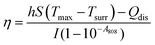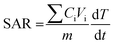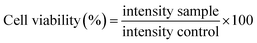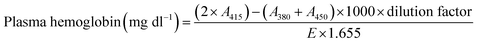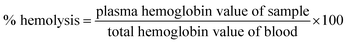Albumin stabilized gold nanostars: a biocompatible nanoplatform for SERS, CT imaging and photothermal therapy of cancer†
Sisini Sasidharana,
Dhirendra Bahadur*b and
Rohit Srivastava*a
aDepartment of Biosciences and Bioengineering, IIT Bombay, Powai, Mumbai, 400076, India. E-mail: rsrivasta@iitb.ac.in
bDepartment of Metallurgical Engineering and Materials Science, IIT Bombay, Powai, Mumbai, 400076, India. E-mail: dhirenb@iitb.ac.in
First published on 30th August 2016
Abstract
Plasmonic nanoparticles have emerged as a multifunctional material for biomedical applications. In comparison to other nanostructures, plasmonic gold nanostars with their anisotropic geometry and photophysical properties have gained immense attention. However, their synthesis based on colloid chemistry has always relied on toxic reagents which raise huge concerns for their use in the body. Moreover, lack of stability and functional group for further bioconjugation limited their use. Herein, gold nanostars (GNS) were synthesized using a simple wet chemistry route without the use of any toxic reagents. These nanostars were further functionalized with albumin to impart stability yielding a monodispersed colloidal solution of particles with ∼120 nm in size. The albumin stabilized gold nanostars (Alb-GNS) tagged with the Raman reporter dye, Rhodamine 6G exhibited a surface enhanced Raman scattering (SERS) property suggesting its potential use for Raman imaging. Alb-GNS exhibited extreme compatibility towards normal (L929) and cancerous (KB) cells with no reactive oxygen species development and hemolytic response towards red blood cells. Additionally, its superior computed tomographic (CT) contrast ability and photothermal activity towards cancer cells bring about Alb-GNS as a single platform for multimodal theragnostic application.
1. Introduction
Cancer tops the list of health concerns all over the world. Early diagnosis and treatment play an important role in the effective cure for cancer.1 To achieve the same, an efficient, minimally invasive, non-surgical method of cancer therapy in combination with a sensitive diagnostic modality is a need of this era. This kind of theragnostic (fusion of therapy and diagnostics) approach can be achieved by either combination of agents on a single platform or a single agent meeting all the prerequisites. Gold nanoparticles having anisotropic structure with its unique properties such as surface plasmon resonance (SPR),2 surface enhanced Raman scattering (SERS)3 and X-ray attenuation4 serve the purpose of a single agent with multiple functionalities. Gold nanostars having branched morphology owing to its high molar extinction coefficient and large absorption cross-section exhibit excellent photothermal effects.5 The sharp and elongated branches of gold nanostars exhibit superior heating in comparison to massive structures due to easy penetration of incoming electric field through thin structure and consequently heating the entire gold matter.5,6 Moreover, its high surface to volume ratio and irregular shape result in drastic enhancement of heat production.5–7 The exceptional confinement and enhancement of electromagnetic field at tips (hot spots) make it the most sought after SERS substrate for Raman imaging and chemical sensing.8 Additionally, these structures find application in real-time particle tracking with strong two-photon luminescence (TPL)9 and photodynamic therapy.10Gold nanostars have been synthesized by either seed mediated or seedless method.11 Synthesis by seedless method is extremely sensitive to reaction conditions, resulting in difficulty to control the size and morphology of nanostructures. On the other hand, preformed seeds act as nucleation points in seeded method and the development of branches upon deposition of gold occurs in a controlled manner.12,13 Though quite a few researchers have reported the synthesis of gold nanostars but involving toxic materials for synthesis such as CTAB,14,15 SDS16 as surfactant or hydroquinone17 as reducing or capping agent and N,N-dimethylformamide (DMF)18,19 as solvent. Although these gold nanostars are promising for biomedical applications, they cannot be used directly after synthesis, major reasons being poor stability at physiological conditions and the lack of functionality required for binding to biomolecules. These limitations underscore the need to develop stable gold nanostars with functional groups. Few researchers have advocated the use of synthetic copolymers,20 polyethylene glycols,21 zwitter ionic surfactants22 for the same. However, protein, especially albumin in addition to being non-toxic, non-immunogenic, biodegradable, cheap,23,24 possess ligand binding properties25–28 and thus can be better utilized for stabilization and functionalization of gold nanoparticles. In order to accomplish the same, we have developed gold nanostars tuned to NIR range (clear window for biological application) by simple reduction with ascorbic acid using seed mediated method9 and stabilized it with albumin (Alb-GNS). The surface enhanced Raman scattering of these particles were analyzed by tagging with Rhodamine 6G (R6G) molecule for Raman imaging. The blood and cell compatibility as well as X ray-CT contrast ability along with photothermal cytotoxicity of these nanostructures were also evaluated.
2. Experimental
2.1 Materials
Tetra chloro auric acid (HAuCl4) [Spectrochem], silver nitrate (AgNO3) [Acros organics] trisodium citrate dihydrate (Na3C6H5O7·2H2O) [Merck], ascorbic acid [Merck], bovine serum albumin [Sigma Aldrich] and Rhodamine 6G [Sigma Aldrich] were used for the study.2.2 Methods
Synthesis of gold seed nanoparticles was carried out by the conventional Turkevich method29,30 with the use of citrate as reducing and capping agent. In a typical experiment, 10 ml of HAuCl4 was boiled and 1.5 ml of 1% trisodium citrate was added. The solution was boiled for 15 min keeping the volume to be stable. The resultant solution was cooled, centrifuged at 6000 rpm for 20 min and redispersed in deionized water. The colloidal solution was then stored at 4 °C.
These gold seed nanoparticles (GS) synthesized with citrate was used to prepare gold nanostars. Typically, 100 μl of GS was added to 10 ml of 0.25 mM HAuCl4 with 10 μl of 1 M HCl. The solution was stirred at room temperature with a speed of 1000 rpm. To this solution, 100 μl of 3 mM AgNO3 and 50 μl of 100 mM ascorbic acid were added simultaneously. The solution was stirred for 30 s and 10 mg ml−1 of BSA was added during the generation of greenish blue color. This resultant solution was then stirred for an hour to yield albumin stabilized gold nanostar (Alb-GNS). The solution was then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and pellet was redispersed in deionized water. Rhodamine 6G was added to the dispersed pellet and incubated for an hour to obtain R6G bound albumin stabilized gold nanostar (R6G-Alb-GNS). The solution was centrifuged, pellet was washed several times with deionized water and stored at 4 °C.
000 rpm for 30 min and pellet was redispersed in deionized water. Rhodamine 6G was added to the dispersed pellet and incubated for an hour to obtain R6G bound albumin stabilized gold nanostar (R6G-Alb-GNS). The solution was centrifuged, pellet was washed several times with deionized water and stored at 4 °C.
Gold nanostars (GNS) were synthesized in a similar fashion without the use of albumin for stabilization.9
2.3 Characterization
The size and stability of GNS and Alb-GNS were evaluated using Dynamic Light Scattering (DLS) particle sizer and zeta potential analyzer (Beckman Coulter, USA). The nanostructures were analyzed using Perkin Elmer spectrophotometer (Lambda 25) for its SPR characteristics using quartz cuvette of 1 ml at a path length of 1 cm. Size and morphology of the samples were analyzed by field emission gun scanning electron microscope – FEG-SEM (JSM-7600F, JEOL) and field emission gun transmission electron microscope FEG-TEM (JEM 2100-F, JEOL). For FEG-SEM imaging, dilute concentration of samples were mounted and air-dried on stubs which was further sputter coated with gold. An acceleration voltage of 5 kV and working distance of 4.5 mm was used for imaging. In case of FEG-TEM, a diluted drop of the samples was placed on carbon coated copper grid. In case of Alb-GNS, 1% of phosphotungstic acid was used as negative staining in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilutions to see the presence of albumin. Imaging was performed at various magnifications and Selected Area Electron Diffraction pattern (SAED) as well as its elemental composition with EDAX were studied. In order to evaluate the conversion of Au3+ to Au and Ag incorporated in the particles, the pellet of nanoparticles obtained after centrifugation was digested with acids and subjected to ICP-AES analysis along with precursor solution and appropriate controls. A Fourier transform infrared (FT-IR) spectrum was employed to assess functional groups on the nanoparticles and was recorded with Fourier Transform infrared Spectrometer (MAGNA 550, Nicolet Instruments Corporation, USA) using KBr method. The spectral scan was carried out over a frequency range of 4000 to 400 cm−1. The amount of albumin grafted on Alb-GNS was determined using CHN elemental analysis with CHNS (O) Analyzer (FLASH EA 1112, Thermo Finnigan). The percentage of protein content was calculated using the following equation:
200 dilutions to see the presence of albumin. Imaging was performed at various magnifications and Selected Area Electron Diffraction pattern (SAED) as well as its elemental composition with EDAX were studied. In order to evaluate the conversion of Au3+ to Au and Ag incorporated in the particles, the pellet of nanoparticles obtained after centrifugation was digested with acids and subjected to ICP-AES analysis along with precursor solution and appropriate controls. A Fourier transform infrared (FT-IR) spectrum was employed to assess functional groups on the nanoparticles and was recorded with Fourier Transform infrared Spectrometer (MAGNA 550, Nicolet Instruments Corporation, USA) using KBr method. The spectral scan was carried out over a frequency range of 4000 to 400 cm−1. The amount of albumin grafted on Alb-GNS was determined using CHN elemental analysis with CHNS (O) Analyzer (FLASH EA 1112, Thermo Finnigan). The percentage of protein content was calculated using the following equation:| % protein = % nitrogen × NF |
The crystallinity of GNS and Alb-GNS were analyzed by X-ray diffraction (XRD) measurements (Philips X'PERT PRO diffractometer with a Cu-Kα source, λ = 1.54056 Å). The spectrum was recorded from 5–85° and phase identification was done with standard JCPDS database.
Surface enhanced Raman effect of Alb-GNS was analyzed by studying the characteristic Raman signal of R6G dye. The amount of R6G bound to Alb-GNS was determined by the indirect method (the unadsorbed dye was calculated spectrophotometrically and subtracted from the original amount). The same amount of dye was used as a control during the analysis. The sample solution was drop casted on a glass slide and was subjected to 514 nm laser at 10 mW power with an accumulation time of 20 s.
The photothermal transduction effect of Alb-GNS was evaluated with 808 nm NIR laser (500 mW, 1.3 W cm−2, PMC, India). In a typical experiment, 200 μl of the Alb-GNS (25 μg ml−1 of gold concentration) and control solution (water and albumin) were added into wells (of a 96-well plate) with due care to avoid any kind of heat transfer. The well plate was floated on a water bath maintained at 37 °C. The laser was passed through the wells containing control and sample solutions at a predetermined distance. The temperature rise was recorded with digital thermometer at 0, 2.5, 5, 7.5 and 10 min and was plotted with 37 °C as baseline. All the experiments were performed in triplicates.
To evaluate the photothermal conversion efficiency, a digital thermometer was clamped and inserted into quartz cuvette containing 2 ml of Alb-GNS solution (25 μg ml−1 of gold concentration) to measure the temperature. The bottom of the cuvette was kept raised from the base to avoid any kind of temperature fluctuation and the probe of the thermometer was submerged into the solution in such a way that it does not fall in the illumination path of light from the laser. The temperature increment of solution was noted while irradiating with laser (500 mW, 1.3 W cm−2) for 20 min and further during cooling period at specified intervals. Water was used as a control solution and experiment was carried out in triplicates. The photothermal efficiency was determined by the following equation:
The photothermal conversion efficiency was also evaluated by the ability of nanoparticle to absorb energy per unit mass, also known as specific absorption rate (SAR) using the following equation:
The photothermal stability of Alb-GNS was evaluated by measuring its absorbance using spectrophotometer (Lambda 25, Perkin Elmer) before and after laser irradiation in the photothermal transduction experiment.
The CT contrast efficiency of Alb-GNS was analyzed using a 64-slice cardiac capable PET-CT Scanner (Biograph mCT-Molecular CT, SIEMENS). Different concentrations (in triplicates) of Alb-GNS were added into 96-well plates along with Omnipaque (clinically approved contrast) as reference and Milli Q water as negative control. The contrast of samples were determined with a tube voltage of 100 kVp, tube current of 200 μA, slice thickness of 1 mm, scan time of 6 s and rotation time of 1 s. X-ray contrast of samples were determined by selecting manually the regions of interest of same diameter and resolving Hounsfield units using ImageJ 1.48 software.
2.4 Cell culture studies
Normal mouse fibroblast cells (L929) and oral epithelial carcinoma cells (KB) were procured from the National Center for Cell Science, Pune, India. Cell lines were grown in a humidified environment of 5% CO2 at 37 °C in a T25 flask in Dulbecco's modified Eagles Medium (DMEM) (HiMedia, India) supplemented with 10% fetal bovine serum (FBS), 50 IU ml−1 penicillin and 50 μg ml−1 streptomycin (HiMedia, India).where, intensity sample and intensity control are the intensity values obtained from cells treated with sample and basal media respectively.
2.5 Blood compatibility studies
The blood compatibility studies were performed according to national health guidelines after obtaining clearance from IIT Bombay institutional ethical and biosafety committee (reference number – IITB-IEC/2016/003) and appropriate informed consent from volunteers. Whole blood (5 ml) was withdrawn from three healthy volunteers aged 22–32 years in vials containing sodium citrate. All studies were carried out in triplicates with fresh blood.Amount of plasma hemoglobin was calculated from the equation as follows,
3. Results and discussion
A gold seed-mediated approach which employs silver ions for directional growth and ascorbic acid as reducing agent with no other hazardous chemicals is used for the generation of gold nanostars which is further stabilized with albumin as seen in Fig. 1. The classical citrate method is the simplest method to produce gold seed nanoparticles.29,30 The addition of citrate in the boiling solution of HAuCl4 resulted in ruby red color colloidal solution confirming the formation of gold seed nanoparticles as depicted in the inset of Fig. 2A. The citrate ions reduce the Au3+ ions to Au and act as both capping and reducing agent resulting in the formation of stable colloidal solution of gold seed nanoparticles.30 These gold seed act as a template on which gold atoms get further reduced by ascorbic acid during synthesis of gold nanostars. Silver ions are added simultaneously forming silver atoms which get adsorbed on to the surface of growing particle and are believed to prevent its isotropic growth yielding bluish green colored GNS. Halide and silver (Ag+) ions have been reported to affect the growth and formation of gold nanostructures.34 Au grew from the active sites on the surface of the GS due to catalytic effect of silver atoms generating GNS. Even the addition of HCl helped in formation of branched structures owing to the role of chloride ions.9 In the absence of silver ions, formation of rods and spheres were noted. Moreover, the Ag+ ions and ascorbic acid need to be added together for proper development of branches, the early addition of Ag+ resulted in precipitation of black silver chloride particles and no branched structures of gold formed on addition of ascorbic acid. If Ag+ is added too late, the added ascorbic acid already formed large gold spheres. Hence the addition of reagents at the appropriate ratio and time played a crucial role in the formation of gold nanostars. The gold nanostars synthesized using this seed-mediated method were unstable if stored for a long time as depicted in the inset of Fig. 2B and possess no free functional groups for further conjugation with biomolecules for its target specific application. Hence to overcome these difficulties, GNS were required to be stabilized and functionalized, for which albumin seemed to be an ideal choice. Albumin is known to adsorb on gold particle by electrostatic interaction or by the linkage of its thiol molecule with gold.26–28 Being a protein, it has lot of functional groups which can be further used for conjugation purposes. In the synthesis procedure of albumin stabilized gold nanostars (Alb-GNS), the addition of albumin at an appropriate time is crucial. It is to be added only when the bluish green colour starts to appear; otherwise its addition in the beginning of synthesis might hinder the precise formation of branched structure. The resulting bluish green solution was found to be very stable for as long as 6 weeks in water as well as physiological solutions as shown in the inset of Fig. 2C and ESI Fig. S1† respectively. The R6G tagged Alb-GNS were synthesized to analyze the SERS activity which can further be explored for Raman imaging. The conversion yield of Au3+ to Au was found to be 88.8 ± 2% with the help of ICP-AES analysis. The loss of particles in the supernatant during centrifugation can also be a reason for the decrease in yield. The amount of silver was found to be 4.6 ± 0.2% of the gold content in Alb-GNS.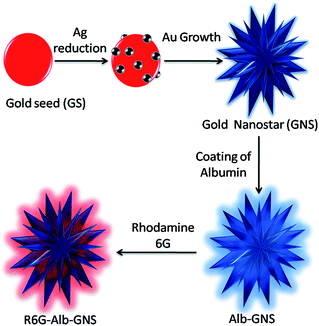 | ||
| Fig. 1 Schematic representation of formation of albumin stabilized gold nanostars (Alb-GNS) using gold seed mediated method. | ||
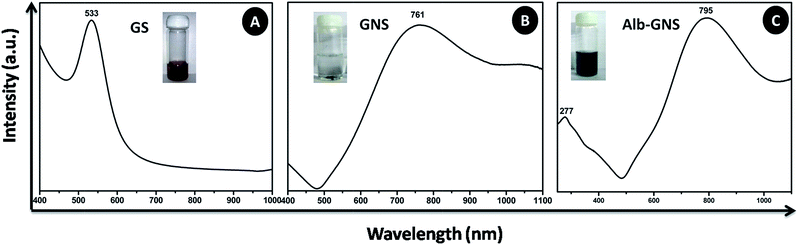
The gold seed nanoparticle synthesized by citrate method showed a SPR λmax at 533 nm as shown in Fig. 2A which confirmed the formation of spherical seed nanoparticles. The aborbance of GNS is very broad and spreads over a wide range from 500 to >1100 nm and can be tuned to obtain the SPR λmax at any region in the NIR by variation in parameters during synthesis such as pH, silver and ascorbic acid concentration. As the 790–810 nm wavelengths in the therapeutic window shows minimum absorbance from biomolecules, this region of SPR λmax is preferred. The GNS synthesized as described in the procedure exhibited SPR λmax at 761 nm (shown in Fig. 2B), however albumin stabilization resulted in sharper peak and a slight red-shift with SPR λmax at 795 nm as shown in Fig. 2C. The absorbance peak around 277 nm in the spectra of Alb-GNS is due to the classic absorbance of aromatic amino acids namely tryptophan and tyrosine of proteins, hence confirming the presence of albumin coat over the nanostructures. Alb-GNS were found to be stable with a zeta potential of −29 ± 3 mV and hydrodynamic diameter of 220 nm whereas the gold nanostars (GNS) exhibit a zeta potential value of −9.3 ± 1 mV only accounting for its instability. The Alb-GNS were more dispersed and stable than GNS owing to the presence of albumin coat which prevents the formation of aggregates. To evaluate the morphology of anisotropic nanostructures being formed, FEG-SEM imaging was carried out for GNS and Alb-GNS. As depicted in Fig. 3A and B the nanostructures are branched in nature with a size of ∼120 nm. The halo region surrounding the branched structure in Fig. 3B reveals the presence of albumin coat in Alb-GNS. To decipher the morphology in detail, FEG-TEM images were taken. The images of gold seed nanoparticles as seen in Fig. 3C confirm its spherical nature with an average size of 50 nm. The images of GNS and Alb-GNS revealed nanostructure with branched morphology and size of ∼120 nm as shown in Fig. 3E and G respectively. The dispersed nature of GS and Alb-GNS and the aggregated nature of GNS can be deciphered from the images taken under low magnification seen as inset of Fig. 3C, G and E respectively. A halo coat on the nanostructures in case of Alb-GNS seen in Fig. 3G is due to the negative staining by phosphotungstic acid which confirms the presence of albumin coat. The lattice structure and electron diffraction patterns of GS, GNS and Alb-GNS as seen in Fig. 3D, F and H suggest its crystalline nature. However, the diffuse rings in the electron diffraction pattern of Alb-GNS, as shown in the inset of Fig. 3H, suggest the presence of amorphous albumin. The presence of gold was revealed in EDAX spectra of the nanostructures as shown in ESI Fig. S2† with no other elemental presence other than that related to copper grid employed for imaging. Similar spectra were obtained for all the nanostructures (data not shown).
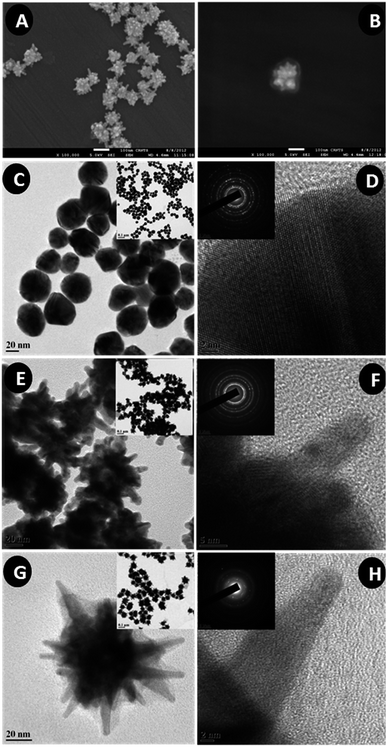
The presence of albumin on Alb-GNS was further confirmed using FTIR analysis (Fig. 4A) by analyzing the functional groups present on the surface of nanostructures. The strong absorption band at 3440 cm−1 in GNS is characteristic of H–O–H bending of water molecules adsorbed on particles. The absorption band at 1638 cm−1 in case of GNS, is also attributed to O–H bending vibrations. As seen in Fig. 4A absorption band in the region of 3000–3500 cm−1 is usually due to O–H bending vibrations, however the peak near 3300 cm−1 can be due to N–H bending vibration present in both albumin and Alb-GNS. Similarly the peak near 2950 cm−1, in the region of 1700–1600 cm−1 and 1600–1500 cm−1 attributed to CH2 stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes (amide I) and mixture of C–N stretching along with N–H bending modes (amide II) respectively in albumin are present in Alb-GNS as well suggestive of the presence of albumin on the particles.35 The albumin content in Alb-GNS was found to be 24 ± 3% as evaluated by CHN elemental analysis. X-ray diffraction of the gold nanostructures as depicted in ESI Fig. S3† exhibited all the peaks which are characteristic of metallic gold in accordance with JCPDS-03-065-2870. Small nanocrystallite size of the synthesized nanoparticles was deciphered from the peak broadening of the XRD spectra. The Raman spectra as shown in Fig. 4B depict the SERS property of Alb-GNS. As can be seen, R6G bound to Alb-GNS exhibited an enhanced spectrum as compared to R6G alone. The band at approximately 613 cm−1 is attributed to the C–C–C ring in-plane vibration mode. The bands at 1126 and 1185 cm−1 are due to the C–H in-plane bend modes. The bands at 1312 and 1545 cm−1 are a result of the N–H in plane bend modes and those at 1748 cm−1 are due to the C–C stretching modes.36 Photothermal transduction experiments were performed to analyze the photothermal efficacy of Alb-GNS, by studying the increase in temperature achieved upon laser irradiation (Fig. 4C). Alb-GNS was able to achieve the critical temperature (for tumor cell death) of 43 °C within 8–9 min; however, water and albumin solution reached only upto 38 °C during the same time duration. The photothermal transduction efficiency measures the efficiency of a material to convert the absorbed light into heat resulting in increased temperature of the surroundings. The efficiency of Alb-GNS was evaluated by irradiating the sample solution of known optical density at ambient temperature for 20 min which resulted in temperature rise (10 °C) and further cooling upon switching off the laser as shown in ESI Fig. S4A.† The thermal time constant was determined from the graph of time versus natural logarithm of temperature from cooling period as depicted in ESI Fig. S4B.† The Alb-GNS exhibited an average photothermal transduction efficiency of 65% (calculations are described in ESI†) and specific absorption rate (SAR) of 6.7 kW g−1 at 1.3 W cm−2 of laser power. Alb-GNS exhibited similar absorbance intensity and SPR λmax both, before and after photothermal transduction experiment suggesting its high photothermal stability.
O stretching modes (amide I) and mixture of C–N stretching along with N–H bending modes (amide II) respectively in albumin are present in Alb-GNS as well suggestive of the presence of albumin on the particles.35 The albumin content in Alb-GNS was found to be 24 ± 3% as evaluated by CHN elemental analysis. X-ray diffraction of the gold nanostructures as depicted in ESI Fig. S3† exhibited all the peaks which are characteristic of metallic gold in accordance with JCPDS-03-065-2870. Small nanocrystallite size of the synthesized nanoparticles was deciphered from the peak broadening of the XRD spectra. The Raman spectra as shown in Fig. 4B depict the SERS property of Alb-GNS. As can be seen, R6G bound to Alb-GNS exhibited an enhanced spectrum as compared to R6G alone. The band at approximately 613 cm−1 is attributed to the C–C–C ring in-plane vibration mode. The bands at 1126 and 1185 cm−1 are due to the C–H in-plane bend modes. The bands at 1312 and 1545 cm−1 are a result of the N–H in plane bend modes and those at 1748 cm−1 are due to the C–C stretching modes.36 Photothermal transduction experiments were performed to analyze the photothermal efficacy of Alb-GNS, by studying the increase in temperature achieved upon laser irradiation (Fig. 4C). Alb-GNS was able to achieve the critical temperature (for tumor cell death) of 43 °C within 8–9 min; however, water and albumin solution reached only upto 38 °C during the same time duration. The photothermal transduction efficiency measures the efficiency of a material to convert the absorbed light into heat resulting in increased temperature of the surroundings. The efficiency of Alb-GNS was evaluated by irradiating the sample solution of known optical density at ambient temperature for 20 min which resulted in temperature rise (10 °C) and further cooling upon switching off the laser as shown in ESI Fig. S4A.† The thermal time constant was determined from the graph of time versus natural logarithm of temperature from cooling period as depicted in ESI Fig. S4B.† The Alb-GNS exhibited an average photothermal transduction efficiency of 65% (calculations are described in ESI†) and specific absorption rate (SAR) of 6.7 kW g−1 at 1.3 W cm−2 of laser power. Alb-GNS exhibited similar absorbance intensity and SPR λmax both, before and after photothermal transduction experiment suggesting its high photothermal stability.
Alb-GNS was analyzed for CT imaging in comparison to Omnipaque (polyiodinated compound). Equal concentrations of iodine and gold were imaged at a tube current and tube voltage of 200 μA and 100 kVp respectively. A visibly brighter contrast is seen at concentrations more than 0.5 mg ml−1 for Alb-GNS in comparison to Omnipaque as seen in Fig. 5A. The signal intensity measured quantitatively in Hounsefield units using ImageJ 1.48 software revealed a 2 times higher intensity for Alb-GNS than Omnipaque at same material concentrations (Fig. 5B). Superior contrast of Alb-GNS is owing to better attenuation of X rays because of higher Z-number of gold.4 This is of clinical significance as lesser amount of nanomaterial can give a better contrast hence limiting toxicity.
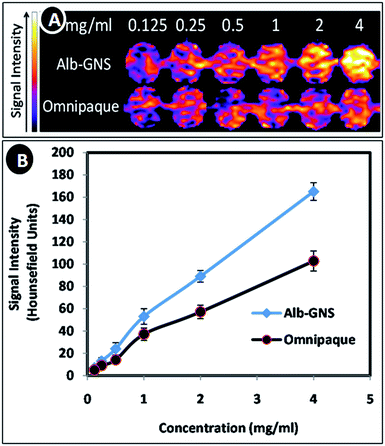 | ||
| Fig. 5 (A) CT contrast images of Alb-GNS and Omnipaque with increasing concentrations (pseudo colored) and (B) graphical representation of computed Hounsefield unit values of Alb-GNS and Omnipaque. | ||
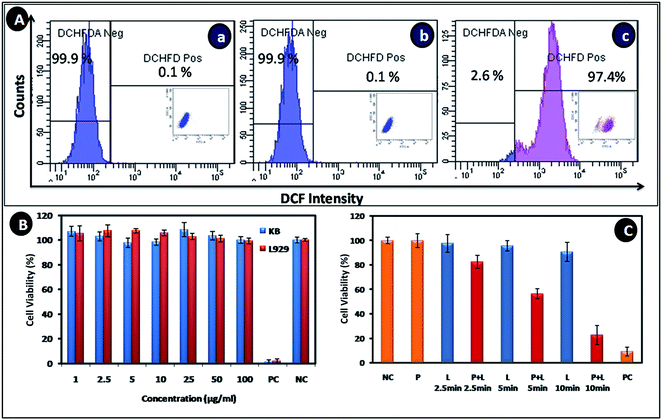
Alb-GNS need to be non-toxic in nature for its biomedical application. They were evaluated for generation of reactive oxygen species using ROS assay by flowcytometry with H2DCFDA and cytotoxicity using Alamar blue assay. As seen in Fig. 6A, KB cells treated with high concentration of Alb-GNS (100 μg ml−1) did not show any significant ROS generation (0.1%) similar to untreated cells owing to the ROS scavenging property of proteins associated with Alb-GNS. However, cells treated with H2O2 (positive control) exhibited high ROS activity with 97.4% of cells being DCFH-DA +ve. Additionally, ICP-AES studies revealed presence of ∼80% of nanoparticles in KB cells which may be a result of high adherence to the cell membranes. Alamar blue cell viability analysis (Fig. 6B) of KB and L929 cells showed more than 90% of cells to be viable and metabolically active even at high concentrations such as 100 μg ml−1. These studies prove the cytocompatible nature of Alb-GNS on different cell lines with no adverse effects on metabolic activity.
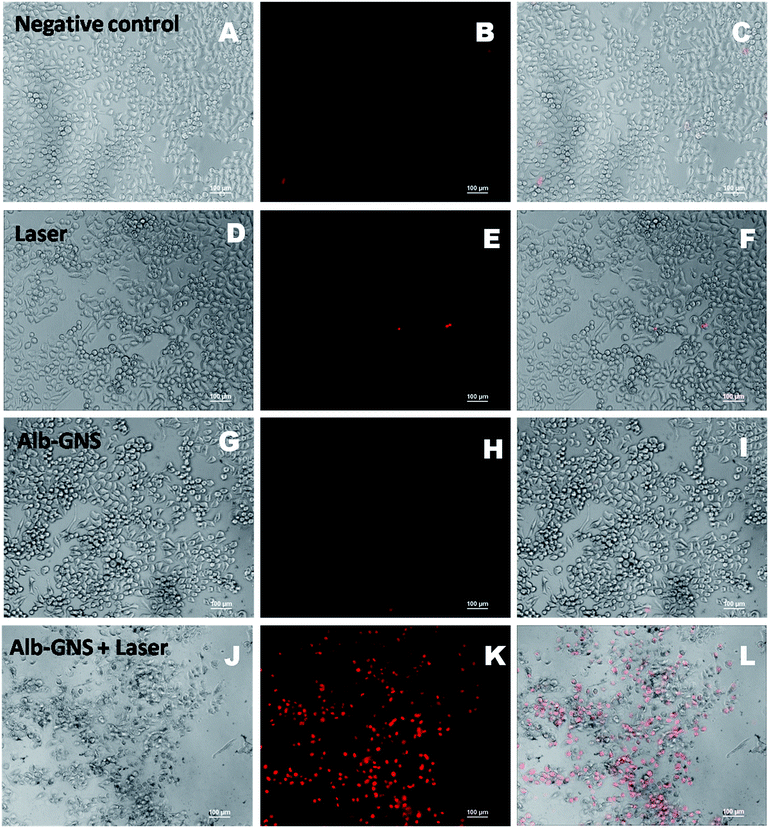
Photothermal mediated cytotoxicity experiment on KB cells revealed that laser irradiation after treatment with Alb-GNS resulted in marked cell death when compared with just laser or particle treatment alone as shown in Fig. 6C. An increase in duration of laser exposure resulted in decrease of percentage of cells which are viable with significant cell death after particle treatment and 10 min of laser exposure. Similarly, cell death after various modes of treatment were analyzed qualitatively using a nuclear staining dye-propidium iodide which mark dead cell as red under fluorescent microscope. DIC (left column), fluorescent showing the PI stain (middle column) and merged (right column) images of KB cells subjected to various types of treatment can be seen in Fig. 7. The cells subjected to just laser treatment or particle (100 μg ml−1 of Alb-GNS) treatment and negative control (no treatment) do not show any significant cell death (no cells were stained with red color). However, upon laser irradiation for 10 min after treatment with Alb-GNS marked cell death can be accounted (dead cells stained red with PI), hence proving the photothermal efficacy of Alb-GNS in cancer treatment.
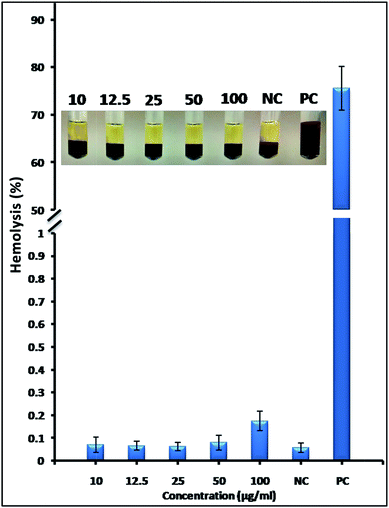
Alb-GNS would come in contact with blood upon intra-tumoral or intravenous administration for its potential application as photothermal and CT contrast agent. This necessitates hemolytic analysis of Alb-GNS.37 Hemolysis is rupturing of erthyrocytes or red blood cells and leakage of hemoglobin into plasma. The hemolytic effect of Alb-GNS on RBC were analyzed by treating the whole blood with varying concentrations (10, 12.5, 25, 50, 100 μg ml−1) of Alb-GNS and evaluation of hemolysis (in percentage). Photograph in inset of Fig. 8 shows leakage of hemoglobin into plasma (supernatant) in case of 1% Triton X-100 (positive control) and undamaged RBC sediment with clear plasma supernatant in blood treated with Alb-GNS similar to negative control (0.9% saline). Quantitative evaluation of the samples by spectrophotometer revealed ∼75% of hemolysis in positive control and no significant hemolysis in blood samples treated even with high concentration (100 μg ml−1) of Alb-GNS similar to negative control (Fig. 8). This confirms the non-hemolytic nature of Alb-GNS.
4. Conclusions
Gold nanostars ∼120 nm in size are synthesized by a simple reduction method and functionalized with albumin resulting in a highly stable solution. They exhibited absorption in the near infra red region with SPR λmax at 795 nm and significant hyperthermic activity as well as superior CT contrast. The compatibility analysis on normal (L929), cancer cell line (KB) and red blood cells revealed its extreme compatibility even at high concentrations (100 μg ml−1) without generation of any reactive oxygen species. These cell and blood compatible albumin stabilized gold nanostars also exhibited marked photothermal cytotoxicity on KB cancer cell line. Hence this study demonstrates the development of highly stable and biocompatible albumin functionalized gold nanostars as a potential SERS, CT contrast and photothermal agent for cancer.Acknowledgements
Authors gratefully acknowledge Nanavati Super Speciality Hospital, Mumbai for CT imaging of samples. Authors also thank sophisticated analytical instrument facility (SAIF), IRCC and Department of Physics, IIT Bombay for the various facilities used for analysis.References
- A. M. Ilbawi and B. O. Anderson, Sci. Transl. Med., 2015, 7, 1–6 CrossRef PubMed.
- X. Huang and M. A. El-Sayed, J. Adv. Res., 2010, 1, 13–28 CrossRef.
- M. V. Yigit and Z. Medarova, Am. J. Nucl. Med. Mol. Imaging, 2012, 2, 232–241 CAS.
- J. F. Hainfeld, D. N. Slatkin, T. M. Focella and H. M. Smilowitz, Br. J. Radiol., 2006, 79, 248–253 CrossRef CAS PubMed.
- F. Hao, C. L. Nehl, J. H. Hafner and P. Nordlander, Nano Lett., 2007, 7, 729–732 CrossRef CAS PubMed.
- G. Baffou, R. Quidant and C. Girard, Appl. Phys. Lett., 2009, 94, 153109 CrossRef.
- W. Hasan, C. L. Stender, M. H. Lee, C. L. Nehl, J. Lee and T. W. Odom, Nano Lett., 2009, 9, 1555–1558 CrossRef CAS PubMed.
- F. Tian, F. Bonnier, A. Casey, A. E. Shanahan and H. J. Byrne, Anal. Methods, 2014, 6, 9116–9123 RSC.
- H. Yuan, C. G. Khoury, H. Hwang, C. M. Wilson, G. A. Grant and T. Vo-Dinh, Nanotechnology, 2012, 23, 75102–75111 CrossRef PubMed.
- S. Wang, P. Huang, L. Nie, R. Xing, D. Liu, Z. Wang, J. Lin, S. Chen, G. Niu, G. Lu and X. Chen, Adv. Mater., 2013, 25, 3055–3061 CrossRef CAS PubMed.
- A. Guerrero-Martínez, S. Barbosa, I. Pastoriza-Santos and L. M. Liz-Marzán, Curr. Opin. Colloid Interface Sci., 2011, 16, 118–127 CrossRef.
- T.-H. Tran and T.-D. Nguyen, Colloids Surf., B, 2011, 88, 1–22 CrossRef CAS PubMed.
- S. Sasidharan, D. Bahadur and R. Srivastava, ACS Appl. Mater. Interfaces, 2016, 8, 15889–15903 CAS.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648–8649 CrossRef CAS PubMed.
- C. L. Nehl, H. Liao and J. H. Hafner, Nano Lett., 2006, 6, 683–688 CrossRef CAS PubMed.
- A. Shiohara, J. Langer, L. Polavarapu and L. M. Liz-Marzan, Nanoscale, 2014, 6, 9817–9823 RSC.
- M. Schutz, D. Steinigeweg, M. Salehi, K. Kompe and S. Schlucker, Chem. Commun., 2011, 47, 4216–4218 RSC.
- C. G. Khoury and T. Vo-Dinh, J. Phys. Chem. C, 2008, 2008, 18849–18859 Search PubMed.
- S. Barbosa, A. Agrawal, L. Rodríguez-Lorenzo, I. Pastoriza-Santos, R. A. Alvarez-Puebla, A. Kornowski, H. Weller and L. M. Liz-Marzán, Langmuir, 2010, 26, 14943–14950 CrossRef CAS PubMed.
- G. Cavallaro, D. Triolo, M. Licciardi, G. Giammona, G. Chirico, L. Sironi, G. Dacarro, A. Donà, C. Milanese and P. Pallavicini, Biomacromolecules, 2013, 14, 4260–4270 CrossRef CAS PubMed.
- P. Pallavicini, E. Cabrini, G. Cavallaro, G. Chirico, M. Collini, L. D'Alfonso, G. Dacarro, A. Donà, N. Marchesi, C. Milanese, A. Pascale, L. Sironi and A. Taglietti, J. Inorg. Biochem., 2015, 151, 123–131 CrossRef CAS PubMed.
- A. Casu, E. Cabrini, A. Donà, A. Falqui, Y. Diaz-Fernandez, C. Milanese, A. Taglietti and P. Pallavicini, Chem.–Eur. J., 2012, 18, 9381–9390 CrossRef CAS PubMed.
- J. M. Irache and S. Espuelas, in Nanotechnologies for the Life Sciences, Wiley-VCH Verlag GmbH & Co. KGaA, 2007 Search PubMed.
- A. O. Elzoghby, W. M. Samy and N. Elgindy, J. Controlled Release, 2012, 157, 168–182 CrossRef CAS PubMed.
- F. A. de Wolf and G. M. Brett, Pharmacol. Rev., 2000, 52, 207–236 CAS.
- O. Horovitz, G. Tomoaia, A. Mocanu, T. Yupsanis and M. Tomoaia-Cotisel, Gold Bull., 2007, 40, 213–218 CrossRef CAS.
- M. Iosin, F. Toderas, P. L. Baldeck and S. Astilean, J. Mol. Struct., 2009, 924–926, 196–200 CrossRef CAS.
- S. Khan, A. Gupta, N. C. Verma and C. K. Nandi, J. Chem. Phys., 2015, 143, 164709 CrossRef CAS PubMed.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- J. Turkevich, P. C. Stevenson and J. Hillier, J. Phys. Chem., 1953, 57, 670–673 CrossRef CAS.
- C. Ayala-Orozco, C. Urban, M. W. Knight, A. S. Urban, O. Neumann, S. W. Bishnoi, S. Mukherjee, A. M. Goodman, H. Charron, T. Mitchell, M. Shea, R. Roy, S. Nanda, R. Schiff, N. J. Halas and A. Joshi, ACS Nano, 2014, 8, 6372–6381 CrossRef CAS PubMed.
- S. Freddi, L. Sironi, R. D'Antuono, D. Morone, A. Donà, E. Cabrini, L. D'Alfonso, M. Collini, P. Pallavicini, G. Baldi, D. Maggioni and G. Chirico, Nano Lett., 2013, 13, 2004–2010 CrossRef CAS PubMed.
- A. Espinosa, A. K. A. Silva, A. Sánchez-Iglesias, M. Grzelczak, C. Péchoux, K. Desboeufs, L. M. Liz-Marzán and C. Wilhelm, Adv. Healthcare Mater., 2016, 5, 1040–1048 CrossRef CAS PubMed.
- M. R. Langille, M. L. Personick, J. Zhang and C. A. Mirkin, J. Am. Chem. Soc., 2012, 134, 14542–14554 CrossRef CAS PubMed.
- J. L. R. Arrondo, A. Muga, J. Castresana and F. M. Goñi, Prog. Biophys. Mol. Biol., 1993, 59, 23–56 CrossRef CAS PubMed.
- R. Li, H. Li, S. Pan, K. Liu, S. Hu, L. Pan, Y. Guo, S. Wu, X. Li and J. Liu, J. Mater. Res., 2013, 28, 3401–3407 CrossRef CAS.
- D. A. Urban, L. Rodriguez-Lorenzo, S. Balog, C. Kinnear, B. Rothen-Rutishauser and A. Petri-Fink, Colloids Surf., B, 2016, 137, 39–49 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Photograph of stability studies, EDAX spectra, X-ray diffraction analysis and calculation of photothermal conversion efficiency. See DOI: 10.1039/c6ra11405a |
| This journal is © The Royal Society of Chemistry 2016 |