K. Brunner, F. Sager and V. Dudler, Food Addit. Contam., Part A, 2013, 30, 587–598 CrossRef CAS PubMed.
- F. A. El-Toufaili, G. Feix and K. H. Reichert, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 1049–1059 CrossRef CAS.
- S. M. Aharoni, Polym. Eng. Sci., 1998, 38, 1039–1047 CAS.
- S. Keresztes, E. Tatár, V. G. Mihucz, I. Virág, C. Majdik and G. Záray, Sci. Total Environ., 2009, 407, 4731–4735 CrossRef CAS PubMed.
- I. Shigemoto, T. Kawakami and M. Okumura, Polymer, 2013, 54, 3297–3305 CrossRef CAS.
- M. Yin, C. Li, G. Guan, D. Zhang and Y. Xiao, J. Appl. Polym. Sci., 2010, 115, 2470–2478 CrossRef CAS.
- Q. Lin, Y. Gu and D. Chen, J. Appl. Polym. Sci., 2013, 129, 2571–2579 CrossRef CAS.
- J. C. Bersot, N. Jacquel, R. Saint-Loup, P. Fuertes, A. Rousseau, J. P. Pascault, R. Spitz, F. Fenouillot and V. Monteil, Macromol. Chem. Phys., 2011, 212, 2114–2120 CrossRef CAS.
- W. Gitzen, Alumina as a Ceramic Material, Wiley-American Ceramic Society, New York, 1970 Search PubMed.
- Z. Xu, J. Yu and M. Jaroniec, Appl. Catal., B, 2015, 163, 306–312 CrossRef CAS.
- L. Liu, W. Huang, Z.-h. Gao and L.-h. Yin, J. Ind. Eng. Chem., 2012, 18, 123–127 CrossRef CAS.
- T. He, L. Xiang, W. Zhu and S. Zhu, Mater. Lett., 2008, 62, 2939–2942 CrossRef CAS.
- C. Clar, A. Scian and E. Aglietti, Thermochim. Acta, 2003, 407, 33–40 CrossRef CAS.
- G. L. Teoh, K. Y. Liew and W. A. Mahmood, J. Sol-Gel Sci. Technol., 2007, 44, 177–186 CrossRef CAS.
- M. Thorp, J. Kruger, S. Oliver, E. Nilssen and C. Prescott, J. Laryngol. Otol., 1998, 112, 925–928 CAS.
- T. Sato, Netsu Sokutei, 1986, 13, 113–122 CAS.
- H. Hou, Y. Xie, Q. Yang, Q. Guo and C. Tan, Nanotechnology, 2005, 16, 741 CrossRef CAS.
- M. Nofz, J. Pauli, M. Dressler, C. Jügeg and W. Altenburg, J. Sol-Gel Sci. Technol., 2006, 38, 25–35 CrossRef CAS.
- L. Allouche, C. Gérardin, T. Loiseau, G. Férey and F. Taulelle, Angew. Chem., 2000, 112, 521–524 CrossRef.
- Y. Koide and A. R. Barron, Organometallics, 1995, 14, 4026–4029 CrossRef CAS.
- E. Rufino and E. Monteiro, Polymer, 2000, 41, 4213–4222 CrossRef CAS.
- P. Raybaud, M. Digne, R. Iftimie, W. Wellens, P. Euzen and H. Toulhoat, J. Catal., 2001, 201, 236–246 CrossRef CAS.
- K. Tomita, Polymer, 1976, 17, 221–224 CrossRef CAS.
- S. K. Ramadugu and S. E. Mason, J. Phys. Chem. C, 2015, 119, 18149–18159 CAS.
- A. G. Ilgen and T. P. Trainor, Environ. Sci. Technol., 2011, 46, 843–851 CrossRef PubMed.
- X. Song, P. Yang, C. Jia, L. Chen and K. Matras-Postolek, RSC Adv., 2015, 5, 33155–33162 RSC.
- S. Hovenkamp and J. Munting, J. Polym. Sci., Part A-1: Polym. Chem., 1970, 8, 679–682 CrossRef CAS.
- W. Hergenrother, J. Polym. Sci., Polym. Chem. Ed., 1974, 12, 875–883 CrossRef CAS.
- C. N. McMahon, L. Alemany, R. L. Callender, S. G. Bott and A. R. Barron, Chem. Mater., 1999, 11, 3181–3188 CrossRef CAS.
- Z. Li, Y. n. Wu, J. Li, Y. Zhang, X. Zou and F. Li, Chem.–Eur. J., 2015, 21, 6913–6920 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |