Facile synthesis of a Fe3O4/MIL-101(Fe) composite with enhanced catalytic performance†
Zhong Wei Jianga,
Fu Qiang Daia,
Cheng Zhi Huang*ab and
Yuan Fang Li*a
aKey Laboratory of Luminescent and Real-Time Analytical Chemistry (Southwest University), Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China. E-mail: liyf@swu.edu.cn; Fax: +86 23 68367257; Tel: +86 23 68254659
bCollege of Pharmaceutical Sciences, Southwest University, Chongqing 400716, China. E-mail: chengzhi@swu.edu.cn
First published on 5th September 2016
Abstract
A magnetic porous material, Fe3O4/MIL-101(Fe), has been successfully fabricated by using simple ultrasound-assisted electrostatic self-assembly technology, and was demonstrated to be a highly active heterogeneous catalyst for the dimerization reaction of o-phenylenediamine (OPD) in the presence of H2O2 via synergistic peroxidase-like activity.
Metal–organic frameworks (MOFs), well-known as a new class of porous materials synthesized by assembly with metal cations/clusters and organic linkers, have attracted significant research interest in recent years,1 not only due to their diversiform topological structure but also due to their many attractive applications in adsorption and separation,2 sensing,3 drug delivery4 and catalysis.5 Recently, Fe-based MOFs have been widely used in catalytic applications due to their catalytically active coordinatively unsaturated Fe(III) sites and the existence of Fe3-μ3-oxo clusters, which endows iron-containing MOFs with photocatalytic activity.6 Moreover, intrinsic peroxidase-like activity, a new activity of Fe-based MOFs, which shows the ability to catalytically decompose hydrogen peroxide (H2O2) to ˙OH radicals via a Fenton-like route has been found recently and it shows a wide range of applications in biosensing platforms.7 As far as we know, there is no report about utilizing the peroxidase-like activity catalytic performance of Fe-based MOFs towards organic synthesis.
Magnetic nanoparticles (MNPs) have received considerable attention because of their power in separation techniques. Among various magnetic particles, iron oxides (such as Fe3O4 MNPs) have drawn the most attention owing to their strong magnetic responsiveness and biocompatibility. As magnetic separation is directly performed on complex samples, the functionalized Fe3O4 MNPs are usually used for the preconcentration and isolation of analytes from complex matrices.8 For instance, MNPs have been used for separating DNA, proteins, cells from samples9 and catalyst recycling in organic synthesis aspect.10 In addition, a research reported that Fe3O4 MNPs exhibit peroxidase-like activity allowing the effective detection of H2O2.11 Since then, Fe3O4 MNPs pose the widespread applications in sensing and water purification by utilizing the peroxidase-like activity.12
Controllable integration of MOFs and functional materials is resulting in the creation of new multifunctional composites, which exhibit new properties that are superior to those of the individual components through the collective behavior of the functional units.13 For the magnetization of MOFs, five different synthesis approaches, including, hydrothermal,14 encapsulation,15 in situ magnetization,16 chemical bonding17 and coprecipitation assembly18 were attempted by previous report. Wherein, the magnetized MOFs were used for catalysis, extraction and separation. Intriguing by these meaningful researches and given the tremendous emerging interest in this field, we present an ultrasound-assisted electrostatic self-assembly strategy that has allowed us to rationally design and fabricate a novel type of magnetic composites in a general approach.
In our strategy, MIL-101(Fe), a kind of Fe-based MOFs, was chose as a matrix for loading Fe3O4 MNPs due to the outstanding peroxidase-like activity among the reported Fe-based MOFs owing to the high BET surface, mesosize cages and superior affinity for H2O2.19 Typically, cysteine (Cys) functionalized Fe3O4 MNPs and MIL-101(Fe) suspensions were mixed together under ultrasonication at room temperature for 10 minutes and the magnetic composite would be obtained by magnetic separation. The Fe3O4/MIL-101(Fe) magnetic composite prepared by this method displays high saturation magnetization value, making this composite more susceptible to magnetic fields and easier to isolate from aqueous solution. Furthermore, the as-synthesized Fe3O4/MIL-101(Fe) exhibits much higher catalytic activity than the single component for the synthesis of 2,3-diaminophenazine (2,3-DPA) due to the synergistic peroxidase-like activity between the multiple active centers, including Fe3O4 MNPs and MIL-101(Fe), which improved the decomposition efficiency of H2O2 and produced abundant reactive oxygen species (ROS) (Scheme 1).
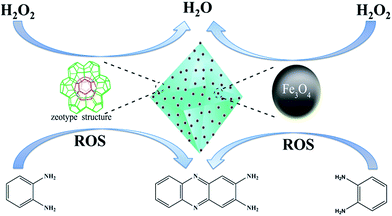 | ||
| Scheme 1 Schematic illustration of the synergistic catalytic behavior of Fe3O4/MIL-101(Fe) for the oxidation of OPD. | ||
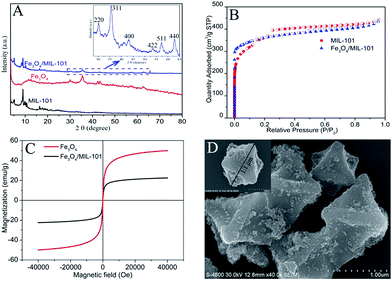
Powder XRD patterns of the parent materials (MIL-101(Fe), Fe3O4) and the hybrid Fe3O4/MIL-101(Fe) are shown in Fig. 1A. The diffraction peaks of MIL-101(Fe) and Fe3O4 are observed in the PXRD patterns of the as-synthesized hybrid Fe3O4/MIL-101(Fe), which confirms that the MIL-101(Fe) represented the major component of the composite, and it also indicated that the hybrid Fe3O4/MIL-101(Fe) preserved the crystalline characters of parent MIL-101(Fe). The diffraction intensity of Fe3O4 in the PXRD patterns of Fe3O4/MIL-101(Fe) was relatively weak, probably because the low content of Fe3O4 in the composite. The Brunauer–Emmett–Teller (BET) surface area of the as-synthesized MIL-101(Fe) and Fe3O4/MIL-101(Fe) were evaluated by nitrogen adsorption–desorption isotherms (Fig. 1B). The BET surface area of the naked MIL-101 and the hybrid Fe3O4/MIL-101(Fe) are calculated to be 1362 m2 g−1 and 1248 m2 g−1, respectively. Obviously, Fe3O4/MIL-101(Fe) still maintained a high specific surface area although the BET surface area of MIL-101(Fe) is minor higher than that of Fe3O4/MIL-101(Fe). Besides, the pore size of Fe3O4/MIL-101(Fe) is similar to that of MIL-101(Fe) with a little change (Fig. S1†).
In addition, the magnetization curve illustrates the superparamagnetic characteristic of Fe3O4 and Fe3O4/MIL-101(Fe) (Fig. 1C). The saturation magnetization values were measured to be 50 emu g−1 and 23 emu g−1 respectively, which is susceptible to magnetic fields and easy to isolate from aqueous solution. The photographs of dispersed MIL-101(Fe) and Fe3O4/MIL-101(Fe) are shown in Fig. S2A and B,† respectively. A clear solution could be obtained after separation of Fe3O4/MIL-101(Fe) by using the external magnet in 20 s (Fig. S2C†). In order to better understand the morphological structure of the hybrid material Fe3O4/MIL-101(Fe), the texture of the samples were observed by scanning electron microscope (SEM). As can be seen from Fig. 1D, the surface of MIL-101(Fe) with a particle size of 1.1 μm has been successfully decorated with Fe3O4 nanoparticles without any influence on the morphology of MIL-101(Fe). The transmission electron microscope (TEM) images also demonstrated that the Fe3O4 MNPs with particle size about 10 nm were attached on the surface of MIL-101(Fe) successfully (Fig. S3†).
As we know, Cys is a kind of ampholyte with an isoelectric point of 5.05. Therefore, the prepared Cys-functionalized Fe3O4 MNPs should exhibit different zeta potential under various pH of the suspension. The as-prepared MIL-101(Fe) shows a strong electropositive. Hence, in order to obtain electronegative Fe3O4 MNPs, the pH of the suspension has been tuned from 6.0 to 10.0 (Fig. 2A). With the increase of the pH, the electronegativity of Cys-Fe3O4 become stronger and the corresponding electropositivity of the as-prepared Fe3O4/MIL-101(Fe) get weaker. Unfortunately, when the pH of Cys-Fe3O4 MNPs suspension was tuned to 10.0, the as-synthesized Fe3O4/MIL-101(Fe) would aggregate seriously. So, the pH of Cys-Fe3O4 MNPs suspension was selected as 9.0 with the purpose of obtaining stable dispersion of Fe3O4/MIL-101(Fe). As shown in Fig. 2B, the zeta potential of Cys-Fe3O4MNPs is −36.87 mV at pH of 9.0, and 35.37 mV for that of original MIL-101(Fe) suspension. The corresponding Fe3O4/MIL-101(Fe) shows weak electropositivity (11.23 mV) without obvious aggregation.
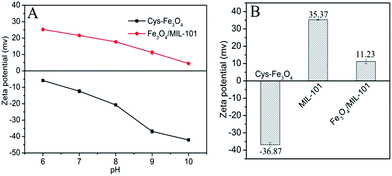 | ||
| Fig. 2 (A) Zeta potential of Fe3O4 and the corresponding Fe3O4/MIL-101(Fe) under various pH; (B) the changes of zeta potential before and after reaction. | ||
Liquid-phase dimerization reaction of OPD to 2,3-DPA in the presence of H2O2 was used as a model reaction system to characterize the catalytic performance of the Fe3O4/MIL-101(Fe). When the Fe3O4/MIL-101(Fe) catalyst was introduced into the solution, the fluorescence of 2,3-DPA increased along with the time's going and showed an intensive yellow-green fluorescence under UV excitation (Fig. S4†). The product 2,3-DPA shows a strong fluorescence emission (558 nm) in methanol under 439 nm excitation. So, the reaction process was monitored by fluorescence standard curve method (Fig. S5†). The standard curve has a good linear relationship (R2 = 0.998) with a linear equation y = 24.60c + 37.52. The reaction proceeds slowly over the Cys-Fe3O4 MNPs and MIL-101(Fe) catalysts, while the catalytic activity of the Fe3O4/MIL-101(Fe) catalyst is noticeably better under the same conditions (Fig. 3). It also indicated that the catalytic efficiency of H2O2 is very low without Fe3O4/MIL-101(Fe). The possible reaction mechanism was shown in Fig. S6.† The Fe3O4/MIL-101(Fe) shows excellent catalytic activity for the decomposition of H2O2 and produces abundant reactive oxygen species (ROS) which was involved in the whole oxidation of OPD.
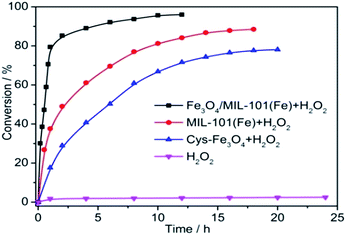 | ||
| Fig. 3 Catalytic conversion of OPD to 2,3-DPA over Cys-Fe3O4 MNPs, MIL-101(Fe) and Fe3O4/MIL-101(Fe), respectively. | ||
Similar to peroxidase, the catalytic activity of Fe3O4/MIL-101(Fe) was found to be closely dependent on pH, temperature, H2O2 concentration, and so on. Therefore, the reaction conditions, such as the concentration of H2O2 and Fe3O4/MIL-101(Fe), pH, temperature, and reaction time, have been optimized (Fig. S7†). Under the optimal reaction conditions, the Fe3O4/MIL-101(Fe) displayed a higher catalytic activity than that of Cys-Fe3O4 MNPs and MIL-101(Fe), achieving 97.79% yield of 2,3-DPA (Fig. S8†). Compared with other existing catalyst (Table S1†), the as-synthesized Fe3O4/MIL-101(Fe) shows highest catalytic efficiency among the reported catalyst for the oxidation of OPD. For the product 2,3-DPA (structure displayed in Fig. S9†), the characterization data of FTIR, 1H NMR, 13C NMR and ESI-TOFMS are showed in Fig. S10–S13,† respectively.
From the results shown above, one can easily draw a conclusion that the catalytic activity of Fe3O4/MIL-101(Fe) is higher than those of Cys-Fe3O4MNPs and MIL-101(Fe) for the same reaction time. This catalytic activity enhancement can be attributed to the synergistic effect of Cys-Fe3O4MNPs and MIL-101(Fe). Furthermore, the recyclability of the Fe3O4/MIL-101(Fe) catalyst was also examined in the dimerization reaction of OPD. The catalyst was isolated by magnetic separation from the reaction mixture and washed with methanol, and reused for the next run under the same conditions. The results indicated that no significant loss of activity for the dimerization reaction of OPD was observed over Fe3O4/MIL-101(Fe) in the five successive catalytic cycles, suggesting that the Fe3O4/MIL-101(Fe) possesses long-term stability (Fig. 4). The atomic absorption spectrometry (AAS) has been used for monitoring the leaching test. The total iron content which leached from the composite would have a relatively high leaching quantity in the first-time using. The highest leaching amount is 1.153 ng mL−1. The leaching amount dropped dramatically with the increase of cycling times, and even no iron was detected after three cycles (Fig. S14†). However, the catalytic efficiency of the composite is still maintained at a high level. It indicates that the catalytic activity is mainly derived from the composite material rather than the leached iron ion. After five recycling use, the PXRD pattern showed that the used Fe3O4/MIL-101(Fe) keeps close to the originality (Fig. S15†). As can be seen from the X-ray photoelectron spectroscopy (XPS) spectrums (Fig. S16†), there were no changes between the fresh and recovered Fe3O4/MIL-101(Fe). It indicates that the composite remains the integrity without any valence state change before and after the reaction. Besides, the morphology of Fe3O4/MIL-101(Fe) has no serious changes and no great influence upon the catalytic activity after five consecutive reaction cycles (Fig. S17†).
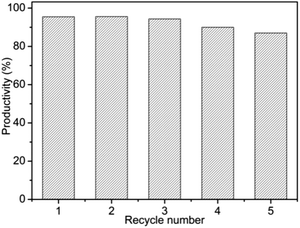 | ||
| Fig. 4 The productivity of 2,3-DPA using Fe3O4/MIL-101(Fe) as catalyst after five consecutive reaction. | ||
In summary, we have developed a facile, general and effective ultrasound-assisted electrostatic self-assembly strategy for immobilizing Fe3O4 MNPs on the surface of MIL-101(Fe). The catalytic property of Fe3O4/MIL-101(Fe) was evaluated by using the dimerization reaction of OPD as a model reaction system. It is noteworthy that the as-prepared Fe3O4/MIL-101(Fe) composite synergistically improves the catalytic activity compared to the Cys-Fe3O4 and MIL-101(Fe). Furthermore, the high catalytic activity was retained after a number of reaction cycles with a slightly change. This study may bring light to new opportunities in the development of high performance heterogeneous catalysts based on the peroxidase-like activity of Fe-based MOFs or nanostructures.
Acknowledgements
The authors gratefully acknowledge thank the financial support of the National Natural Science Foundation of China (NSFC, No. 21175109).Notes and references
- W. G. Lu, Z. W. Wei, Z. Y. Gu, T. F. Liu, J. Park, J. Park, J. Tian, M. W. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H. C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC
.
-
(a) G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC
; (b) Z. Y. Gu, C. X. Yang, N. Chang and X. P. Yan, Acc. Chem. Res., 2012, 45, 734–745 CrossRef CAS PubMed
; (c) N. Chang, Z.-Y. Gu and X.-P. Yan, J. Am. Chem. Soc., 2010, 132, 13645–13647 CrossRef CAS PubMed
; (d) J. J. Gutiérrez-Sevillano, A. Martín-Calvo, D. Dubbeldam, S. Calero and S. Hamad, RSC Adv., 2013, 3, 14737 RSC
.
-
(a) D. Wang, L. Sun, C. Hao, Y. Yan and Z. Liang, RSC Adv., 2016, 6, 57828–57834 RSC
; (b) B. Ding, C. Guo, S. X. Liu, Y. Cheng, X. X. Wu, X. M. Su, Y. Y. Liu and Y. Li, RSC Adv., 2016, 6, 33888–33900 RSC
; (c) J. F. Guo, R. M. Fang, C. Z. Huang and Y. F. Li, RSC Adv., 2015, 5, 46301–46306 RSC
.
-
(a) P. Horcajada, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. KyuHwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. C. a. R. Gref, T. Chalati, C. Serre, B. Gillet and C. Sebrie, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed
; (b) J. Zhuang, C.-H. Kuo, L.-Y. Chou, D.-Y. Liu, E. Weerapana and C.-K. Tsung, ACS Nano, 2014, 8, 2812–2819 CrossRef CAS PubMed
.
-
(a) H. T. H. Nguyen, O. T. K. Nguyen, T. Truong and N. T. S. Phan, RSC Adv., 2016, 6, 36039–36049 RSC
; (b) A. R. Oveisi, A. Khorramabadi-zad and S. Daliran, RSC Adv., 2016, 6, 1136–1142 RSC
; (c) K. Huang, Y. Xu, L. Wang and D. Wu, RSC Adv., 2015, 5, 32795–32803 RSC
; (d) S. Beheshti and A. Morsali, RSC Adv., 2014, 4, 41825–41830 RSC
.
-
(a) D. Wang, R. Huang, W. Liu, D. Sun and Z. Li, ACS Catal., 2014, 4, 4254–4260 CrossRef CAS
; (b) K. G. Laurier, F. Vermoortele, R. Ameloot, D. E. De Vos, J. Hofkens and M. B. Roeffaers, J. Am. Chem. Soc., 2013, 135, 14488–14491 CrossRef CAS PubMed
; (c) D. Wang, M. Wang and Z. Li, ACS Catal., 2015, 5, 6852–6857 CrossRef CAS
.
-
(a) L. Ai, L. Li, C. Zhang, J. Fu and J. Jiang, Chem.–Eur. J., 2013, 19, 15105–15108 CrossRef CAS PubMed
; (b) Z. Jiang, Y. Liu, X. Hu and Y. Li, Anal. Methods, 2014, 6, 5647–5651 RSC
; (c) Z. Jiang, P. Gao, L. Yang, C. Huang and Y. Li, Anal. Chem., 2015, 87, 12177–12182 CrossRef CAS PubMed
; (d) Y. L. Liu, X. J. Zhao, X. X. Yang and Y. F. Li, Analyst, 2013, 138, 4526–4531 RSC
; (e) Y. Liu, P. Gao, C. Huang and Y. Li, Sci. China: Chem., 2015, 58, 1553–1560 CrossRef CAS
.
-
(a) X. L. Zhang, H. Y. Niu, W. H. Li, Y. L. Shi and Y. Q. Cai, Chem. Commun., 2011, 47, 4454–4456 RSC
; (b) K. Aguilar-Arteaga, J. A. Rodriguez and E. Barrado, Anal. Chim. Acta, 2010, 674, 157–165 CrossRef CAS PubMed
.
-
(a) I. Safarik and M. Safarikova, BioMagnetic Research and Technology, 2004, 2, 7 CrossRef PubMed
; (b) C. Bergemann, D. Müller-Schulte, J. Oster, L. à. Brassard and A. S. Lübbe, J. Magn. Magn. Mater., 1999, 194, 45–52 CrossRef CAS
; (c) M. Franzreb, M. Siemann-Herzberg, T. J. Hobley and O. R. Thomas, Appl. Microbiol. Biotechnol., 2006, 70, 505–516 CrossRef CAS PubMed
.
-
(a) Z. Zhu, Z. Lu, D. Wang, X. Tang, Y. Yan, W. Shi, Y. Wang, N. Gao, X. Yao and H. Dong, Appl. Catal., B, 2016, 182, 115–122 CrossRef CAS
; (b) A. Habibi-Yangjeh and A. Akhundi, J. Mol. Catal. A: Chem., 2016, 415, 122–130 CrossRef CAS
.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang and N. Gu, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed
.
-
(a) M. Liang, K. Fan, Y. Pan, H. Jiang, F. Wang, D. Yang, D. Lu, J. Feng, J. Zhao, L. Yang and X. Yan, Anal. Chem., 2013, 85, 308–312 CrossRef CAS PubMed
; (b) N. Ding, N. Yan, C. Ren and X. Chen, Anal. Chem., 2010, 82, 5897–5899 CrossRef CAS PubMed
; (c) Y. Shi, P. Su, Y. Wang and Y. Yang, Talanta, 2014, 130, 259–264 CrossRef CAS PubMed
; (d) H. Wang, H. Jiang, S. Wang, W. Shi, J. He, H. Liu and Y. Huang, RSC Adv., 2014, 4, 45809–45815 RSC
.
- Q. L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC
.
-
(a) Y.-F. Huang, M. Liu, Y.-Q. Wang, Y. Li, J.-M. Zhang and S.-H. Huo, RSC Adv., 2016, 6, 15362–15369 RSC
; (b) Y.-F. Huang, Y.-Q. Wang, Q.-S. Zhao, Y. Li and J.-M. Zhang, RSC Adv., 2014, 4, 47921–47924 RSC
; (c) S. Zhang, Z. Jiao and W. Yao, J. Chromatogr. A, 2014, 1371, 74–81 CrossRef CAS PubMed
.
-
(a) Z. Jin, Y. Luan, M. Yang, J. Tang, J. Wang, H. Gao, Y. Lu and G. Wang, RSC Adv., 2015, 5, 78962–78970 RSC
; (b) M. Babazadeh, R. Hosseinzadeh-Khanmiri, J. Abolhasani, E. Ghorbani-Kalhor and A. Hassanpour, RSC Adv., 2015, 5, 19884–19892 RSC
.
- S. H. Huo and X. P. Yan, Analyst, 2012, 137, 3445–3451 RSC
.
-
(a) F. Farzaneh and Y. Sadeghi, J. Mol. Catal. A: Chem., 2015, 398, 275–281 CrossRef CAS
; (b) Y. L. Hu, Z. L. Huang, J. Liao and G. K. Li, Anal. Chem., 2013, 85, 6885–6893 CrossRef CAS PubMed
.
-
(a) M. Saikia, D. Bhuyan and L. Saikia, New J. Chem., 2015, 39, 64–67 RSC
; (b) Z. Jiang and Y. Li, J. Taiwan Inst. Chem. Eng., 2016, 59, 373–379 CrossRef CAS
.
-
(a) D. Chen, B. Li, L. Jiang, D. Duan, Y. Li, J. Wang, J. He and Y. Zeng, RSC Adv., 2015, 5, 97910–97917 RSC
; (b) F. Cui, Q. Deng and L. Sun, RSC Adv., 2015, 5, 98215–98221 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19170c |
| This journal is © The Royal Society of Chemistry 2016 |