Carbazole-based two-photon fluorescent probe for selective imaging of mitochondrial hydrogen peroxide in living cells and tissues†
Kai
Zhang‡
bd,
Wei
Wu‡
a,
Yinhui
Li
*ab,
Mingtai
Sun
c,
Huan
Yu
*c and
Man Shing
Wong
*b
aCollege of Chemistry, Xiangtan University, Xiangtan, 411105, People's Republic of China. E-mail: yinhuili16@163.com
bDepartment of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong, People's Republic of China. E-mail: mswong@hkbu.edu.hk
cInstitute of Intelligent Machines, Chinese Academy of Sciences, Hefei, Anhui 230031, People's Republic of China. E-mail: hyu@iim.ac.cn
dCollege of Preclinical Medicine, Southwest Medical University, Luzhou, 646000, People's Republic of China
First published on 28th November 2016
Abstract
This paper reported a two-photon fluorescent probe for mitochondrial H2O2 detection and imaging based on the incorporation of a sensing boronate ester and an organelle-targeting triphenylphosphonium moiety onto the carbazole fluorophore. The probe exhibits a fast “turn on” response, good selectivity toward H2O2 and high specificity for mitochondria.
As a typical ROS, hydrogen peroxide (H2O2) can mediate diverse physiological responses and serve as the second messenger in normal cellular signal transduction.1 In mammalian cells, mitochondria have been demonstrated as main venues for the production of H2O2 and the generated H2O2 can participate in redox signaling through reversible oxidation of cysteine residues in target proteins.2 Excessive production of H2O2 in mitochondria will induce oxidative damage and cause physiological changes and diseases, such as cancer, cardiovascular disorders, and Alzheimer's disease.3 Therefore, specific, localizable and readily deployable tools for reliable and precise intracellular measurement of H2O2 are imperative. Up to now, a variety of analytical means, such as spectrophotometry, electrochemistry, and MRI4 together with recognition principles have been used to develop effective hydrogen peroxide sensors. The fluorescence assay for H2O2 detection in human physiology and pathology, which employs H2O2-triggered oxidative cleavage of boronate to phenol, sulfonate group hydrolysis,5 triphenylphosphine oxidation,6 Baeyer–Villiger type reaction7 and metal complex mediated reaction,8 has shown an extensive prospect because of the high sensitivity and specificity as well as capability of real-time and in situ imaging analysis. Although fluorescent probes were well competent to image the spatial and temporal distribution of cellular H2O2, how to maintain the cell viability following prolonged exposure to excitation illumination was still a challenge.
Two-photon microscopy (TPM) can record the most subtle internal structure deep into tissue with low cell damage by using two near-infrared photons for the excitation, which provides greater tissue penetration depth and higher spatial resolution.9 A variety of TP probes equipped with an organelle-targeting moiety such as tertiary amine, pyridinium or triphenyl-phosphonium group for labeling lysosomes and mitochondria have been developed which provided organelle-specific imaging with good spatial and temporal resolution. The superior tracing and imaging functions of TPM can be used for determining the spatial and temporal dynamics of reactive oxygen metabolites in a living system, especially for elucidating the diverse roles of H2O2 in a complex biological environment. It is particularly beneficial to use TPM for reactive oxygen species (ROS) and reactive nitrogen species (RNS) detections in a complex biological system. Some TP fluorescent probes have been shown to image hypochlorous acid,10 superoxide anion11 and nitric oxide12 in cellular environment. Up to date, there are only a handful of TP probes designed for intracellular H2O2 imaging,13 especially for mitochondrial or lysosomal H2O2.14 Because of the rapid metabolic process and constantly changed concentration of intracellular H2O2, there is a great demand for developing TPM probes to monitor the H2O2 production and consumption in mitochondria with fast response time and low detection limit to better understand the cellular and physiological roles of mitochondrial H2O2.
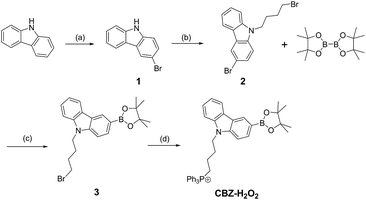
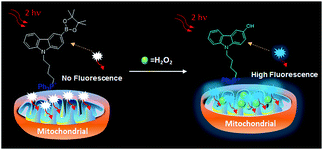
In this work, a TP fluorescent probe CBZ-H2O2 was synthesized by incorporating a boronate functionality as a H2O2 recognition site and the triphenylphosphonium group as mitochondrial targeting moiety into the fluorescent carbazole framework. The presence of boronic ester group at the 3-position of a carbazole scaffold would quench the TP fluorescence of CBZ-H2O2. After treatment with H2O2, a highly fluorescent product would be subsequently yielded due to the chemospecific deprotection of boronate to hydroxyl functionality. Compared with the existing TP probes for mitochondrial H2O2, CBZ-H2O2 exhibited a fast response time (∼15 min), improved sensitivity and low interference from other ROS. Importantly, it can be applied for direct imaging of mitochondrial H2O2 in living cells and frozen slice of rat liver with satisfactory imaging results.
The TP probe, CBZ-H2O2 was developed for localized detection of H2O2 concentration variations in mitochondrial. The synthetic routes and recognition principle were shown in Schemes 1 and 2, respectively. The molecular structure of CBZ-H2O2 was fully characterized with 1H NMR and 13C NMR as well as 31P NMR (Fig. S1–S4 in the ESI†). We then investigated the absorption and fluorescence properties of CBZ-H2O2 in PBS/DMSO (4/1 v/v, 20 mM, pH 7.4) solution at room temperature. As shown in Fig. S5,† the maximum absorbance at 340 nm of CBZ-H2O2 was gradually shifted to 360 nm after adding H2O2, with slightly enhanced absorbance. The same trend was followed for the maximum fluorescence intensity at 396 nm. In the presence of H2O2, the emission band exhibited an apparent 35 nm red shift and the fluorescence intensity dramatically increased nearly four times. The sensitive spectral response confirmed the specific reaction between CBZ-H2O2 and H2O2.
In order to optimize the pH value for the best response, we then investigated the fluorescence response of CBZ-H2O2 toward H2O2 in buffer solution at different pH ranging from 4.0 to 10.0. The intensity of CBZ-H2O2 remained consistent at different pH values. Upon treatment with H2O2, compared with the acidic region, the fluorescence intensity displayed a more noticeable enhancement in the alkaline region between 7.0 and 10.0 as shown in Fig. S6.† Therefore, it is advantageous to carry out the experiments in the buffer solution with pH value of 7.4, which is promising for biological applications.
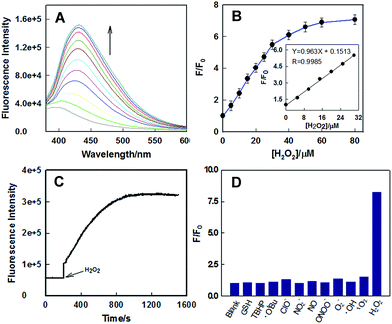
Fluorescence titration of H2O2 was conducted using 1.0 μM of CBZ-H2O2 and different concentration of H2O2 in PBS (20 mM, pH 7.4) containing 20% DMSO. As shown in Fig. 1A, the fluorescence response of probe was highly sensitive to the amount of H2O2. When the concentration of H2O2 went to 80 μM, the fluorescence enhancement reached a plateau, and further increase in the concentration would not boost the signal. Moreover, the dose-dependent fluorescence enhancement showed a good linearity between the F/F0 and the concentrations of H2O2 in a wide range from 0 to 30 μM with a detection limit (3σ/slope) of 1.96 μM (Fig. 1B, inset), where F0 and F corresponded to the fluorescence intensities at 430 nm of CBZ-H2O2 before and after adding H2O2, respectively. Compared with the reported fluorescent probes for H2O2, CBZ-H2O2 presents an improved detection sensitivity, which exhibited the potential to be used in monitoring physiological concentration of mitochondrial H2O2.
The real-time kinetic response of CBZ-H2O2 toward H2O2 was measured by recording the fluorescence emission as a function of time. As shown in Fig. 1C, in the first three minutes, the fluorescence intensity of CBZ-H2O2 remained steady and constant under irradiation. Upon the addition of H2O2, a drastic fluorescence enhancement was observed and the intensity reached plateaus within 15 minutes, indicating that the reaction proceeded faster than a majority of reported H2O2 probes. Considering that ROS and RNS are involved in a wide range of physiological and pathological processes, the selectivity of CBZ-H2O2 over ROS, RNS and other potentially competing species was investigated. Treatment of CBZ-H2O2 with other ROS (O2˙−, HO˙, ClO−, 1O2), RNS (NO, NO2−, ONOO−), TBHP, OtBu, and GSH afforded negligible effects on the fluorescence intensity of CBZ-H2O2 as shown in Fig. 1D. Only the presence of H2O2 could induce dramatically intensity enhancement, suggesting that CBZ-H2O2 exhibited a good selectivity toward H2O2 in complex physiological conditions.
To investigate the reaction mechanism of CBZ-H2O2 with H2O2, the differences of 1H NMR spectra of CBZ-H2O2 and the corresponding H2O2-reaction product were carefully analyzed and compared as shown in Fig. S7.† Upon the addition of H2O2, the resonance signal of CBZ-H2O2 at 8.44 ppm gradually moved to the high field, while a new and distinct resonance signal appeared and increased in intensity at 9.04 ppm over a period of 30 min. These changes of NMR signals corresponded to the phenolic proton of the reaction product, supporting the transformation of carbazole boronate to its corresponding phenol in the presence of H2O2.
With a good understanding of the sensitivity and selectivity of spectroscopic responses of the CBZ-H2O2 probe for detecting H2O2in vitro, we sought to apply it for the detection of H2O2 in living cells. Before carrying out the cell imaging experiments, the cytotoxicity of CBZ-H2O2 with concentrations from 0 to 20 μM and its reaction product with H2O2 were determined using MTT viability assay. As shown in Fig. S8,† CBZ-H2O2, and its reaction product with H2O2 showed low toxicity to HeLa cells, with relatively high cell viability greater than 85% after incubation for 24 h. The results suggested that CBZ-H2O2 probe showed superior biocompatibility for cell imaging. To further investigate the application of CBZ-H2O2 in bio-imaging, the TP action cross-section (Φδmax) was determined from the two-photon excited fluorescence (TPEF) spectra of CBZ-H2O2 in the absence and presence of H2O2 by using rhodamine-6G in methanol as the reference, as shown in Fig. S9,† the Φδmax of CBZ-H2O2 is 4.8 GM at 720 nm, but Φδmax increased to 65 GM after addition of H2O2. Such a drastic enhancement in TPEF indicates that CBZ-H2O2 is a potential two-photon probe for imaging H2O2 in living specimens.
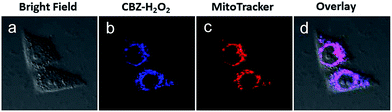
To investigate the mitochondria-targeting function of CBZ-H2O2 and its application as a TP probe for detecting H2O2 level changes in mitochondria, a co-localization experiment was carried out to confirm mitochondria-localization specificity of CBZ-H2O2 in HeLa cells by co-staining it with Mito Tracker red (1.0 μM), a commercially available mitochondria tracker. As shown in Fig. 2, the red fluorescent from Mito Tracker (2c, red channel, λex = 590 nm; λem = 620–660 nm) and the blue fluorescent from CBZ-H2O2 in H2O2 solution (2b, blue channel, λex = 720 nm; λem = 420–460 nm) overlapped very well, and the Pearson's co localization coefficient was calculated at 0.925. The results indicated that CBZ-H2O2 was distributed mainly in the mitochondria with mitochondria-positioning capability.
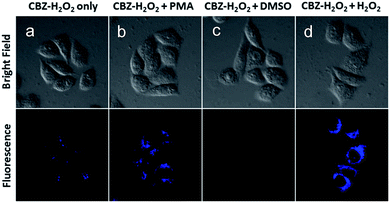
To demonstrate the potential bio-applications of CBZ-H2O2, the ability of probe to track endogenous burst of H2O2 produced within HeLa cells was evaluated. Firstly, CBZ-H2O2 was incubated with live HeLa cells for 30 min to guarantee completely entry in cells. As shown in Fig. 3, two-photon confocal fluorescence images taken at an excitation of 720 nm for HeLa cells incubated with 10.0 μM CBZ-H2O2 indicated that the probe was cell-permeable. When HeLa cells were stained with CBZ-H2O2 only, the image displayed extremely weak fluorescence induced by the intrinsic H2O2 produced by cell metabolism. Further more, the endogenous H2O2 was produced by stimulating HeLa cells with phorbol myristate acetate (PMA), which induced a phagocytosis-associated H2O2 generation.15 And remarkable fluorescence enhancement from the cells was observed when the cells was stimulated with PMA (1.0 μg mL−1) before introducing CBZ-H2O2. It is evident that the obvious signal enhancement is mainly due to the reaction between CBZ-H2O2 and endogenous H2O2. In order to further validate the main functions of H2O2 for signal increases, HeLa cells were then treated with DMSO (0.5%) as a ROS scavenger before loading CBZ-H2O2 for 30 min.16 The presence of CBZ-H2O2 in cells did not show any fluorescence response due to the clearance of intracellular H2O2.
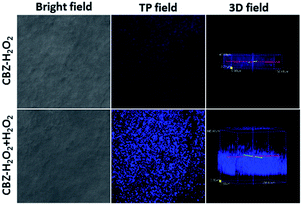
Encouraged by the success of cell imaging with CBZ-H2O2 for mitochondrial H2O2, we further investigated whether it could be used for H2O2 imaging in deep-tissue. The images were obtained from 1.0 mm-thick rat liver frozen slice which was treated with CBZ-H2O2 before and after incubating with 50 μM H2O2 at 37 °C. As shown in Fig. 4, the slice treated with CBZ-H2O2 only exhibited feeble fluorescence signal, whereas the addition of H2O2 to the slice distinctly intensified the fluorescence signal, clearly demonstrating the capability of CBZ-H2O2 for H2O2 detection in tissue. Subsequently, to further investigate the utility for deep tissue imaging, the 3D two-photon confocal fluorescence imaging along the z-direction were taken by treating the slice with CBZ-H2O2 in the absence and presence of H2O2. The Z-scanning confocal imaging showed that luminescence emission of CBZ-H2O2 present in the penetration depth of 40 μm, while the presence of H2O2 in tissue made the emission deeper to 105 μm, indicating that CBZ-H2O2 has excellent capacity of monitoring H2O2 at a greater depth of tissue using two-photon microscopy.
Conclusions
In summary, we have designed and synthesized a new two-photon fluorescent “turn-on” probe for mitochondrial H2O2 detection. The probe consists of a boronate ester functionality as a sensing site, a carbazole derivative as a fluorophore and a triphenylphosphonium moiety as a mitochondrial-targeting localizer. It exhibits not only fast response and high selectivity over other ROS, RNS and related biomolecules but also low detection limit of 1.96 μM. Co-localization experiments clearly demonstrated that CBZ-H2O2 selectively localizes to and accumulates in the mitochondria. CBZ-H2O2 is also sensitive to both exogenous and endogenous H2O2. By using two-photon fluorescence microscopy, it can detect H2O2 in living cells and tissues at a depth of 105 μm for a long period of time with excellent imaging quality. This probe will allow in-depth investigation of the roles and functions of H2O2 in complex biological systems.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21305036, 21507135), Hunan Provincial Natural Science Foundation of China (2015JJ3035), China Postdoctoral Science Foundation funded project (2015M580685) and Anhui Province Natural Science Foundation of China (1608085MB30).Notes and references
- B. Halliwell and J. M. C. Gutteridge, Free Radicals in Biology and Medicine, Clarendon Press, Oxford, UK, 3rd edn, 1999 Search PubMed.
- L. J. Chen, R. Na, M. J. Gu, A. B. Salmon, Y. H. Liu, H. Y. Liang, W. B. Qi, H. V. Remmen, A. Richardson and Q. T. Ran, Aging Cell, 2008, 7, 866 CrossRef CAS PubMed.
- (a) H. K. Seitz and F. Stickel, Nat. Rev. Cancer, 2007, 7, 599 CrossRef CAS PubMed; (b) H. Ohshima, M. Tatemichi and T. Sawa, Arch. Biochem. Biophys., 2003, 417, 3 CrossRef CAS PubMed; (c) A. M. Shah and K. M. Channon, Heart, 2004, 90, 486 CrossRef CAS PubMed; (d) M. T. Lin and M. F. Beal, Nature, 2006, 443, 787 CrossRef CAS PubMed.
- (a) S. L. Hempel, G. R. Buettner, Y. Q. O'Malley, D. A. Wessels and D. M. Flaherty, Free Radical Biol. Med., 1999, 27, 146 CrossRef CAS; (b) M. Y. Hua, H. C. Chen, R. Y. Tsai, Y. L. Leu, Y. C. Liu and J. T. Laie, J. Mater. Chem., 2011, 21, 7254 RSC.
- (a) A. R. Lippert, K. Y. Keshari, J. Kurhanewicz and C. J. Chang, J. Am. Chem. Soc., 2011, 133, 3776 CrossRef CAS PubMed; (b) G. C. Van de Bittner, C. R. Bertozz and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 1783 CrossRef CAS PubMed; (c) M. C. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392 CrossRef CAS PubMed; (d) B. C. Dickinson, C. Huynh and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 5906 CrossRef CAS PubMed; (e) E. W. Miller, A. E. Albers, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2005, 127, 16652 CrossRef CAS PubMed; (f) H. Maeda, Y. Fukuyasu, S. Yoshida, M. Fukuda, K. Saeki, H. Matsuno, Y. Yamauchi, K. Yoshida, K. Hirata and K. Miyamoto, Angew. Chem., Int. Ed., 2004, 43, 2389 CrossRef CAS PubMed; (g) K. Xu, B. Tang, H. Huang, G. Yang, Z. Chen, P. Li and L. An, Chem. Commun., 2005, 48, 5974 RSC.
- N. Soh, O. Sakawaki, K. Makihara, Y. Odo, T. Fukaminato, T. Kawai, M. Irie and T. Imato, Bioorg. Med. Chem., 2005, 13, 1131 CrossRef CAS PubMed.
- M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629 CrossRef CAS PubMed.
- (a) T. Zhang, H. Fan, G. Liu, J. Jiang, J. Zhou and Q. Jin, Chem. Commun., 2008, 5414 RSC; (b) O. S. Wolfbeis, A. Dü rkop, M. Wu and Z. Lin, Angew. Chem., Int. Ed., 2002, 41, 4495 CrossRef CAS.
- (a) H. M. Kim and B. R. Cho, Acc. Chem. Res., 2009, 42, 863 CrossRef CAS PubMed; (b) F. Helmchen and W. Denk, Nat. Methods, 2005, 2, 932 CrossRef CAS PubMed; (c) J. M. Squirrell, D. L. Wokosin, J. G. White and B. D. Bavister, Nat. Biotechnol., 1999, 17, 763 CrossRef CAS PubMed.
- (a) W. R. Zipfel, R. M. Williams and W. W. Webb, Nat. Biotechnol., 2003, 21, 1369 CrossRef CAS PubMed; (b) L. Li, X. Shen, Q. Xu and S. Q. Yao, Angew. Chem., Int. Ed., 2013, 52, 424 CrossRef CAS PubMed; (c) H. Ahn, K. E. Fairfull-Smith, B. J. Morrow, V. Lussini, B. Kim, M. V. Bondar, S. E. Bottle and K. D. Belfield, J. Am. Chem. Soc., 2012, 134, 4721 CrossRef CAS PubMed; (d) H. C. Heo, K. H. Kim, H. J. Kim, S. H. Baik, H. Song, Y. S. Kim, J. Lee, I. Mook-jung and H. M. Kim, Chem. Commun., 2013, 49, 1303 RSC; (e) Q. L. Xu, C. H. Heo, G. Kim, H. W. Lee, H. M. Kim and J. Yoon, Angew. Chem., Int. Ed., 2015, 54, 4890 CrossRef CAS PubMed.
- P. Li, W. Zhang, K. X. Li, X. Liu, H. B. Xiao, W. Zhang and B. Tang, Anal. Chem., 2013, 85, 9877 CrossRef CAS PubMed.
- (a) X. H. Dong, C. H. Heo, S. Y. Chen, H. M. Kim and Z. H. Liu, Anal. Chem., 2014, 86, 308 CrossRef CAS PubMed; (b) H. B. Yu, Y. Xiao and L. J. Jin, J. Am. Chem. Soc., 2012, 134, 17486 CrossRef CAS PubMed.
- (a) C. Chung, D. Srikun, C. S. Lim, C. J. Chang and B. R. Cho, Chem. Commun., 2011, 47, 9618 RSC; (b) K. M. Zhang, W. Dou, P. X. Li, R. Shen, J. X. Ru, W. Liu, Y. M. Cui, C. Y. Chen, W. S. Liu and D. C. Bai, Biosens. Bioelectron., 2015, 64, 542 CrossRef CAS PubMed; (c) H. C. Guo, H. Aleyasin, S. S. Howard, B. C. Dickinson, V. S. Lin, R. E. Haskew-Layton, C. Xu, Y. Chen and R. R. Ratan, J. Biomed. Opt., 2013, 18, 106002 CrossRef PubMed.
- (a) G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518 RSC; (b) J. Jing and J. L. Zhang, Chem. Sci., 2013, 4, 2947 RSC.
- (a) H. R. Petty, Biochim. Biophys. Acta, 1989, 1012, 284 CrossRef CAS PubMed; (b) P. Bellavite, Free Radical Biol. Med., 1988, 4, 225 CrossRef CAS.
- M. Y. Song, L. Z. Zeng, X. J. Hong, Z. H. Meng, J. F. Yin, H. L. Wang, Y. Liang and G. B. Jiang, Environ. Sci. Technol., 2013, 47, 2886–2891 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21260c |
| ‡ These author contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |