Effect of boron–carbon–nitride as a negative additive for lead acid batteries operating under high-rate partial-state-of-charge conditions†
S. Mithin Kumara,
Uday Venkat Kiranb,
Arockia Kumar Rajub,
S. Ambalavanana and
Sundar Mayavan*a
aLead Acid Battery Section, CSIR-Central Electrochemical Research Institute, Karaikudi, India. E-mail: sundarmayavan@cecri.res.in
bMetallurgical & Material Engineering Dept, National Institute of Technology, Warrangal, India
First published on 27th July 2016
Abstract
The consequences of including boron carbon nitride (BCN) in the negative plates of lead acid batteries have been investigated after exposure to high rate partial state of charge duty cycles. For BCN-NAM, the dominating elementary process is the formation of PbO instead of PbSO4. The BCN disintegrates to form carbon and nitrogen species on cycling that improve the performance of BCN-NAM under HRPSoC cycling.
Start–stop hybrid vehicles use internal combustion engines mated with lead acid batteries (LABs). The mated LAB typically operates under high rate partial state of charge (HRPSoC) conditions, involving short durations of charge and discharge with high current rates.1,2 Pristine LAB technology cannot cope with the HRPSoC mode and it fails prematurely from low charge acceptance and the progressive sulfation of negative active materials (NAMs). Various additives have been used to overcome the mechanism responsible for progressive sulfation. Moseley et al. and Pavlov et al. have previously explained the role of carbon in NAMs under HRPSoC conditions.3,4 A variety of carbon structures have been studied as NAM additives under HRPSoC cycling.5–7 Our group has shown that LAB performance under HRPSoC conditions can be improved by replacing conventional carbon black with a multi-walled carbon nanotube (MWNT) conductive additive.8,9 Active research is currently devoted towards finding novel additives for NAMs under HRPSoC conditions.
Among various nanostructured materials, boron carbon nitride (BCN) 2D sheets offer excellent properties and show great promise in various applications ranging from electronics to catalysis.10,11 Recently, our group have successfully synthesized BCN 2D sheets that show potential application for fuel cell catalysis.12 To the best of our knowledge, their utilization as NAM additives for LABs operating under HRPSoC conditions has not been studied yet. In this study we examine the prospects of BCN incorporation into NAMs to meet HRPSoC demands. This study is of great significance as it will allow us to identify the structural changes and elementary processes that take place at the negative plates under HRPSoC cycling and to evaluate the applicability of BCN as an additive for NAMs under our experimental conditions.
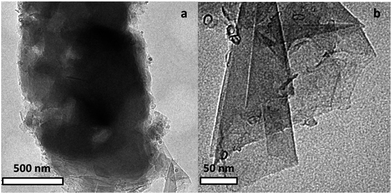
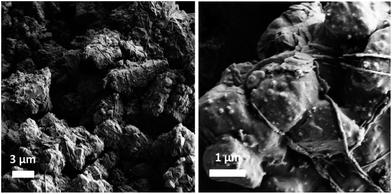
The BCN sheets were prepared using a glycine–nitrate combustion method described elsewhere. In brief, calculated quantities of glycine, zinc nitrate (ZnNO3), and boron oxide (B2O3) were thermally treated at 500 °C for 2 h under a controlled atmosphere to form ZnO-containing BCN sheets (BCN) (experimental details are in the ESI†). TEM images of the as-prepared BCN sheets are shown in Fig. 1, which reveal the graphene-like morphology of the aggregated nanosheets. EDX confirms the presence of B, C, N, O and Zn species (Fig. S1†). The XRD spectrum shows characteristic BCN diffraction peak at around 26° and 42° (Fig. S2†). In order to evaluate the consequences of including BCN (0.25 wt%) sheets in the NAM of LABs during HRPSoC cycling, lead acid test cells (2 V/3 A h) were assembled comprising one negative (with 0.25% BCN) and two positive plates, using a lead–calcium alloy (see NAM preparation and cell assembly in the ESI†). Accelerated cycling for the test cell was performed under HRPSoC conditions, mimicking micro-hybrid driving mode.13 The cells were subjected to cycling according to the following schedule: charging at a 2C rate for 60 s; rest for 10 s; discharging at a 2C rate for 60 s; and rest for 10 s. The test was stopped when the end voltage reached 1.83 V or when the upper voltage limit of 2.83 V was reached. The above-described cycling steps comprise one cycle-set of the test. After this cycle-set, the cell was fully re-charged (to a 100% SoC) and the C20 capacity was measured then followed by a second cycle-set.13 The charge–discharge tests were carried out using a Bitrode life cycle tester. Scheme 1 presents a summary of the NAM prepared with BCN, and the type of tests and analyses performed within this study. Fig. 2 shows FE-SEM images of the as-prepared NAM (before cycling) with the BCN additive. Microstructural characterization of BCN-NAM reveals aggregates of a 2–3 micron size. The addition of BCN leads to a median pore radius of 0.17 μm (measured using a mercury porosimeter) i.e., a negative active mass acquires a membrane-sized pore structure as compared to the micron sized pore structure obtained with the addition of conventional carbon additives and nano-structured carbon additives, like SWNT and MWNT.14
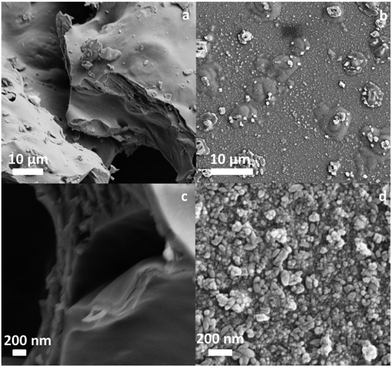
In order to understand the electrochemical properties of the NAM with and without the BCN additive, cyclic voltammetry (CV) was performed at a scan rate of 5 mV s−1 at room temperature as shown in Fig. S3.† The CV curves consist of an oxidation peak and a reduction peak, corresponding to the charging reaction and the discharge reaction of the Pb/Pb2+ redox couple. The anodic peak current for the NAM with BCN is comparatively higher than for carbon black, which indicates a higher utilization of leady oxide (i.e., NAM incorporated with BCN) as compared to carbon black. Fig. 3a shows the cycling performance for a cell with BCN-NAM at a 20 h rate. The cell capacity gradually increases during the initial cycles and then stabilizes. At the end of 10 cycles, the capacity is 3.17 A h. The cell is then subjected to HRPSoC cycling. BCN completed 8 consecutive cycle-sets with 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 804 cycles (Fig. 3b). Fig. 3c shows the behaviour of the cell capacity (at C20) after each HRPSoC cycle test. The cell with BCN-NAM shows a gradual decrease in discharge capacity. These data clearly show that BCN can be used as a potential additive in NAMs under a HRPSoC. Failed test cells (as per HRPSoC conditions) were cut open for analysis. The negative plate was taken out of the cell, washed and dried. Samples were analysed using FE-SEM, XRD, Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). The FE-SEM images of BCN-NAM (Fig. 4a and b) reveal the formation of spherical structures of a 1–2 micron size. EDX spectroscopy was performed to identify the elements present in the cycled BCN-NAM. EDX spectra (Fig. 4c) confirm the presence of Pb, O and C species. The chemical composition and phases present on the electrode surfaces were analysed using XRD. XRD data (Fig. 5a) indicates the formation of PbO diffraction peaks along with lead peaks. Fig. 5b shows the Raman spectrum of BCN-NAM. The NAM with BCN does not show any peak relating to PbSO4 but reveals characteristic PbO bands at 151, 281 and 367 cm−1.15 XPS was done to understand the chemical state of BCN-NAM after cell failure. Fig. 5c shows the Pb 4f peaks of BCN-NAM. The Pb 4f spectra of BCN-NAM show two broad singlet peaks corresponding to Pb 4f7/2 and Pb 4f5/2 centered around binding energy values of 138.8 eV and 143.9, eV respectively.9 No sulphur peak is evident. High resolution C 1s spectra (Fig. 5d) show a major peak at 285 eV (which corresponds to a C–C bond) with a small shoulder at 289.7 eV. The small shoulder at 289.7 eV relates to C
804 cycles (Fig. 3b). Fig. 3c shows the behaviour of the cell capacity (at C20) after each HRPSoC cycle test. The cell with BCN-NAM shows a gradual decrease in discharge capacity. These data clearly show that BCN can be used as a potential additive in NAMs under a HRPSoC. Failed test cells (as per HRPSoC conditions) were cut open for analysis. The negative plate was taken out of the cell, washed and dried. Samples were analysed using FE-SEM, XRD, Raman spectroscopy and X-ray photoelectron spectroscopy (XPS). The FE-SEM images of BCN-NAM (Fig. 4a and b) reveal the formation of spherical structures of a 1–2 micron size. EDX spectroscopy was performed to identify the elements present in the cycled BCN-NAM. EDX spectra (Fig. 4c) confirm the presence of Pb, O and C species. The chemical composition and phases present on the electrode surfaces were analysed using XRD. XRD data (Fig. 5a) indicates the formation of PbO diffraction peaks along with lead peaks. Fig. 5b shows the Raman spectrum of BCN-NAM. The NAM with BCN does not show any peak relating to PbSO4 but reveals characteristic PbO bands at 151, 281 and 367 cm−1.15 XPS was done to understand the chemical state of BCN-NAM after cell failure. Fig. 5c shows the Pb 4f peaks of BCN-NAM. The Pb 4f spectra of BCN-NAM show two broad singlet peaks corresponding to Pb 4f7/2 and Pb 4f5/2 centered around binding energy values of 138.8 eV and 143.9, eV respectively.9 No sulphur peak is evident. High resolution C 1s spectra (Fig. 5d) show a major peak at 285 eV (which corresponds to a C–C bond) with a small shoulder at 289.7 eV. The small shoulder at 289.7 eV relates to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionality.16 The formation of C
O functionality.16 The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O may be due to the oxidation of carbon under acidic conditions. The N 1s peak (Fig. 5e) shows a major peak at 412.3 eV and a small shoulder at 408.5 eV which corresponds to nitrogen oxide species adsorbed on the Pb.17 This indicates that the majority of lead appears to be present as lead oxide. This confirms the formation of PbO (or the conversion of Pb to PbO) under HRPSoC duty cycles with the BCN additive.
O may be due to the oxidation of carbon under acidic conditions. The N 1s peak (Fig. 5e) shows a major peak at 412.3 eV and a small shoulder at 408.5 eV which corresponds to nitrogen oxide species adsorbed on the Pb.17 This indicates that the majority of lead appears to be present as lead oxide. This confirms the formation of PbO (or the conversion of Pb to PbO) under HRPSoC duty cycles with the BCN additive.
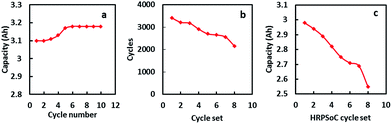 | ||
| Fig. 3 (a) Cycling performance of a BCN added cell. (b) Completed HRPSoC cycles per set for a cell with BCN. (c) Cell capacity after each HRPSoC cycle set. | ||
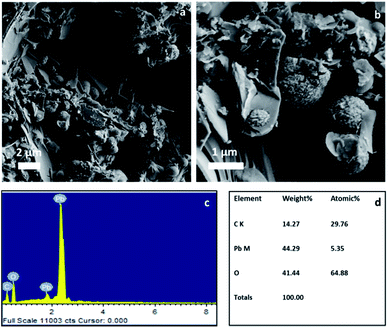 | ||
| Fig. 4 (a, b) FE-SEM images of cycled BCN-NAM (after cell failure) at different magnifications, and (c) EDX spectra, and (d) elemental composition of cycled BCN-NAM. | ||
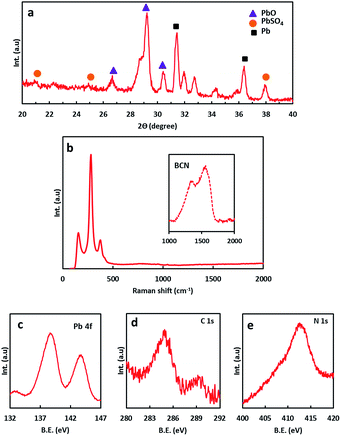 | ||
| Fig. 5 (a) XRD spectrum of cycled BCN-NAM; (b) Raman spectra of cycled BCN-NAM; and high resolution Pb 4f (c), C 1s (d), and N 1s (e) spectra of cycled BCN-NAM. | ||
To understand the failure mechanism of the cell without BCN, a control cell with a carbon black (CB) additive (in the NAM) was constructed and studied under HRPSoC cycling conditions. Failed control cells were cut open for analysis. The control cell with a CB additive under HRPSoC conditions results in the formation of unconverted lead sulfate (PbSO4) crystals (SEM and EDX data shown in the ESI in Fig. S4†). PbSO4 formed on the surface progressively accumulates in the negative plates, which leads to the formation of coarse crystals of irreversible lead sulphate. But on the contrary to BCN addition, lead oxide (PbO) crystals are formed instead of PbSO4 (XRD and EDX data). Through XRD, Raman, FESEM-EDX and XPS data we have clearly shown that the dominating elementary process is the formation of PbO (instead of PbSO4 as is observed with a conventional CB additive) for BCN-NAM.
The formation of PbO and PbSO4 depends upon the nature of the additive. Additives can reduce the pore size within the NAM and the diffusion of sulphuric acid (H2SO4) into the pores becomes increasingly difficult below a pore size of 1.5 μm, hence lead oxide (PbO), not PbSO4, is formed during operation.14 In our case, with the addition of BCN the pore size narrows down to 0.17 μm which may act as a semi-permeable membrane and block/impede the movement of large sized SO42− and HSO4 ions into the pores. Thus the solution inside the pores becomes alkaline resulting in the formation of PbO.1 However, with carbon black additives, large sized pores (2.6 μm) are formed which freely allow the movement of large sized SO42− and HSO4 ions (into the pores), thus favouring the formation of PbSO4 (Fig. S4†).14
XPS spectra of as-prepared BCN sheets (before addition to the NAM) show peaks relating to B, C, N, O and Zn. But, XPS data for BCN-NAM does not show B and Zn species. We did a control experiment to understand the stability of BCN in 1.260 specific gravity (sp.gr) H2SO4 acid. A calculated quantity of BCN was added to 1.260 sp.gr acid. BCN readily dissolved in acid to form a homogenous solution. FE-SEM images of BCN before and after dissolution in acid are shown in Fig. 6. The BCN sheets broke into smaller spherical pieces with sizes of less than 300 nm (Fig. 6b and d), while those of the original BCN sheets were usually over a few micrometers (Fig. 6a and c). FE-SEM images confirm the disintegration of the BCN sheets into nanoparticles in contact with 1.26 sp.gr H2SO4 acid (via oxidative etching). Under HRPSoC cycling mode, BCN-NAM is constantly exposed to H2SO4. Based on the XPS data, we postulate that prolonged exposure (of the BCN present in the NAM to acid) would have oxidized boron (evaporable) and dissolved Zn species (leached out) with the generation of carbon and nitrogen species.18 XPS data confirms the presence of carbon and nitrogen species along with Pb (with the absence of boron and zinc species). The presence of carbon and nitrogen species may contribute towards improving the performance of NAMs under HRPSoC conditions.14
Conclusions
Through our study, for the first time we have shown that BCN can be used as a potential additive to NAMs to meet the HRPSoC demands of LABs. Moreover, this study allowed us to identify the elementary processes that take place at the negative plates on HRPSoC cycling. For BCN-NAM, the dominating elementary process is the formation of PbO instead of PbSO4. The BCN disintegrates to form carbon and nitrogen species on cycling that improve the performance of BCN-NAM under a HRPSoC.AcknowledgementsThis work was supported by Council of Scientific and Industrial Research (CSIR), India – TAPSUN project NWP 56A. We thank CSIR-CECRI Central Instrumentation Facility for the analytical support and Er V. Prabhu, Er A. Rathishkumar and Er J. Kennedy for FE-SEM, TEM and XPS analyses.Acknowledgements
This work was supported by Council of Scientific and Industrial Research (CSIR), India – TAPSUN project NWP 56A. We thank CSIR-CECRI Central Instrumentation Facility for the analytical support and Er V. Prabhu, Er A. Rathishkumar and Er J. Kennedy for FE-SEM, TEM and XPS analyses.Notes and references
- L. T. Lama, R. Louey, N. P. Haigh, O. V. Lima, D. G. Vella, C. G. Phyland, L. H. Vu, J. Furukawa, T. Takada, D. Monmab and T. Kano, J. Power Sources, 2007, 174, 16 CrossRef.
- P. T. Moseley, J. Power Sources, 2009, 191, 134 CrossRef CAS.
- P. T. Moseley, R. F. Nelson and A. F. Hollenkamp, J. Power Sources, 2006, 157, 3 CrossRef CAS.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2011, 196, 5155 CrossRef CAS.
- K. Nakamura, M. Shiomi, K. Takahashi and M. Tsubota, J. Power Sources, 1996, 59, 153 CrossRef CAS.
- M. Shiomi, T. Funato, K. Nakamura, K. Takahashi and M. Tsubota, J. Power Sources, 1997, 64, 147 CrossRef CAS.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2011, 196, 5155 CrossRef CAS.
- M. Saravanan, P. Sennu, M. Ganesan and S. Ambalavanan, J. Electrochem. Soc., 2013, 160, A70 CrossRef CAS.
- S. Mithin Kumar, S. Ambalavanan and S. Mayavan, RSC Adv., 2014, 4, 36517 RSC.
- Y. Wang, Y. Shao, D. W. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790 CrossRef CAS PubMed.
- L. Qu, Y. Liu, J. B. Baek and L. Dai, ACS Nano, 2010, 4, 1321 CrossRef CAS PubMed.
- A. Yamuna, A. Mandalam, A. Karthigaiselvi, M. Balasubramanian, B. Thiruparasakthi, S. Ravichandran and S. Mayavan, RSC Adv., 2015, 5, 69394 RSC.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2010, 195, 4444 CrossRef CAS.
- D. Pavlov and P. Nikolov, J. Power Sources, 2013, 242, 380 CrossRef CAS.
- D. A. Ciomartan, R. J. H. Clark, L. J. McDonald and M. Odlyha, J. Chem. Soc., Dalton Trans., 1996, 1, 3639 RSC.
- Y. Wang, X. Tong, X. Guo, Y. Wang, G. Jin and X. Guo, Nanotechnology, 2013, 24, 475602 CrossRef PubMed.
- J. Baltrusaitis, P. M. Jayaweera and V. H. Grassian, Phys. Chem. Chem. Phys., 2009, 11, 8295 RSC.
- Y. Lia, K. Tu, X. Han, L. Hu, J. W. Connell, Z. F. Chen and Y. Lin, Sci. Rep., 2015, 5, 14510 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD and EDX spectra of ZnO–BCN, and CV data. See DOI: 10.1039/c6ra13458k |
| This journal is © The Royal Society of Chemistry 2016 |