Gold nanosheets synthesized with red marine alga Actinotrichia fragilis as efficient electrocatalysts toward formic acid oxidation
Safieh Momeni*a,
Afsaneh Safavib,
Raheleh Ahmadib and
Iraj Nabipoura
aPersian Gulf Marine Biotechnology Research Center, The Persian Gulf Biomedical Sciences Research Institute, Bushehr University of Medical Sciences, Bushehr 75147, Iran. E-mail: safieh.momeni@gmail.com; Fax: +98 77 33328724
bDepartment of Chemistry, College of Sciences, Shiraz University, Shiraz 71454, Iran
First published on 3rd August 2016
Abstract
In this study, a simple and green synthesis of gold nanosheets was explained when red marine alga extract (Actinotrichia fragilis) collected from the Persian Gulf was used as both a reducing and shape controlling agent. Various nanosheet shapes such as a triangle, truncated triangle, hexagon and polygon were synthesized via this seaweed extract. The size, morphology and crystal nature of the as-prepared nanostructures were characterized by field emission scanning electron microscopy (FESEM), atomic force microscopy (AFM), X-ray diffraction (XRD) and FT-IR spectroscopy. AFM analysis showed that the Au nanosheets have a thickness ranging from 10–15 nm. The as-prepared Au nanosheets were used as an electrocatalyst for the fabrication of an electrochemical sensor for oxidation of formic acid. The Au nanosheets modified carbon ionic liquid electrode (AuNS/CILE) was designed and its catalytic activity was investigated toward formic acid oxidation. The results imply that the AuNS/CILE shows excellent electrocatalytic activity and good stability for formic acid oxidation in a direct pathway without CO poisoning. These results, a direct pathway formic acid oxidation reaction without CO poisonous intermediates together with a simple and green route for the synthesis of Au nanosheets, show that the AuNS/CILE is one of the most promising systems for application in direct full cells.
Introduction
Nanomaterials with no spherical shapes such as cube, wire/rod, plate and belt have shown physical and chemical properties that are distinctly different from conventional spherical nanoparticles.1–3 On the other hand, the properties of nanomaterials are strongly dependent on their size, shape and crystallinity.4 As a result, shape controlled synthesis of nanostructures has been paid increasing attention and there is fundamental interest in developing new methods for generating anisotropic nanostructures. Gold nanostructures with controllable morphologies have many identified applications in catalysts,5 optics,6 Surface-Enhanced Raman Substrate (SERS),7,8 tip-enhanced Raman substrate9 and medical10 applications. Among the various morphologies of gold nanoparticles, two-dimensional Au nanosheets are a particularly interesting class of nanostructures with a desired degree of anisotropy.11–15 The main interest in these gold nanosheets relies on their potential applications in gas sensors, inducing hyperthermia in tumors, infrared absorbing optical coatings, and basic building block for nanodevices.16–18 Therefore, the fabrication of gold nanosheets with effective but simple preparative methods has been paid considerable attention.Generally, chemical techniques have been employed to prepare gold nanosheets, particularly seed-mediated growth approach,19 template-assisted synthesis,20 microwave heating21 and one-pot procedure.22 Currently, there is increasing interest in the fabrication of nanomaterials using biomolecules and bioorganisms.23 However, there are only few reports on the biosynthesis of gold nanostructures with high anisotropy. In biological synthesis, various microorganism and plant extracts24 were known to produce nanomaterials either intracellularly or extracellularly. Due to the complexity of biological reactions, the actual mechanisms of their performance and the presence of any effective structure-directing agent in the synthesis of nanoparticles are often not clear. Marine biological species such as yeast, fungi, sponge and alga have been found to be capable of synthesizing nanostructures.25 Multicellular brown alga (Sargassum sp.)26 and unicellular green alga were used for synthesis of gold nanosheets by Lee et al.27 They showed that a protein with a molecular weight of approximately 28 kDa in crude extract of green alga participates in the nucleation and growth of gold nanostructures into the specific shape and size.27 Sastry et al. reported a method for synthesis of gold nanoplates by using an extract of Lemongrass leaves as a reducing and shape-driving agent.28
Fuel cells are potential devices in the conversion between chemical energy and electronic energy using hydrogen or small organic molecules as fuels. Electro-oxidation of small organic molecules such as formic acid,29 ethanol30 and methanol31–34 has been greatly investigated for the development of direct formic acid fuel cells (DFAFCs) and direct methanol fuel cells (DMFCs).35–37 Direct liquid fuel cells such as DMFCs and DFAFCs are considered as potential power generator for portable electronic devices such as laptop computers. Compared with methanol, formic acid has attracted much more attention due to its high theoretical open circuit voltage, less toxicity, non-flammability and low crossover flux through polymer membrane.38 These unique characteristics of formic acid allow DFAFC to have a combination of higher voltage and higher power densities. Moreover, alkaline fuel cells (AFCs) with the use of alkaline anion exchange membranes and oxygen reduction reaction (ORR) on the cathode have attracted much attention.39–41
Electro-oxidation of formic acid (FA) to CO2 follows a dual-path mechanism. The first involves the dehydrogenation path (direct pathway) with formate anion as the reactive intermediate; while, the other pathway proceeds by the dehydration of FA (indirect pathway) with the formation of the CO poisoning intermediate and subsequent oxidation to CO2 at higher potentials.42 Platinum (Pt) and palladium (Pd) have been considered as the most common catalysts for formic acid oxidation.35,36 However, pure Pt can be easily poisoned by CO intermediates (indirect pathway is predominant on Pt) and its catalytic activity decreased significantly and inhibits the direct FA oxidation. In contrast, Pd possesses higher catalytic activity than Pt for FA oxidation, but is much less stable in acidic solutions. As shown in a previous investigation, the formation of CO intermediates needs continuous Pt surface, while for the direct oxidation pathway, the presence of discrete Pt atoms is sufficient.43 Unfortunately, Pt and Pd are expensive metals and their available resources in the earth are limited. Thus, many reports are focused on the synthesis of economical and active catalysts to further decrease the cost of fuel cells.
A common strategy to improve the catalytic activity in dehydrogenation pathway of FA oxidation is to make the Pt–M (M = Au, Pd, Ru, Bi, etc.) alloy or bimetallic core–shell nanoparticles, which could enhance CO-tolerance capability during the FA oxidation.44–48 These facts show that a Pt-based catalyst has reinforced the direct dehydrogenation pathway and has weaken the indirect dehydration pathway simultaneously. Among various Pt-based catalysts, bimetallic Pt–Au nanocatalysts with different structures such as Pt–Au alloy, Pt modified Au and Au@Pt have attracted more interest.49–51 Introducing Au dopant could reduce the number of adsorption sites for CO through the segregation of Pt sites and thus improves the catalytic activity toward FA oxidation.43 In addition, the presence of Au could enhance the stability against dissolution by raising the Pt oxidation potential. To further decrease the consumption of noble metals and improve their catalytic activity, the key method is the binding of nanocatalyst onto the surface of conductive substrates such as carbon black,52 carbon nanotube53 and graphene nanosheets.54 Zhang et al.55 prepared graphene nanosheets supported Pt–Au nanoparticles and found that the Pt–Au/graphene has superior catalytic activity for FA oxidation. To the best of our knowledge, there have been only few reports on using Au nanostructures for the electrocatalytic oxidation of FA till now.56 Ramaraj et al.56 showed that the morphology of Au nanostructures is an important factor in the electrocatalytic oxidation of FA. In their report, Au nanoparticles electrodeposited on electrochemically reduced graphene oxide modified glassy carbon electrode (GCE) showed electrocatalytic activity for formic acid oxidation but single Au nanostructure-modified GCE failed to catalyze the formic acid oxidation.
According to the previous reports, oxidation of formic acid has been intensively investigated in acidic media and only a few studies have focused on the oxidation of formic acid in alkaline media.57,58 Osawa et al. investigated the electrooxidation of HCOOH/HCOO− over a wide range of solution pH.59 Their results showed that the FA oxidation current exhibits a volcano-shape pH dependence with a peak at a pH close to the pKa of formic acid (3.75). On the other hand, the best oxidation performance of formic acid can be achieved at pH ≈ pKa. Further examination in single cell experiments was performed by Lee et al. over a wide pH range.60 They showed that the maximum cell performance was found at a pH slightly higher than the pKa of FA. The peak current of FA oxidation is then suppressed in the alkaline media due to OH adsorption on the surface and surface oxidation.
In this report, we describe a rapid, green and one-step wet chemical technique to produce large-scale gold nanosheets using crude extract of red marine alga (Actinotrichia fragilis) as a green reducing and shape controlling agent. Various nanosheet shapes such as triangular, truncated triangular, hexagon and polygon were synthesized via this seaweed extract. Detailed investigations on the influences of temperature and reagent concentrations have been found to arrive the optimal growth conditions for the gold nanosheets. The as-prepared Au nanosheets were used as an electrocatalyst for the fabrication of electrochemical sensor for oxidation of formic acid. Herein, we designed Au nanosheets modified carbon ionic liquid electrode (AuNS/CILE) catalyst and investigated its electrocatalytic activity toward formic acid oxidation. Au nanosheets showed an excellent electrocatalytic activity for FA oxidation on the surface of AuNS/CILE. One of the most distinctive features of electrooxidation of FA on AuNS/CILE is elimination of the indirect FA oxidation pathway and the significant tolerance against CO poisoning.
Experimental
Materials
Formic acid, sodium hydroxide (NaOH), chloroauric acid (HAuCl4), ethanol, 1-iodooctane, pyridine and diethyl ether were used as received from Merck. Graphite powder (mesh size < 100 lm), ammonium hexafluorophosphate was purchased from Fluka. All compounds were used as received without further treatment. Double distilled water was used throughout the experiment.Instrumentation
The morphology and size of the synthesized gold nanosheets were examined by field emission scanning electron microscope (Hitachi S-4160 FESEM) at an accelerating voltage of 20 kV. Structural characterization was performed by X-ray diffraction using an X-ray diffractometer (D8, Advance, Bruker, axs diffractometer). The diffraction angle covered 20–90 degree with a step size of 0.05 degree. The thickness and surface topography of nanosheets were characterized by atomic force microscopy (JPK, Nano Wizard II AFM) using a silicon probe at room temperature. The UV-vis-NIR spectroscopy was measured on a Specord analytic jena spectrophotometer. The electrochemical measurements were carried out in a conventional three electrode cell. A carbon ionic liquid electrode (CILE) and Au nanosheets modified CILE (1.8 mm in diameter) were used as the working electrodes. The counter electrode and the reference electrode were platinum wire and Ag/AgCl electrode, respectively. The electrochemical properties were recorded with an Auto lab system (Eco-Chemie, Utrecht, The Netherlands) equipped with GPES software (Eco-Chemie, Utrecht, The Netherlands). All of the electrochemical measurements were carried out at room temperature. The electrochemical measurements were performed in a 0.1 M NaOH solution.Electrode preparation
Carbon ionic liquid electrode was prepared by hand-mixing of graphite powder and n-octyl pyridinum hexafluoro phosphate (OPyPF6) with a ratio of 50/50 (w/w) as described previously.61 Au nanosheets modified carbon ionic liquid electrode was prepared using of graphite powder, ionic liquid, and Au nanosheets (50%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40%
40%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%, wt%), respectively. The paste was packed into the cavity of a Teflon tube (1.8 mm diameter) and the electrical contact was established via a stainless steel handle.
10%, wt%), respectively. The paste was packed into the cavity of a Teflon tube (1.8 mm diameter) and the electrical contact was established via a stainless steel handle.
Synthesis of Au nanosheets
Actinotrichia fragilis red alga was collected along the Bushehr coast of the Persian Gulf (southwestern Iran) in May 2015. The red alga was transported to the laboratory, cleaned carefully in fresh water and then by distilled water to remove sand and salts. After cleaning, the algae were dried in shade at room temperature for 6 days. Prior to the experiments, the dried seaweed was ground to a fine powder. About 2.0 g of the red alga powder was added to 200 mL of deionized water and the mixture was boiled for 15 min. The seaweed extract was passed through Whatman No. 1 filter paper and the filtrates were centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The prepared extract was used for synthesis of Au nanosheets.
000 rpm for 10 min. The prepared extract was used for synthesis of Au nanosheets.
In a typical synthesis experiment of Au nanosheets at room temperature, crude extract (9.5 mL) was added to different volumes of an aqueous solution of chloroauric acid (HAuCl4, 50–500 μL, 0.05 M) and the volume was adjusted to 10 mL by the addition of an appropriate volume of distilled water. The reaction was carried out under stirring at 500 rpm at room temperature for 10 h. The color of the mixture of algal extract and aqueous HAuCl4 solution changed from yellow to shimmery yellow state, indicative of changes in the metal oxidation state. The resulting products were isolated by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min). The process of centrifugation and redispersion were repeated several times with water and ethanol to remove residual reactants in the crude extract. The product was then dried. The prepared Au nanosheets were subjected for the detailed characterization to determine their properties.
000 rpm for 15 min). The process of centrifugation and redispersion were repeated several times with water and ethanol to remove residual reactants in the crude extract. The product was then dried. The prepared Au nanosheets were subjected for the detailed characterization to determine their properties.
Results and discussion
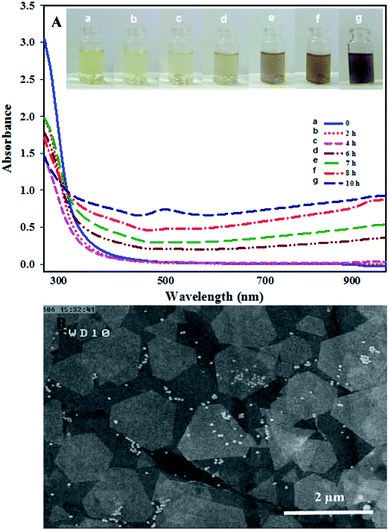
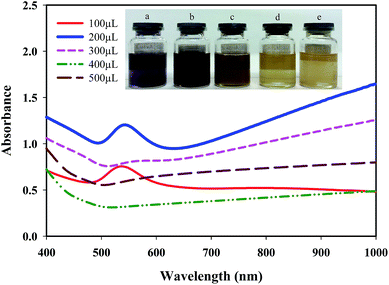
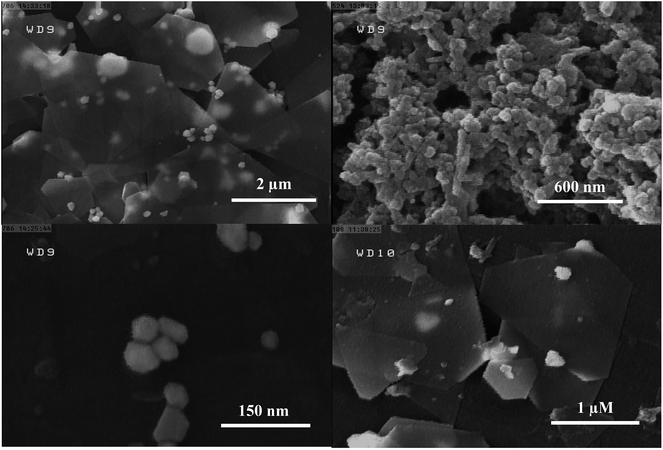
The current study is focused on the synthesis of gold nanosheets using red marine alga extract and their application as an electrocatalyst for the fabrication of an electrochemical sensor for oxidation of formic acid. The algal crude extract was a light-yellow liquid. After mixing of HAuCl4 (300 μL, 0.05 M) with 9.5 mL of seaweed extract at room temperature, the color gradually changed during reaction time (10 h) with the appearance of glittering Au nanosheets. The UV-vis absorption spectra and digital photographs of a mixture of gold ions and crude extract were obtained at multiple time intervals from the beginning of the reductive reaction as shown in Fig. 1A. The spectroscopic change indicates a change in the gold oxidation state (Au(III) was reduced to Au(0)). It is well known that optical properties of Au nanoparticles are strongly dependent on their size and shape.62 In general, spherical gold nanoparticles exhibit only one surface plasmon resonance peak around 550 nm, whereas anisotropic Au nanoparticles have more than two adsorption peaks because of mutually different dipole resonance.62 As has been illustrated in Fig. 1A, the band at 315 nm attributed to HAuCl4 ion solution gradually decreases and a broad band over 500 nm begins to appear that implies the formation of nanostructures with anisotropic shape and very wide size distribution. The morphology of the prepared Au nanosheets was characterized by FESEM to confirm the sheet nature of the products. Fig. 1B shows the FESEM images of gold nanosheets prepared using red marine alga extract. FESEM images present the sheet structure with different shapes including hexagon, triangles, truncated triangular and polygons well as small spheres. The size of gold nanosheets was in the micrometer scale (0.5–3 μm along their longest edge) and their thickness was in the nanometer scale with flat surface. It is worth noting that the synthesized Au nanosheets were transparent which could be attributed to their thin nature (10–20 nm in thickness). Generally, red marine alga extract contains both reducing and stabilizing agents, so that the in situ synthesis of gold nanoparticles is simply feasible.The generation of Au nanosheets was found to be dependent on the appropriate concentration of the precursor (HAuCl4). The effect of the amount of HAuCl4 on the size and morphology of the synthesized Au nanosheets was investigated at different amounts of HAuCl4 (50–500 μL, 0.05 M) in the presence of 9.5 mL of red marine alga extract. The surface plasmon resonance (SPR) spectra change gradually with varying the amount of HAuCl4. As shown in Fig. 2, the variation in the SPR of Au nanoparticles synthesized by the reduction of different concentrations of HAuCl4 solution exhibits that Au nanostructures with different shapes have been synthesized. At low concentrations of HAuCl4 (100 μL of 0.05 M), a purple colloidal stable gold nanostructure was obtained with an intense SPR band at 540 nm that is mainly due to the presence of Au nanospheres. At HAuCl4 amounts above 100 μL, the absorbance spectrum displayed a broad band in the visible and near-infrared region and gold nanosheets were obtained that resulted in golden glittering precipitate (Fig. 2, inset photograph). With increasing the amounts of HAuCl4, the SPR band at longer wavelengths extended to the infrared region that indicates the decrease in the number of nanospheres and formation of large gold nanosheets. Fig. 3 shows the FESEM images of gold nanostructures prepared with different amounts of HAuCl4. Fig. 3A shows that the resulting gold product at a low concentration of HAuCl4 (100 μL, 0.05 M) is mainly consisted of nanospheres with size of about 30–50 nm. Fig. 3B–D show the FESEM images of the obtained products at HAuCl4 amounts above 100 μL. FESEM images show that the products are dominated by micro-sized Au nanosheets with regular shapes (hexagon, triangles, truncated triangular) and low amounts of nanospheres.
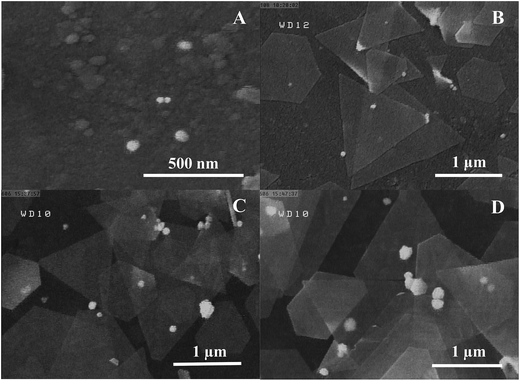 | ||
| Fig. 3 FESEM images of synthesized Au nanostructures with addition of (A) 100, (B) 200, (C) 300, (D) 400 μL HAuCl4 (0.05 M) to a 9.5 mL of red marine alga extract solution. | ||
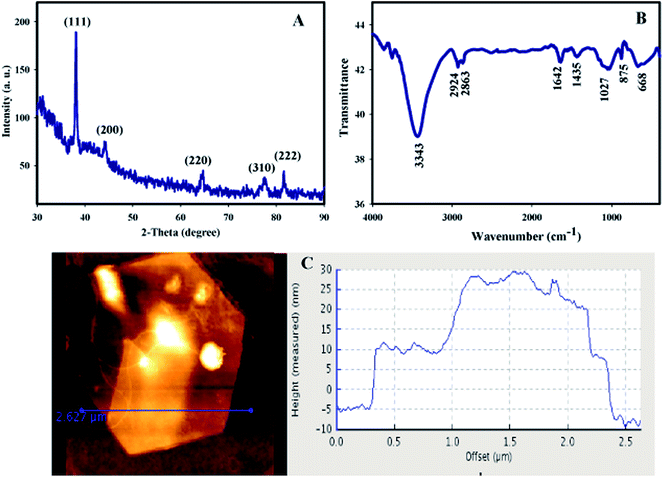
To investigate the crystalline structure of Au nanosheets, XRD analysis was performed. XRD analysis was used for the product obtained from the addition of 300 μL HAuCl4 (0.05 M) to 9.5 mL of red marine alga extract. As shown in Fig. 4A, the XRD peaks appeared at (111), (200), (220) and (311) plane (2θ 35–100) that are assigned to the face centered cubic (fcc) structure of gold nanoparticles (JCPDS card no. 04-0783). In addition, the relative diffraction intensities of either (200)/(111) and (220)/(111) are much lower than the conventional values (0.1 versus 0.53 and 0.15 versus 0.33, respectively). These results imply that the preferential growth direction of Au nanosheets is along the gold (111) facets.
FTIR analysis was performed to recognize the nature of biomolecules that bound specifically on the surface of Au nanosheets (Fig. 4B). The strong peak at 3343 cm−1 is attributed to the free O–H group. The band at 2924 cm−1 is due to the alkane C–H stretching or is assigned to the secondary amine. The peak appearing at 1435 cm−1 is assigned to the –C–O– group and a band at 1642 cm−1 might result from conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the red alga polysaccharides. The absorption band at 1027 cm−1 can be assigned to the S
O group of the red alga polysaccharides. The absorption band at 1027 cm−1 can be assigned to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and the band at 875 cm−1 is corresponded to –C–O–SO3 of the sulfated polysaccharides.63 These results suggest that polysaccharide containing compounds in the crude extract are adsorbed on the surface of Au nanosheets.
O stretching and the band at 875 cm−1 is corresponded to –C–O–SO3 of the sulfated polysaccharides.63 These results suggest that polysaccharide containing compounds in the crude extract are adsorbed on the surface of Au nanosheets.
Surface topography and thickness of the Au nanosheets were investigated by AFM. Fig. 4C shows the AFM image of the synthesized Au nanosheets with the addition of 300 μL HAuCl4 to 9.5 mL of seaweed extract. AFM showed atomically flat surface and thickness on the scale of nanometers which give them optical transparency. In AFM analysis, a number of Au nanosheets produced by this method were examined and showed a thickness ranging from 10–20 nm with an edge length of 0.5–4 μm.
Temperature plays an important role in the generation of Au nanosheets. In fact, the kinetic of gold ion reduction and the rate of the nucleation and growth of the nanoparticles are strongly dependent on the temperature. The selected temperatures were 0, 25 and 50 °C. The effect of temperature was investigated at two concentrations of HAuCl4 (100 and 300 μL of 0.05 M) solution. At room temperature, a solution of 1.5 mM HAuCl4 (300 μL, 0.05 M) produced gold nanosheets with well-defined hexagonal and triangular shapes as shown in Fig. 5A. By increasing the temperature from 25 °C to 50 °C, the reduction reaction rate increased sharply and the color of the solution changed from yellow to purple. Fig. 5B shows the FESEM image of gold nanostructures obtained at 50 °C, which show at 50 °C, the products were predominantly spherical nanoparticles and the yield of Au nanosheets decreased dramatically. In a second experiment, the temperature of the reaction was decreased to 0 °C for a gold ion solution with a concentration of 0.5 mM (100 μL, 0.05 M HAuCl4). At this concentration, mainly spherical gold nanoparticles were produced at room temperature. By decreasing the temperature to 0 °C, the rate of HAuCl4 reduction reaction considerably decreased, which resulted in the formation of well-defined hexagonal, truncated triangular and triangular Au nanosheets. Fig. 5D shows the SEM image of the product obtained at 0 °C, which contains triangular and hexagonal nanosheets.
Red alga extract was used as a cheap, nontoxic, and green reagent for seed-less synthesis of Au nanosheets. To further evaluate the influence of the amount of crude extract used on the generation of Au nanosheets, five samples were prepared by adding different amounts of crude extract (1.0 mL, 2.5 mL, 5.0 mL, 7.5 mL and 9.5 mL of extract (2.0 g red alga in 200 mL water)) to the HAuCl4 solution while other experimental conditions were kept at their optimum values. As shown in Fig. 6, the reduction of gold ions and formation of gold nanoparticles occurred at every employed concentration of seaweed extract and the main products were Au nanosheets with smooth surfaces for HAuCl4 amount of 300 μL. However, by increasing the amount of crude extract, the yield of production of Au nanosheets was increased. Higher yields of gold nanosheets were obtained for crude extract amounts of 7.5–9.5 mL.
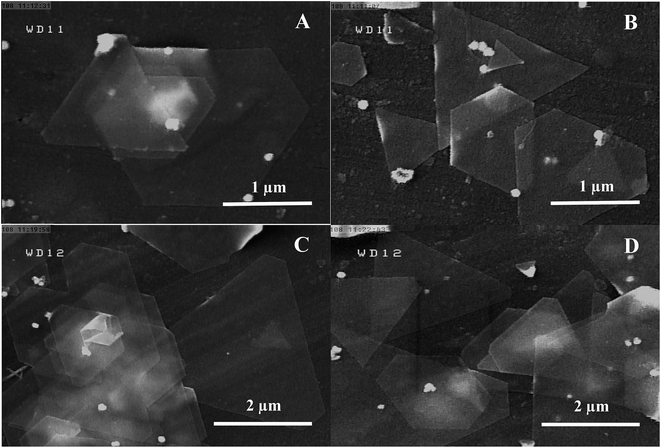 | ||
| Fig. 6 FESEM images of synthesized gold nanostructures with addition of 300 μL HAuCl4 (0.05 M) to a 10 mL solution containing (A) 1.0 (B) 2.5, (C) 5.0 and (D) 7.5 mL of red marine alga extract. | ||
In this study, synthesis of gold nanosheets was performed using red marine alga extract. As shown in previous reports, algal extract contains various compounds such as polysaccharides (carrageenan), polyphenols, alkaloids, amides and amines.64,65 It is worth noting that the crude extract of red alga provided both the reducing agent(s) and the shape controlling agent for synthesis of anisotropic gold nanoparticles. The formation of Au nanosheets was initiated by the generation of seeds and then the appropriate capping agents are adsorbed on the selected facets of gold seeds and greatly decrease the growth on these facets relative to the others. As a result, anisotropic Au nanosheets with different shapes were formed. However, the exact growth mechanism of Au nanosheets has not been thoroughly explored due to the diversity of compounds available in a crude extract of red marine alga. According to the literature, Chlorella vulgaris green alga and Sargassum sp. brown alga were used for synthesis of Au nanosheets. In this study, it is shown that Actinotrichia fragilis red marine alga could also be used for synthesis of Au nanosheets in large scale.
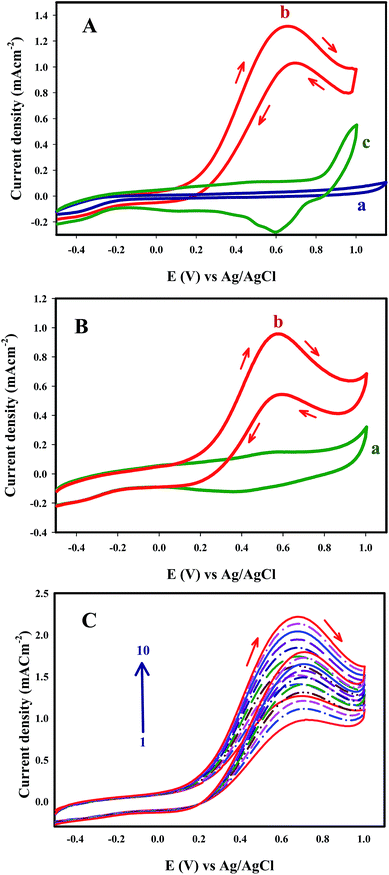
The catalytic activities of the as-prepared Au nanosheets were investigated for FA oxidation with electrochemical measurements. Au nanosheets synthesized by the proposed method were used for construction of an Au nanocomposite electrode (AuNS/CILE). The catalytic activity of AuNS/CILE toward FA oxidation was investigated in acidic and alkaline media. But the best oxidation performance of FA was achieved in 0.1 M NaOH solution containing FA (0.1 M). After addition of FA to the NaOH solution, pH of solution was measured that reached to 5.5. These results were in agreement with previous reports that FA oxidation exhibits a pH dependence and shows the best performance at a pH close to the pKa. The cyclic voltammograms, at 50 mV s−1 in a potential window of −0.5 to 1.0 V versus Ag/AgCl are presented in Fig. 7A. For comparison the result for FA oxidation with CILE under the same conditions has also been shown in Fig. 7A, curve a. However, at the bare CILE electrode, no peak was observed for the FA oxidation (Fig. 7A curve a). FA oxidation on the surface of AuNS/CILE (Fig. 7A, curve b) is appeared with onset potential at 0.3 V and a sharp peak centered at around 0.64 V in the forward scan (peak I) and a second peak that is often related to the CO poisoning was not shown in forward scan. In the reverse scan, a large anodic peak was observed almost at about the same peak potential (0.67 V) of the anodic peak in the forward scan (peak II). The anodic peak in the reverse scan can also be associated with the direct FA oxidation and assigned to the same reaction. Cyclic voltammogram of AuNS/CILE electrode in the 0.1 M NaCl solution (pH 5.5) in the absence of FA is shown in Fig. 7A, curve c. It can be seen that AuNS/CILE shows two peaks at around +1.0 and +0.6 V in 0.1 M NaCl solution (pH 5.5) in the absence of FA. The peaks that appear in the base line of AuNS/CILE are attributed to the oxidation and reduction of Au nanosheets in the structure of electrode. The catalytic activity of AuNS/CILE toward FA oxidation was investigated in 0.5 M phosphate buffer solution (PBS, pH 5.5), (Fig. 7B). As shown in Fig. 7, the modification of CILE with AuNS could enhance the catalytic activity of the electrode for FA oxidation. Surprisingly, when AuNS was used in the construction of the electrode, the oxidation of FA was performed exclusively with the direct pathway (dehydrogenation mechanism) and the indirect peak (dehydration mechanism, CO oxidation peak) was not appeared. In fact, Au nanosheets with sharp edges and corners can increase the rate of oxidation of FA via a direct CO2 pathway.
| Direct pathway: HCOOH → HCOOads + H+ + e− → CO2 + 2H+ + 2e− (dehydrogenation) |
| Indirect pathway: HCOOH → COads + H2O → CO2 + 2H+ + 2e− (dehydration) |
As shown in previous reports,45 the morphology of Au nanostructures is an important factor in the electrocatalytic oxidation of FA. Ramaraj et al. showed that Au nanoparticles electrodeposited on electrochemically reduced graphene oxide modified GCE showed electrocatalytic activity for formic acid oxidation; but Au nanostructures modified GCE failed to catalyze the formic acid oxidation. In our study, Au nanosheets with (111) high index facets were evaluated as a catalyst for oxidation of FA. However, one of the most distinctive features of this study for electrooxidation of FA on AuNS/CILE is the elimination of the indirect FA oxidation pathway. Table 1 shows a comparison between AuNS/CILE and previously reported electrodes for oxidation of formic acid.
| Electrode | Electrolyte | Ep in forward scan (V) | J (mA cm−2) | Ref | |
|---|---|---|---|---|---|
| Ep1 | Ep2 | Jp1/Jp2 | |||
| Pt–Au/RGO/CF | 0.5 M H2SO4 | 0.33 V | 0.69 V | 2.57 | 69 |
| Pt–Au–Cu alloy NPs | 0.5 M H2SO4 | 0.32 V | 0.6 V | ∼1 | 70 |
| Au NPs/RGO/GCE | 0.5 M KOH | 0.46 V | 0.69 V | ∼1 | 56 |
| Pt50–Au50 alloyed NPs | 0.5 M H2SO4 | 0.5 V | — | — | 50 |
| NiOx/Au/Pt/GC | 0.5 M Na2SO4 | 0.28 V | — | ∞ | 41 |
| AuPt@Pd/C | 0.5 M Na2SO4 | 0.2 V | 0.65 V | ∼2 | 71 |
| Pt-on-Au/SnO2–CNTs/GC | 1 M HClO4 | 0.3 V | — | ∼2 | 72 |
| Porous Au/Pt alloy NPs | 0.5 M H2SO4 | 0.3 V | 0.7 V | ∼2 | 73 |
| Pd–Au NPs/graphene nanoplatelets | 0.5 M H2SO4 | 0.3 V | 0.65 V | ∼2 | 54 |
| AuNS/CILE | 0.1 M NaCl | 0.64 V | — | ∞ | This work |
Fig. 7C shows ten repetitive cyclic voltammograms recorded at AuNS/CILE in a formic acid solution. It can be seen that the peak current density increased during successive scans. The increase in the oxidation current might be due to the preconcentration of formic acid into the ionic liquid component of the electrode66 prior to any electron transfer process.
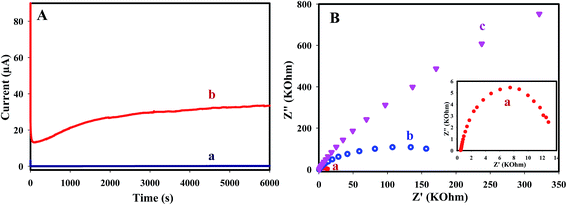
The stability and the activity of the electrodes were investigated by chronoamperometry. Chronoamperometric measurements of oxidation of FA at the bare CILE and AuNS/CILE were examined by maintaining the electrode at a potential of 0.6 V for 6000 s continuous electrolysis. Fig. 8A shows a typical chronoamperometric response of 0.1 M FA and 0.1 M NaOH at the surface of CILE and AuNS/CILE. The current density of FA oxidation at AuNS/CILE is higher than CILE and the current increased slowly within 0–3000 s and remained efficiently unchanged after 6000 s of continuous electrolysis. These results indicate the high catalytic activity and stability of AuNS/CILE and the significant tolerance against CO poisoning.
Electrochemical impedance spectroscopy has been used to investigate the electrocatalytic activity of AuNS toward FA oxidation. Fig. 8B shows the Nyquist plot of FA (0.1 M FA in 0.1 M NaOH) oxidation on CILE and AuNS/CILE at open circuit potential (OCP) and 0.3 V. The diameter of the impedance arc dramatically decreased in AnNS/CILE which indicates that the Rct on the AuNS/CILE is much smaller than CILE. In Fig. 8, no impedance behavior was appeared in the negative part of Nyquist plot, indicating a good tolerance towards CO poisoning intermediates. With increasing the electrode potential from OCP to 0.3 V, the diameter of the arc decreased that shows faster electron transfer kinetic at the onset potential.
The electrocatalytic activity of metal nanostructures is strongly dependent on their size, shape and exposed surfaces. The anisotropic effects of gold nanostructures were studied in a previous report and emphasized the importance of the anisotropic effects of crystallographic plane.67 Chen et al. showed that the reactive sites of gold nanoplates are mainly low-coordination metal sites and follow the trend of corner regions > edge regions > flat surface facet regions.68 In this study, due to the presence of sharp edges and corners and high conductivity of Au nanosheets, AuNS/CILE shows excellent electrocatalytic activity toward formic acid oxidation with elimination of the indirect pathway.
Conclusion
In summary, micrometer-sized Au nanosheets were efficiently synthesized by a one-step room temperature reduction of HAuCl4 aqueous solutions with red marine alga extract. Au nanosheets with well-defined hexagonal, truncated triangular and triangular shapes were synthesized in high yields with the lateral size in the micrometer scale (0.5–4 μm along their longest edge) and thickness in the nanometer scale with flat surface. This preparation method is very simple, green and economical and only the crude extract of red marine alga is required. The as-prepared Au nanosheets were used as an electrocatalyst for fabrication of electrochemical sensor for oxidation of formic acid. AuNS/CILE exhibited substantially efficient catalytic activity, good stability and strong poisoning tolerance to CO intermediate during the electrooxidation of formic acid in alkaline media. Thus, the direct pathway FA oxidation reaction without the formation of poisonous intermediates together with a simple and ecofriendly synthesis of Au nanosheets by red marine alga extract seems to be effective for application in direct full cells.Acknowledgements
The authors wish to express their gratitude to Shiraz University Research Council, for the support of this work. This project was partly supported by Iran National Science Foundation (Research Chair Award No. 95/INSF/44913).References
- L. Pei, K. Mori and M. Adachi, Langmuir, 2004, 20, 7837 CrossRef CAS PubMed.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS PubMed.
- Y. Sun, B. Mayers and Y. Xia, Nano Lett., 2003, 3, 675 CrossRef CAS.
- M. A. El-Sayed, Acc. Chem. Res., 2001, 34, 257 CrossRef CAS PubMed.
- Y. Zhang, X. Cui, F. Shi and Y. Deng, Chem. Rev., 2012, 112, 2467 CrossRef CAS PubMed.
- K. Ai, Y. Liu and L. Lu, J. Am. Chem. Soc., 2009, 131, 9496 CrossRef CAS PubMed.
- J. Y. Xu, J. Wang, L. T. Kong, G. C. Zheng, Z. Guo and J. H. Liu, J. Raman Spectrosc., 2011, 42, 1728 CrossRef CAS.
- L. Rodríguez-Lorenzo, R. A. Álvarez-Puebla, F. J. García de Abajo and L. M. Liz-Marzán, J. Phys. Chem. C, 2010, 114, 7336 Search PubMed.
- P. Pienpinijtham, X. X. Han, T. Suzuki, C. Thammacharoen, S. Ekgasit and Y. Ozaki, Phys. Chem. Chem. Phys., 2012, 14, 9636 RSC.
- Y. Su, X. Wei, F. Peng, Y. Zhong, Y. Lu, S. Su, T. Xu, S.-T. Lee and Y. He, Nano Lett., 2012, 12, 1845 CrossRef CAS PubMed.
- S. Nootchanat, C. Thammacharoen, B. Lohwongwatanab and S. Ekgasit, RSC Adv., 2013, 3, 3707 RSC.
- L. Wang, X. Wu, X. Li, L. Wang, M. Pei and X. Tao, Chem. Commun., 2010, 46, 8422 RSC.
- C.-C. Chen, C.-H. Hsu and P.-L. Kuo, Langmuir, 2007, 23, 6801 CrossRef CAS PubMed.
- L. Bi, Y. Rao, Q. Tao, J. Dong, T. Su, F. Liu and W. Qian, Biosens. Bioelectron., 2013, 43, 193 CrossRef CAS PubMed.
- W.-L. Huang, C.-H. Chen and M. H. Huang, J. Phys. Chem. C, 2007, 111, 2533 CAS.
- C. Li, W. Cai, B. Cao, F. Sun, Y. Li, C. Kan and L. Zhang, Adv. Funct. Mater., 2006, 16, 83 CrossRef CAS.
- D. H. Dahanayaka, J. X. Wang, S. Hossain and L. A. Bumm, J. Am. Chem. Soc., 2006, 128, 6052 CrossRef CAS PubMed.
- C. S. Ah, Y. J. Yun, H. J. Park, W.-J. Kim, D. H. Ha and W. S. Yun, Chem. Mater., 2005, 17, 5558 CrossRef CAS.
- J. E. Millstone, G. S. Métraux and C. A. Mirkin, Adv. Funct. Mater., 2006, 16, 1209 CrossRef CAS.
- K. Banu and T. Shimura, New J. Chem., 2012, 36, 2112 RSC.
- Z. Li, Z. Liu, J. Zhang, B. Han, J. Du, Y. Gao and T. Jiang, J. Phys. Chem. B, 2005, 109, 14445 CrossRef CAS PubMed.
- M. Tohidi, F. AghakhaniMahyari and A. Safavi, RSC Adv., 2015, 5, 32744 RSC.
- V. Kumar and S. K. Yadav, J. Chem. Technol. Biotechnol., 2009, 84, 151 CrossRef CAS.
- G. S. Ghodake, N. G. Deshpande, Y. P. Lee and E. S. Jin, Colloids Surf., B, 2010, 75, 584 CrossRef CAS PubMed.
- N. Asmathunisha and K. Kathiresan, Colloids Surf., B, 2013, 103, 283 CrossRef CAS PubMed.
- B. Liu, J. Xie, J. Y. Lee, Y. P. Ting and J. Paul Chen, J. Phys. Chem. B, 2005, 109, 15256 CrossRef CAS PubMed.
- J. Xie, J. Y. Lee, D. I. C. Wang and Y. P. Ting, Small, 2007, 3, 672 CrossRef CAS PubMed.
- S. S. Shankar, A. Rai, A. Ahmad and M. Sastry, Chem. Mater., 2005, 17, 566 CrossRef CAS.
- Y. Lu and W. Chen, ACS Catal., 2012, 2, 84 CrossRef CAS.
- M. Liu, S. He and W. Chen, Electrochim. Acta, 2016, 199, 218 CrossRef CAS.
- Y. Lu, Y. Jiang and W. Chen, Nanoscale, 2014, 6, 3309 RSC.
- M. Liu, Y. Lu and W. Chen, Adv. Funct. Mater., 2013, 23, 1289 CrossRef CAS.
- Y. Lu, Y. Jiang, H. Wu and W. Chen, J. Phys. Chem. C, 2013, 117, 2926 CAS.
- W. Wei and W. Chen, J. Power Sources, 2012, 204, 85 CrossRef CAS.
- A. Cheng and P. Holt-Hindle, Chem. Rev., 2010, 110, 3767 CrossRef PubMed.
- C. Bianchini and P. Shen, Chem. Rev., 2009, 109, 4183 CrossRef CAS PubMed.
- M. Liu, R. Zhang and W. Chen, Chem. Rev., 2014, 114, 5117 CrossRef CAS PubMed.
- A. M. Mohammad, G. A. El-Nagar, I. M. Al-Akraa, M. S. El-Deab and B. E. El-Anadouli, Int. J. Hydrogen Energy, 2015, 40, 7808 CrossRef CAS.
- M. Liu and W. Chen, Nanoscale, 2013, 5, 12558 RSC.
- Y. Lu, Y. Jiang, X. Gao, X. Wang and W. Chen, J. Am. Chem. Soc., 2014, 136, 11687 CrossRef CAS PubMed.
- Y. Lu, Y. Jiang and W. Chen, Nano Energy, 2013, 2, 836 CrossRef CAS.
- S. Zhang, Y. Y. Shao, G. P. Yin and Y. H. Lin, Angew. Chem., Int. Ed., 2010, 49, 2211 CrossRef CAS PubMed.
- S.-Y. Lee, N. Jung, J. Cho, H.-Y. Park, J. Ryu, I. Jang, H.-J. Kim, E. Cho, Y.-H. Park, H. C. Ham, J. H. Jang and S. J. Yoo, ACS Catal., 2014, 4, 2402 CrossRef CAS.
- R. Iyyamperumal, L. Zhang, G. Henkelman and R. M. Crooks, J. Am. Chem. Soc., 2013, 135, 5521 CrossRef CAS PubMed.
- Y. Holade, A. Lehoux, H. Remita, K. B. Kokoh and T. W. Napporn, J. Phys. Chem. C, 2015, 119, 27529 CAS.
- L.-B. Wang, Y.-C. Wang, H.-Y. Guo, J.-L. Huang, Y.-L. Zhao, Q.-Y. Liu, X. Wu and J. Zeng, Part. Part. Syst. Charact., 2015, 32, 295 CrossRef CAS.
- A. Sáez, E. Expósito, J. Solla-Gullón, V. Montiel and A. Aldaz, Electrochim. Acta, 2012, 63, 105 CrossRef.
- S. Hua, L. Scudierob and S. Ha, ECS Trans., 2014, 64, 1121 CrossRef.
- R. Wang, J. Liu, P. Liu, X. Bi, X. Yan, W. Wang, X. Ge, M. Chen and Y. Ding, Chem. Sci., 2014, 5, 403 RSC.
- D. N. Oko, J. Zhang, S. Garbarino, M. Chaker, D. Ma, A. C. Tavares and D. Guay, J. Power Sources, 2014, 248, 273 CrossRef CAS.
- M. D. Obradović, J. R. Rogan, B. M. Babić, A. V. Tripković, A. R. S. Gautam, V. R. Radmilović and S. Lj. Gojković, J. Power Sources, 2012, 197, 72 CrossRef.
- I.-S. Park, K.-S. Lee, D.-S. Jung, H.-Y. Park and Y.-E. Sung, Electrochim. Acta, 2007, 52, 5599 CrossRef CAS.
- V. Selvaraj, A. Nirmala Grace and M. Alagar, J. Colloid Interface Sci., 2009, 333, 254 CrossRef CAS PubMed.
- T. Maiyalagan, X. Wang and A. Manthiram, RSC Adv., 2014, 4, 4028 RSC.
- P. Gai, Y. Ji, Y. Chen, C. Zhu, J. Zhang and J.-J. Zhu, Analyst, 2015, 140, 1822 RSC.
- P. Rameshkumar, R. Praveen and R. Ramaraj, J. Electroanal. Chem., 2015, 754, 118 CrossRef CAS.
- J. John, H. Wang, E. D. Rus and H. D. Abruña, J. Phys. Chem. C, 2012, 116, 5810 CAS.
- J. Jiang, J. Scott and A. Wieckowski, Electrochim. Acta, 2013, 104, 124 CrossRef CAS.
- J. Joo, T. Uchida, A. Cuesta, M. T. M. Koper and M. Osawa, J. Am. Chem. Soc., 2013, 135, 9991 CrossRef CAS PubMed.
- J. Joo, M. Choun, J. Jeong and J. Lee, ACS Catal., 2015, 5, 6848 CrossRef CAS.
- N. Maleki, A. Safavi and F. Tajabadi, Anal. Chem., 2006, 78, 3820 CrossRef CAS PubMed.
- H. C. Chu, C. H. Kuo and M. H. Huang, Inorg. Chem., 2006, 45, 808 CrossRef CAS PubMed.
- H. M. El-Rafie, M. H. El-Rafie and M. K. Zahran, Carbohydr. Polym., 2013, 96, 403 CrossRef CAS PubMed.
- M. F. De Jesus Raposo, A. M. B. De Morais and R. M. S. C. De Morais, Mar. Drugs, 2015, 13, 2967 CrossRef PubMed.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2014, 31, 160 RSC.
- M. Opallo and A. Lesniewski, J. Electroanal. Chem., 2011, 656, 2 CrossRef CAS.
- Y. Chen, W. Schuhmann and A. W. Hassel, Electrochem. Commun., 2009, 11, 2036 CrossRef CAS.
- N. M. Andoy, X. Zhou, E. Choudhary, H. Shen, G. Liu and P. Chen, J. Am. Chem. Soc., 2013, 135, 1845 CrossRef CAS PubMed.
- Z. Yao, R. Yue, F. Jiang, C. Zhai, F. Ren and Y. Du, J. Solid State Electrochem., 2013, 17, 2511 CrossRef CAS.
- M. Wang, Y. He, R. Li, Z. Ma, Z. Zhang and X. Wang, Electrochim. Acta, 2015, 178, 259 CrossRef CAS.
- X. Lu, F. Luo, H. Song, S. Liao and H. Li, J. Power Sources, 2014, 246, 659 CrossRef CAS.
- W. Jianshe, X. Jingyu, Z. Lei, Z. Jiujun, G. Xun, Z. Jianhong, S. Chengying and W. Liucheng, Electrochim. Acta, 2013, 112, 480 CrossRef.
- D. Lee, H. Y. Jang, S. Hong and S. Park, J. Colloid Interface Sci., 2012, 388, 74 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |