Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Aerobic oxidations in flow: opportunities for the fine chemicals and pharmaceuticals industries
Asterios
Gavriilidis
a,
Achilleas
Constantinou†
a,
Klaus
Hellgardt
b,
King Kuok (Mimi)
Hii
*b,
Graham J.
Hutchings
c,
Gemma L.
Brett
c,
Simon
Kuhn
 d and
Stephen P.
Marsden
e
d and
Stephen P.
Marsden
e
aDepartment of Chemical Engineering, University College London, Torrington Place, London WC1E 7JE, UK
bDepartment of Chemistry, Department of Chemical Engineering, Imperial College London, South Kensington, London SW7 2AZ, UK. E-mail: mimi.hii@imperial.ac.uk
cSchool of Chemistry, Cardiff University, Main Building, Park Pl, Cardiff CF10 3AT, UK
dDepartment of Chemical Engineering, KU Leuven, Celestijnenlaan 200F bus 2424, B-3001 Leuven, Belgium
eSchool of Chemistry and Institute of Process Research and Development, University of Leeds, Woodhouse Lane, Leeds LS2 9JT, UK
First published on 22nd September 2016
Abstract
Molecular oxygen is without doubt the greenest oxidant for redox reactions, yet aerobic oxidation is one of the most challenging to perform with good chemoselectivity, particularly on an industrial scale. This collaborative review (between teams of chemists and chemical engineers) describes the current scientific and operational hurdles that prevent the utilisation of aerobic oxidation reactions for the production of speciality chemicals and active pharmaceutical ingredients (APIs). The safety aspects of these reactions are discussed, followed by an overview of (continuous flow) reactors suitable for aerobic oxidation reactions that can be applied on scale. Some examples of how these reactions are currently performed in the industrial laboratory (in batch and in flow) are presented, with particular focus on the scale-up strategy. Last but not least, further challenges and future perspectives are presented in the concluding remarks.
1. Introduction
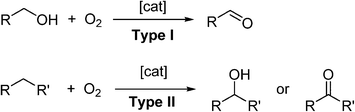
Molecular oxygen (O2) is unquestionably the most important constituent of our planet's atmosphere. The photosynthesis of cyanobacteria began some 2.7 billion years ago, generating sufficient O2 in the atmosphere to support the evolution of more complex life forms. Today, many important biological functions are sustained by redox processes involving reactive oxygen species (ROS),1 including metabolism,2,3 signalling4,5 and enzymatic functions.5O2 can be considered as the ideal oxidant. It is readily abundant, has a low-molecular weight and, in most cases, generates only water as a benign by-product. Triplet, ground-state oxygen does not normally react with organic molecules under ambient conditions. However, the mixture may ignite under certain conditions to liberate CO2 and H2O, which is a highly exergonic process. Therefore, a catalyst is often required to control partial oxidation of a molecule selectively. In recent years, the demand for greener and more sustainable synthetic methods has generated much interest in the use of O2 as a reagent in synthesis.6 The subject of transition-metal catalysed oxidation of organic molecules has been comprehensively reviewed;7–9 including, more recently, development of photocatalytic reactions (involving O2) driven by visible light,10 and the use of microreactors for liquid-phase oxidation chemistry.11 In general, there are two types of aerobic oxidation reactions, depending on the role played by O2 in the reaction (Scheme 1).
In Type I aerobic oxidation reactions, O2 is not incorporated into the product; its role is to regenerate the active catalyst and thus enable turnover to be achieved. By far, the dehydrogenation of alcohols to aldehydes and ketones constitutes the largest class of type I aerobic oxidation reactions. A wide variety of homogeneous and heterogeneous catalysts have been reported for this transformation, and the subject has been reviewed quite exhaustively. Aerobic oxidation also allows alcohols to be used as a latent source of carbonyl compounds which can be transformed into other functional groups (‘tandem oxidation processes’, TOP).
In Type II oxidation reactions, one or both of the oxygen atom(s) of O2 is/are incorporated into the product. The most common type II oxidation reactions are the oxygenation of C–H bonds, which proceed through free radical intermediates.12,13 The oxygenation of benzylic and allylic sp3 C–H bonds are particularly facile as they are highly susceptible to attack by free-radicals, which can be generated using cheap and abundant first-row transition metal salts (such as Fe, Co and Cu), N-hydroxyimides, light sensitive molecules, and even doped carbon materials.14 Enantioselective C–H oxygenation reactions can be achieved using enzymes,15 or their artificial mimics,16,17 based on Fe.
Historically, organic chemists tend to eschew oxidation reactions in multistep organic synthesis as they are considered to be incompatible with the requirements of an ‘ideal synthesis’.18 Many oxidants, such as chromates and manganate, are often not chemoselective, and are difficult to implement on a large-scale. In an essay on redox economy in organic synthesis the authors advocated that the number of non-strategic redox steps should be avoided in multistep reactions.110 This view is very much echoed in an analysis of industrial reactions used for the preparation of 128 drug candidates between Pfizer, AstraZeneca and GlaxoSmithKline, where the need for more efficient oxidation chemistry has been identified as a key bottleneck in their processes: “…there are relatively few atom efficient, chemoselective and environmentally acceptable oxidation methods…oxidations are often designed out of syntheses. The discovery of new chemoselective oxidations, particularly if catalytic, would greatly increase flexibility in synthetic design”.19
Yet, paradoxically, oxidation reactions are commonly used processes for converting petrochemical feedstocks into useful chemical products. Similarly, there is also substantial interest in using aerobic oxidation reactions to convert biomass into platform chemicals.20 Currently, there are 109 industrial oxidation processes with a capacity of >1000 tonnes per annum that are listed in Ullmann's Encyclopedia of Industrial Chemistry, including commodities, large intermediates and specialities (but excluding agrochemicals and pharmaceuticals).21 A survey of the processes listed (Fig. 1) shows that O2 is used as an oxidant in the majority of these processes (61.4%).
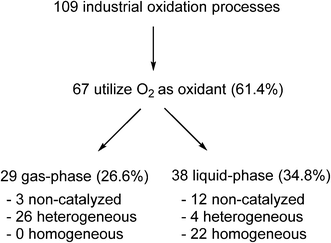 | ||
| Fig. 1 Analysis of industrial oxidation processes with capacities of >1000 tonnes per annum.21 | ||
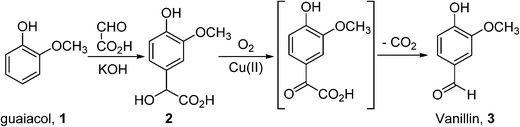
In general, bulk chemicals are simple molecules with few functional groups and low molecular weights/boiling points. This allows some reactions to be performed in the gas-phase at elevated temperatures and pressures (e.g. oxidation of ethylene to ethylene oxide), which are not compatible with the synthesis of more complex organic molecules required by the fine chemicals and pharmaceutical industries. Notably, the liquid-phase processes mostly utilise homogeneous catalysts: a large number of these are conducted in aqueous solutions, thus avoiding flammability issues associated with the use of organic solvents. An example is the production of synthetic vanillin. Currently, 95% of the world supply of this important flavouring agent is produced by the oxidation of petrochemical-based stock, mostly using the Riedel process (Scheme 2). Electrophilic substitution of guaiacol (1) with glyoxylic acid produces mandelic acid (2), which is oxidised to a vanilglyoxylic acid intermediate that decarboxylates spontaneously to vanillin (3). The conversion of 2 to 3 is carried out using a copper(II) hydroxide-oxygen system in an aqueous alkaline medium at 80–130 °C.
Indeed, very few homogeneous reactions listed in Fig. 1 are performed in organic solvents; a notable exception is the Amoco process for the production of terephthalic acid (which will be discussed in the following section). In fact, the lack of aerobic oxidation methodologies is more often due to safety concerns associated with aerated organic solvents. This may be demonstrated by menadione (4), a synthetic chemical compound widely used as an antihemorrhagic agent, as well as a nutritional supplement (Vitamin K3). This is currently prepared on an industrial scale by the oxidation of 2-methyl naphthalene (5) using chromate reagents in sulfuric acid (Scheme 3).22 However, the aerobic oxidation of 5 to 4 can also proceed uncatalyzed at 80 °C in toluene.23 Presumably, despite its obvious disadvantages (poor atom economy, toxic reagent, metal waste) the anaerobic oxidation is preferred as the exotherm can be controlled (by the slow addition of the reagent, for example), compared to performing an uncontrollable autooxidation process in a flammable solvent.
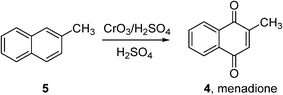 | ||
| Scheme 3 Commercial synthesis of menadione (BASF).22 | ||
2. Aerobic oxidations in the production of fine chemicals & pharmaceuticals
Very often, H2O is formed as the only byproduct of aerobic oxidation reactions. This is especially advantageous for the production of active pharmaceutical ingredients (APIs), where the impurities allowed in the products are tightly regulated. The ability to deploy aerobic oxidations would alleviate substantial operational and environmental costs associated with the work-up of post-reaction mixtures, which can consume more material (particularly solvents24) and energy than the reactions themselves.25 However, in a review of large-scale oxidation processes in the pharmaceutical industry, it was concluded that: “Oxygen gas has the advantage of being the least expensive and most readily available oxidant, but the safety issues surrounding its use make it one of the least frequently used reagents”.26The key reason for the lack of aerobic oxidation reactions in the fine chemicals and pharmaceutical sectors can mainly be attributed to differences in scale and mode of operation. Economy of scale allows bulk chemical processes to be performed in dedicated facilities in a highly integrated manner, with attendant operational efficiencies and synergies. In comparison, pharmaceutical and fine chemicals are typically produced in multiple steps in limited quantities, using multipurpose batch reactors in the liquid phase. Process flexibility is therefore often achieved at the expense of efficiency. Aerobic oxidations fall into a unique category of reactions that simply cannot be performed in batch, due to the considerable headspace within the reactors.
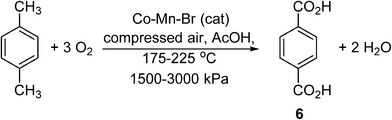
To illustrate these differences, we shall consider the current industrial process used to produce terephthalic acid (TA), (6) (Scheme 4).27 Contrary to many other petrochemical oxidation reactions, TA is produced industrially using an organic solvent (acetic acid), and thus may serve as a useful process to gain transferrable insight for the small scale processing of pharmaceuticals. Driven by global demand, the production of purified terephthalic acid (PTA) is estimated to reach 66 MMt per annum by 2025.28 The commercial process was developed by Amoco, involving the catalytic oxidation of p-xylene, performed using O2 as a terminal oxidant in acetic acid. Catalyzed homogeneously by Co and Mn salts in the presence of a radical initiator (Br−),29 the reaction is highly exothermic, liberating 2 × 108 J per kg of p-xylene consumed.
The Amoco process demonstrates that, in principle, from a (reaction) engineering point of view, there appear to be no insurmountable obstacles or operational restrictions to the performance of safe and effective partial oxidation reactions in organic solvents using oxygen. However, if one examines the process with the view to adopting this for the transformation of APIs, a number of issues emerge that need to be addressed, which are summarised below:
1. Reaction conditions: relatively harsh conditions are employed (175–225 °C and 15–30 bar), beyond the flash point of acetic acid (see Table 1 below), and are incompatible with the synthesis of highly functionalised (thermally sensitive) molecules in batch systems, intimating the need for a flow system.
| Solvent | AIT/°C | Flash point/°C |
|---|---|---|
| a Obtained from MSDS. b Dichloromethane has no flash point in a conventional (closed) tester, but forms flammable vapour–air mixtures at >100 °C. | ||
| Acetic acid | 427 | 40 |
| Dichloromethane | 556 | Noneb |
| Diethyl ether | 160 | −45 |
| Methyl tert-butyl ether | 443 | −28 |
| N,N-Dimethylformamide | 445 | 58 |
| N,N-Dimethylacetamide | 490 | 63 |
| Ethyl acetate | 410 | −3.3 |
| Butyl acetate | 421 | 22 |
| Tetrahydrofuran | 321 | −17.2 |
| 2-Methyltetrahydrofuran | 270 | −23 |
| Ethanol | 365 | 16.6 |
| Isopropanol | 399 | 12 |
| Toluene | 530 | 6 |
| p-Xylene | 530 | 27 |
| n-Hexane | 225 | −26 |
| n-Heptane | 215 | −4.0 |
2. Selectivity: equally, high temperatures and radical reactions are also unlikely to offer the level of selectivity required for the oxidation of complex molecules. In the case of xylene, radical chemistry dictates the expected complete oxidation of the methyl groups. Under these conditions, the acetic acid solvent is also oxidised (‘solvent burning’), leading to the competitive formation of carbon oxides (CO, CO2, formaldehyde).29 This causes a significant solvent loss from the process (0.05 tonne of acetic acid consumed per tonne of TA produced).
3. Stoichiometry: high pressures are required to solubilise oxygen in the solvent. For kinetic reasons the temperature also needs to be increased, reducing in turn the solubility of oxygen in the solvent. This can lead to sub-stoichiometric availability of oxygen and thus undesirable degrees of oxidation.
4. Safety: mixtures of pure oxygen and organic solvents can potentially generate highly flammable mixtures.30 In the Amoco process, the dedicated reactor is designed such that the O2 content of the off-gas is kept near to 0%. The resultant O2 deficiency can lead to a significant compromise in selectivity. Dilution of oxygen with inert gases to counteract this leads, in turn, to sub-stoichiometry and large reactor sizes.
5. Workup: TA precipitates from the reaction mixture, forming a three-phase mixture of solid product, liquid reactants/solvents and a vapour phase containing unreacted oxygen. Product separation and purification are highly optimised through a series of recrystallizations at different pressures/temperatures, which are not always possible using multipurpose reactors.
6. Capital investment: the highly corrosive bromine-acetic acid environment requires special materials to be used in the construction of reaction vessels, e.g. titanium or special alloys, which are not easily available as standard process equipment in the pharmaceutical industry.
The additional complexity and higher-purity requirements for pharmaceutical products pose two main challenges for the development of aerobic oxidation processes: (i) identification of catalysts that are able to catalyse different oxidative reactions chemoselectively under mild conditions; and (ii) design of reactors and process integration that are compatible with the use of organic solvents, and that can deliver O2 efficiently and safely without compromising catalyst activity and selectivity.31
In this Review, we present the hazards and safety issues of aerobic oxidation reactions in the liquid phase using organic solvents, followed by a brief survey of the types of reactors that can be used. This is followed by examples of aerobic oxidation reactions that are currently achieved on a large scale in industrial laboratories, particularly for the production of speciality chemicals and APIs. In each case, safety aspects of the reactions are highlighted. Finally, the challenges and future directions and opportunities are identified.
3. Hazards and safety issues of aerobic oxidation reactions
Of paramount importance for performing any process at scale is to establish safe operational conditions. Invariably, aerobic oxidation reactions involve a combination of oxygen with flammable materials under high temperature and pressure. This warrants extensive risk assessment to identify safe reaction conditions and apparatus, as well as special measures in case of catastrophic failure. In this section, the specific hazards associated with aerobic oxidations are highlighted.3.1 Reaction exotherm
In general, aerobic oxidations are highly exothermic. For example, the oxidation of benzyl alcohol to benzaldehyde by molecular oxygen generates a net heat of reaction of −187 kJ mol−1 (calculated based on the standard enthalpy of formation of each species).32 As such, these reactions are associated with a considerable adiabatic temperature rise, which needs to be considered for safe operation. Effective management of the heat released from the reaction is vital for any scale-up process. Therefore, it is often advantageous to perform aerobic oxidations in continuous flow, as the internal volume of the reaction system is limited and thus allows better heat dissipation (through the reactor wall) for safer operation.The inability to efficiently remove the reaction heat can lead to explosive chemical reactions, and their theoretical description is addressed in the seminal works of Semenov33 and Frank-Kamenetskii.34 The underlying basis of both theories is that the ignition of a reactive mixture is attributed to the fact that the production rate of heat (i.e. the net heat of reaction) is larger compared to the dissipation rate of heat (i.e. heat transfer to the ambient). This imbalance will lead to an unsteady thermal state, which will finally cause a reaction mixture to exceed its autoignition temperature and spontaneously combust (see section 3.2).
The theory of Semenov assumes a well-mixed reaction medium, with no spatial gradients in temperature or chemical composition in the reactor. Heat transfer to the ambient is then described via a (uniform) heat transfer coefficient applied as boundary condition on the vessel wall. However, this assumption of a well-mixed reaction medium does not hold for large reactors or heterogeneous reactions. As an extension of the original Semenov theory, Frank-Kamenetskii included the effect of developing temperature gradients and introduced thermal conduction in the reaction medium as main source of heat transfer (assuming a constant reactor wall temperature). Based on the steady-state energy conservation equation, balancing the heat produced from the reaction with the heat removed via conduction, the dimensionless parameter δ can be derived:
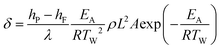 | (1) |
h P, hF enthalpies of the product (P) and feed (F)
λ thermal conductivity of the reaction mixture
ρ density of the reaction mixture
L characteristic dimension of the reactor
A pre-exponential factor
E A activation energy
T W wall temperature
From the energy conservation equation, a critical value δcrit can be derived, which is geometry-dependent, e.g. δcrit = 2.0 for an infinite cylinder.34 It follows that no explosion occurs as long as δ < δcrit. Hence, the Frank-Kamenetskii theory can be used to calculate the maximum tolerable wall temperature for different vessel geometries and vessel sizes L when the reaction mixture properties are known.
Both the Semenov and Frank-Kamenetskii theories can provide guidelines for the temperature management of a reactor vessel to ensure safe operation. However, their predictions always need to be evaluated against their underlying assumptions, particularly in terms of the dominating heat transfer mechanism and temperature independent reaction mixture properties. Most importantly, for continuous flow operation, e.g. in packed-bed reactors, heat transfer via convection will dominate, and one would need to extend the theories to account for this.
3.2 Flash point, autoignition and limiting oxygen concentration
When the concentration of an organic solvent is within the flammability limits, it can ignite in the presence of O2. This can be avoided by operating below the limiting oxygen concentration (LOC), defined as the concentration of O2 below which a fuel-oxidant explosion cannot occur.35 By limiting the O2 concentration below this value, combustion cannot occur irrespective of the concentration of the organic solvent. It is also important to note that these limiting concentrations are linked to the process temperature and pressure: for each 100 °C increase, the lower flammable limits and the LOCs at 1 atm decrease by about 8% of their values at near normal room temperature; the upper flammable limits increase by approximately 8% for the same conditions.35 The US National Fire Protection Association (NFPA) has provided some guidelines for recommended safety margins.36 For LOCs of ≥5%, the O2 concentration should not exceed 60% of the LOC, but with continuous monitoring the O2 may be kept 2% below the LOC.Quantification of the LOC requires the detailed knowledge of the flammability region as a function of the fuel, oxidant, and (if present) inert gas concentrations.37 The flammability limits and the LOC can be graphically represented in a flammability diagram, which for the case of the presence of fuel/oxidant/inert will be triangular, but can also be represented in orthogonal shape omitting e.g. the inert concentration as it can be calculated from the mass balance (the sum of all molar fractions equals 1). Using such a diagram allows to determine if a flammability risk exists for the given process conditions. However, it has to be noted that each diagram needs to be constructed experimentally, and is only valid for a certain temperature and pressure.
Furthermore, it is important to note that mixtures of a flammable material and O2 can spontaneously combust without an ignition source, as long as the external temperature is enough to attain the required activation energy (‘thermal ignition’ or ‘autoignition’). The autoignition temperature (AIT) must not be confused with the flash point of the solvent (the lowest temperature at which the vapour will form a combustible mixture with air). Some AITs and flash points of selected organic solvents commonly used in the academic laboratory and industrial processes are listed in Table 1.38 It is important to note that these values are recorded at ambient conditions, and will change substantially at elevated temperatures and pressures. For example, while no flash point is recorded for dichloromethane, it is able to form a flammable mixture with air when heated above 100 °C. Similarly, AIT can be lowered substantially by pressure39 and the size of the reaction vessel;40 these issues must be considered in designing scale-up processes for aerobic oxidation reactions.
Many industrial processes are conducted at elevated pressure; for liquid-phase aerobic oxidation reactions, pressure is commonly applied to increase the availability of dissolved oxygen. Given that AIT is an inverse function of pressure, it is important to predict the AIT depression as a function of pressure. For several hydrocarbons, this relationship may be represented by the following equation:41
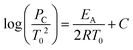 | (2) |
P c and T0 = initial pressure and temperature at the critical condition;
E A = activation energy of the reaction;
R = universal gas constant; and
C = a constant that includes different factors such as surface/volume ratio of the reaction vessel and heat transfer coefficient.
Critically, this relationship is only applicable over a limited pressure range, since the activation energy is also a function of pressure and temperature. This means that, in reality, the functional relationship between AIT and pressure/reaction temperature needs to be determined experimentally. This is particularly relevant in terms of risk assessment for aerobic oxidation reactions, as the application of O2 pressure is desirable, but reliance on standard measurements of autoignition temperatures is not advisable.
When dealing with gaseous mixtures of organic compounds, AIT can be predicted assuming an ideal mixture contribution method:42
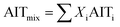 | (3) |
This may be used to predict the AIT of the gaseous mixtures at the reactor exit (particularly in the context of flow chemistry). For the headspace in a batch reactor, the composition of the vapour phase above the reaction mixture and the relative volatilities of the liquid phase must also be taken into account.
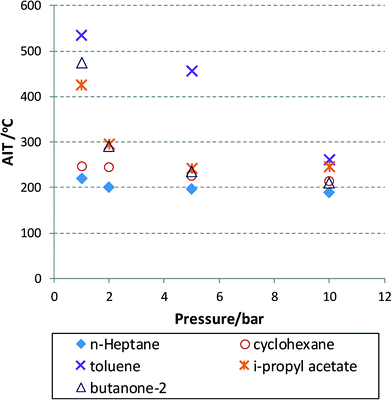
Experimental data are very hard to find that show the AIT as a function of temperature or oxygen partial pressure for that matter. Modelling approaches exist for commonly encountered fuels, with the involved model parameters fitted to experimental data and thus also limited to certain process conditions.39 Some of the available data for a number of solvents40 are plotted in Fig. 2 for aerobic oxidation reactions. In this figure, the AIT of various compounds is plotted as a function of air pressure ranging from 1 to 10 bar. The data obey a Semenov analysis (see eqn (2)) but it is clear that different chemical structures will exhibit different gradients (or EA). Nevertheless, we can conclude that at useful operating pressures, i.e. 10 bar (in terms of oxygen solubility) most solvent will exhibit an AIT of around 200 °C. This is concerning, as high pressures will no doubt reduce this temperature even further, thus moving the AIT into the realm of desirable reaction temperatures leading to heightened risks, unless headspace and exit mixture composition can be adequately controlled.
Currently, a priori prediction of LOCs is difficult, as it is limited by available experimental data. In a recent publication,30 the LOCs of 9 organic solvents commonly employed in the pharmaceutical industry (acetic acid, N-methylpyrrolidone, dimethyl sulfoxide, tert-amyl alcohol, ethyl acetate, 2-methyltetrahydrofuran, methanol, acetonitrile, and toluene) were experimentally determined. For this a flammability apparatus was constructed, consisting of a 5.3 L spherical vessel with an exploding fuse wire as ignition source positioned at the centre of the vessel. The temperatures inside the vessel were recorded using thermocouples, and a pressure transducer monitored the pressure increase associated with the ignition of a flammable mixture. Based on the experimentally determined flammability diagrams the LOCs of the 9 solvents were thus quantified at elevated pressures and temperatures. While this study is a very valuable contribution, the solvent selection and the investigated pressure and temperature conditions are limited by operational limits of the experimental design. This serves to highlight the challenges in performing measurements at the working temperature, pressure and concentration ranges that can be adopted for industrial processes.
3.3 Partial pressure (solubility) of O2
For heterogeneously catalysed oxidations, the Henry's law constant can be used to quantify the oxygen solubility in the liquid, which is an essential quantity for reactor design and optimization. It can be either determined experimentally or predicted based on thermodynamic equations of state.43 However, it is important to note that Henry's law only applies when chemical equilibrium has been reached in a dilute solution (typically <1% solute concentration). Thus, care must be applied in extrapolating these values to solutions containing a synthetically-relevant amount of O2. While the solubility of O2 in aqueous solutions has been studied extensively, similar studies in organic solvents are more limited, particularly for hydrocarbons and alcohols containing more than 5 carbons.443.4 Process safety
Based on the discussion above, we will provide some concluding remarks on how process safety can be ensured. In general, moving from batch to continous flow improves safety as the internal reactor volume (i.e. the reactive volume) is decreased by up to 3 orders of magnitude, thus reducing the impact in case of failure.45 In addition, scaling-down also leads to increased heat and mass transfer processes, in turn reducing the risk of hot spot formation.11 Furthermore, the volumetric flow rates associated with this scale of operation allow using ancillary tubing with inner dimensions below the flame propagation threshold.46 Independent of the scale of operation, the reaction stream temperature needs to be cooled and kept below its flash point, and diluting the outlet with inert gas will avoid the formation of explosive mixtures downstream of the reactor.45 In terms of the product and exhaust streams leaving the reactor, any unreacted oxygen needs to be vented, and the use of condensers will limit the amount of evaporated solvent in the exhaust gas stream. Finally, the process control strategy needs to ensure that the temperature in the entire reactor and associated process streams is below the flash point and AIT. Thus, efficient cooling or dilution measures need to be implemented in the process.4. Flow reactors for aerobic oxidation reactions
4.1 ‘Little and large’
Perhaps unsurprisingly, the reactor configuration is known to affect the selectivity of oxidation reactions, since it can affect mass transfer due to differences in gas–liquid–solid contacting.47 In terms of efficient heat management, the enhanced heat transfer characteristics of microreactors is particularly valuable, due to the large surface-to-volume ratio of between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m2 m−3.48 Furthermore, the residence time can be precisely controlled by changing the flow speed or the length of the channels. This has enabled their application to several reactions with the potential for thermal run-away. Successful implementations of hazardous reactions in microreactors are highlighted in recent reviews, including continuous flow oxidation reactions in the liquid phase.11 However, the productivity of microreactors is generally not compatible with industrial production rates. On the other hand, the type of reactors employed in the commodity industry for catalytic oxidation reactions are often dedicated to tonne-scale processes, designed to be operated continuously over the lifetime of the chemical plant. Thus, for speciality chemicals, there is a need for new reactor designs that can overcome the heat management issue, while maintaining high selectivity, ideally offering flexible productivity rates.
000 m2 m−3.48 Furthermore, the residence time can be precisely controlled by changing the flow speed or the length of the channels. This has enabled their application to several reactions with the potential for thermal run-away. Successful implementations of hazardous reactions in microreactors are highlighted in recent reviews, including continuous flow oxidation reactions in the liquid phase.11 However, the productivity of microreactors is generally not compatible with industrial production rates. On the other hand, the type of reactors employed in the commodity industry for catalytic oxidation reactions are often dedicated to tonne-scale processes, designed to be operated continuously over the lifetime of the chemical plant. Thus, for speciality chemicals, there is a need for new reactor designs that can overcome the heat management issue, while maintaining high selectivity, ideally offering flexible productivity rates.
In this section, different reactor designs suitable for performing aerobic oxidation reactions at the appropriate scale for fine chemicals and pharmaceutical companies are described, along with their advantages and disadvantages. The different reactors are summarised in Table 2, differing in the way the catalyst (if used) is incorporated in the reactor, as well as the mode of O2 delivery. Homogeneous catalysts can be more selective compared to heterogeneous catalysts, and no internal diffusion resistances exist since they are molecularly dispersed within the reaction medium. However, it can be difficult and expensive to recover the catalyst, since a downstream separation section is required.
| Homogeneous/no catalysts | Heterogeneous catalysts |
|---|---|
| Segmented flow | Packed bed |
| Membrane | Wall-coated |
| Membrane |
4.2 Segmented flow reactors
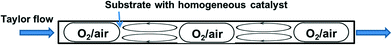
The segmented flow reactor is easy to construct and it is also the most common system employed for reactions performed with or without a catalyst. In this system, the gas (pure O2 or diluted in N2) is premixed with the liquid in a T-junction followed by a tubular reactor which results in segmented (i.e. Taylor or slug) flow, which is characterised by liquid recirculation inside the slugs (Fig. 3). This ensures good mass transfer of the gas in the liquid. In fact, Taylor flow liquid side volumetric mass transfer coefficients (kLa) up to 10 s−1 have been obtained (for channels smaller than 1 mm), which is typically one order of magnitude higher than traditional contactors such as bubble columns, packed columns etc. Furthermore, such reactors offer efficient heat exchange with the reactor walls, a residence time distribution characteristic of plug flow, and allow easy operation at higher temperature and pressure compared to conventional round bottom flasks.49,50 In many cases Taylor flow is adopted, and this can be ascertained visually due to the transparency of the plastic tubes usually employed. When stainless steel tubes with larger diameters are used, however, the precise flow pattern is much more difficult to establish. Nevertheless, higher velocities of the liquid and gas can be achieved in larger reactors, which can contribute to even better gas–liquid mixing.Supported by a pre-competitive consortium of pharmaceutical companies (Eli Lilly, Pfizer and Merck), a research group led by Stahl at University of Wisconsin-Madison (MadOx Consortium) has been developing effective strategies for aerobic oxidation reactions. In their earlier work, a segmented flow reactor was employed to oxidise various primary and secondary alcohols into aldehydes and ketones using a Pd(OAc)2/pyridine homogenous catalyst.51 A 6.35 mm O.D. (5 mL) stainless steel tube was initially used at the lab scale, while larger tubes were used to produce acetophenone from 1-phenylethanol in near quantitative yield at 25 g and 1 kg scales, respectively. Operation of the 5 mL reactor with a single volume of solution and continuous flow of oxygen (representing batch reaction) provided identical yield as compared with continuous flow of liquid, showing that this reaction could be easily translated to continuous flow. However, batch reaction in a flask equipped with an O2-filled balloon, at lower temperature and pressure, required more than an order of magnitude longer time to reach the same yield. To operate safely, diluted oxygen (8% O2 in N2) was used, which required high total gas pressures (25–35 bar) in order to maintain a sufficiently high O2 concentration. More recently, similar reactors were utilised by the same group for the oxidation of benzylic, aliphatic and activated alcohols to the corresponding aldehydes, catalysed by a (bpy)CuI/TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxyl) catalyst.52
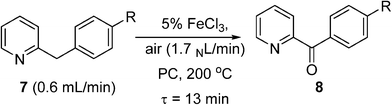
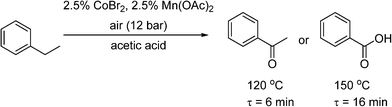
Pieber and Kappe used a stainless steel coil (0.8 mm I.D.) for oxidation of 2-benzylpyridines (7) to the corresponding ketones (8) with synthetic air at 200 °C, using inexpensive iron(III) chloride as a catalyst and propylene carbonate (PC) as a non-toxic and thermally inert solvent (Scheme 5).53 The reaction was enhanced significantly using continuous flow under high temperature and pressure leading to much shorter reaction time than in batch systems. The reactor was replaced with a PFA (perfluoroalkoxy) coil (to prevent corrosion) to perform a variation of the Amoco process, where ethylbenzene was oxidised in the presence of a Co/Mn bimetallic catalyst (Scheme 6). Using 12 bar of synthetic air, the reaction time necessary for complete oxidation of ethylbenzene was 6–7 min, at temperatures 110–120 °C. The acetophenone was formed in 80–84% selectivity, and virtually pure acetophenone was isolated in 66% product yield. The higher pressure and temperature, which were possible in the continuous flow reactor, provided higher selectivity and much smaller reaction time, as compared to batch reaction in a stirred open vial (selectivity to acetophenone ∼74%, at a conversion of ethylbenzene ∼96%, 150 min reaction time, 80 °C). More forcing conditions led to benzoic acid as the major product. It is worth noting that in both of these systems, the need to remove high-boiling PC solvent (Scheme 5), and the low selectivity (Scheme 6), make them unsuitable for industrial applications for the fine and pharmaceutical industries.
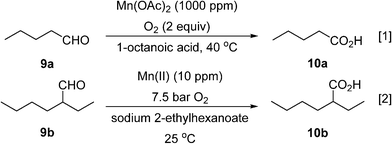
Two different types of segmented flow reactors have been utilised for manganese-catalysed aerobic oxidation of aliphatic aldehydes to acids. The first was reported by Baumeister et al.,54 who used a 4.7 mL microstructured reactor (one-A Engineering, Austria) for the conversion of valeraldehyde (9a) to valeric acid (10a) with pure oxygen with catalytic manganese(II) acetate at 40 °C (Scheme 7, eqn (1)). The addition of octanoic acid as a co-feed is essential to maintain higher productivity and selectivity. Generation of gas–liquid interface areas was facilitated by periodic narrowings along the 1 mm I.D. stainless steel reactor channel. Using a large molar excess of oxygen (2 equivalents with respect to the aldehyde), the dominant phase within the reactor is believed to operate in an annular flow pattern. Conversion of 95% at superficial liquid residence time of 82 s with high selectivity (80–85%) was obtained with a high productivity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290 kg h−1 m−3). Attempt to scale-up the reactor to include 3 mm channels was unsuccessful.
290 kg h−1 m−3). Attempt to scale-up the reactor to include 3 mm channels was unsuccessful.
In recent work, Vanoye et al. showed that neat 2-ethylhexanal (9b) can be oxidised safely to the corresponding carboxylic acid (10b) in a PFA tube reactor (1.65 mm I.D.), using pure oxygen at 7.5 bar at ambient temperature and a homogeneous Mn(II) catalyst (Scheme 7, eqn (2)).55 The system was limited by the low heat transfer coefficient of the PFA which did not allow efficient management of the heat produced by the reaction. This was overcome by lowering the catalyst loading (to 10 ppm) and increasing the O2 pressure to 7.5 bar to maintain 94% selectivity. This was sufficient to maintain a productivity of 130 g h−1 of 2-ethylhexanoic acid, without further purification or solvent separation.
In principle, it is possible to deploy heterogeneous catalysts in segmented flow reactors by suspending catalyst (nano)particles in the reaction mixture, creating a triphasic (gas/liquid/solid) system, as long as the hydrodynamics of the reactor can maintain the catalyst suspension. Further challenges include feeding of liquid/solid slurries and avoiding particle trapping in the reactor or the associated piping. This has been partly demonstrated in a report by Alex et al.,56 who used a PTFE (polytetrafluoroethylene) tube (1 mm I.D.) for the oxidation of benzyl alcohol to benzaldehyde using PVP (polyvinylpyrrolidone)-stabilised Pd and Au/Pd nanoparticles with air. Selectivity of 97% at 91% conversion at a mean residence time of 15 min was obtained at 50 °C and ca. atmospheric pressure under continuous flow (in batch, quantitative conversion with 96% selectivity was achieved under similar conditions at a longer reaction time of 40 min). In this system, the nanoparticles were dispersed in the reaction solution and were precipitated at the end of the reaction. The recovered catalyst could be redispersed for reuse, but showed evidence of deactivation.
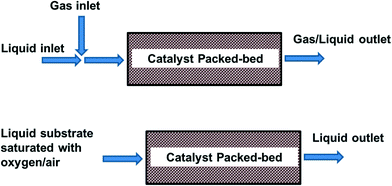
4.3 Packed bed reactors
In packed bed reactors the heterogeneous catalyst is packed and retained in a particulate form in a cartridge, tube, or microchannel. In this way catalyst separation from the reaction mixture is not required. A packed bed reactor offers easy catalyst replacement in the case of deactivation, or if another reaction needs to be performed. It also allows easier control of reaction (residence) time as compared to a batch reactor, and thus avoiding over-oxidation. In this arrangement, the size of the catalyst particles is typically in the order of 100 μm to avoid excessive pressure drop. The liquid can either be pre-saturated in a plug flow, or fed separately in a trickle bed (Fig. 4). Thus, depending on the conditions, the reactor operates at either triphasic (solid–liquid–gas) or biphasic (solid–liquid) conditions.For synthetic applications, milliscale packed bed reactors (<10 mm I.D.) are generally favoured. The most common design uses a segmented flow of oxygen/liquid feed through the catalyst bed. This has been used in the assessment of many catalysts (TEMPO,57 Au,47,58 Pd and Pt59) on different supports, typically in the oxidation of benzyl alcohol to benzaldehyde. For example, the MadOx team recently attempted a scale-up of the aerobic oxidation of alcohols using a heterogeneous Ru(OH)x/Al2O3 catalyst in a stainless steel tube.46 The liquid and gas (8% O2 in N2) were mixed with a T-piece and fed into the reactor in an upflow direction, operating within the slug flow regime and using tubing with diameter below the flame propagation threshold. Under these trickle-bed conditions, catalyst deactivation was significant. Nevertheless, provided that the operating conditions were adjusted accordingly, high steady state yields in the aerobic oxidation of 2-thiophene methanol could be maintained over 72 h to achieve a large yield of aldehyde product. Reaction rates of various alcohols, as well as deactivation characteristics, were found to be similar in batch conditions. A similar trickle-bed arrangement was also adopted by Kobayashi and co-workers to achieve aerobic oxidation of a number of primary and secondary alcohols with mixed solvents using polymer incarcerated gold-based nanocluster catalysts.60 The catalysts were packed with an optimised amount of Celite in a glass column (0.5 cm I.D.) to prevent obstruction by swelling of the catalysts. In this work, the reactions required the presence of inorganic bases, which were delivered in an aqueous phase in a down flow to the column with the packed catalyst bed, along with a solution of the reactant dissolved in an organic solvent, and O2. Good conversion and selectivity were obtained in a single pass with different catalysts (Au–Pt was found to be selective for aldehydes and ketones in trifluorobenzene, while Au–Pd favoured the formation of methyl esters in methanol), under optimised operating conditions. However, the productivity of the system is too low (space-time-yields up to 9.93 μmol mL−1 h−1) for commercial applications. Comparison with batch systems showed higher selectivities in the flow reactor, because over-oxidation could be prevented by controlling the residence time. To tackle the problem of catalyst deactivation, Muzen et al. described a reactor (4 cm I.D.) operated with ON-OFF liquid flow modulation.61 Gas and liquid streams were fed onto a packed-bed (filled with glass beads) in order to achieve thermal and vapour pressure equilibrium before entering the trickle-bed containing 0.4 kg of Pt/γ-Al2O3. The catalytic oxidation of ethyl and benzyl alcohols can be achieved under mild operating conditions (70 °C). Alcohol conversion can be improved by modulating the split and cycle period.
Mass and heat transfer efficiency of packed-bed reactors can be improved by modifying the catalyst supports. The use of ceramic fibre catalyst supports has received attention in recent years, as they can offer a large-surface-to-volume ratio without compromising on pressure drop. A Ru(OH)x catalyst deposited on a ‘paper-structured’ alumina/silica composite has been described.62 Ten of these disk-shaped porous catalyst disks were stacked in a stainless steel flow reactor, for the selective aerobic oxidation of aromatic and aliphatic alcohols to their corresponding aldehydes and ketones. Better performance was recorded compared to beaded catalysts, which was attributed to the formation of thinner liquid film layers.
Better productivity can also be achieved by improved reactor design. Bavykin et al. employed a multichannel reactor containing static mixers and heat-transfer channels, thus integrating mixing, heat transfer and reaction functionalities.63 The reactor consisted of 5 parallel packed-bed channels of 2 × 2, 3 × 3 and 5 × 5 (mm × mm) cross section and 10 cm length, each preceded by a static mixer to mix the gas and liquid streams before their entry to the catalyst bed. The reactor allowed staged injection of oxygen which was shown to be beneficial due to the development of a more uniform hydrodynamic regime of two-phase flow along the packed reaction channel. The reactor was shown to operate isothermally despite the significant heat formation from the exothermic reaction. In this work, Ru/Al2O3 was used as catalyst for oxidation of benzyl alcohol. Yields up to 55% and selectivity of 99% were achieved in a single pass conversion.
Another way to improve the safety in catalytic packed bed reactors is to saturate the substrate solution with oxygen before reaching the catalyst packed bed. The solubility of oxygen may pose a limitation; for this reason, this approach is only suitable for dilute solutions and high pressures. This was first described by Zotova et al. using a commercially-available X-Cube™ reactor,32 consisting of a stainless steel cartridge packed with Ru/γ-Al2O3. The mobile phase was saturated with O2 at different pressures (5–25 bar) before passing it through the catalyst bed. Using the device, a variety of primary and secondary alcohols could be converted to their corresponding carbonyl compounds in good yields and high selectivities. The system could be operated safely under continuous recirculation, and the turnover frequency was comparable to that achieved with other flow reactors. More recently, such reactor was also utilised by Osako et al. for aerobic oxidation of alcohols in water catalysed by Pt nanoparticles dispersed in an amphiphilic polystyrene–poly(ethylene glycol) resin;64 primary and secondary alcohols including aliphatic, aromatic and heteroaromatic alcohols were efficiently oxidised at 40–70 bar. Similarly, an H-Cube™ reactor fitted with an external gas module was used to oxidise benzyl alcohol using Fe/Al-SBA15, with TEMPO as a co-catalyst.65 Conversions of up to 42% in a single pass could be achieved with high selectivity, while continuous recirculation was used to obtain full conversion.
In packed bed reactors, temperature control is usually implemented by external heating elements. An interesting packed bed reactor was described by Kirschning and co-workers,66 where heating was applied inductively directly to the catalyst particles (Au supported on Fe3O4-containing core with SiO2 shell particles) contained within a polyether ether ketone (PEEK) tubing. The liquid phase was pre-saturated with gas by the use of a tube-in-tube AF-2400 device (see section 4.5 below) before entering the packed-bed. The system was used to oxidise allylic and benzylic alcohols where, in almost all cases, full conversion was achieved in a single pass. The system was scaled up to convert 2.5 g of 4-bromobenzyl alcohol to its corresponding aldehyde, with no over-oxidation to the acid. However, the productivity was quite low (operating at a flow rate of 0.2 mL min−1), and catalyst deactivation due to leaching was also observed at the beginning of the process. The same catalyst gave very low conversion of 4-bromobenzylalcohol, when tested under batch conditions in a sealed vial using an external oil bath, possibly due to inefficient oxygen mass transfer.
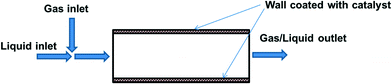
4.4 Catalytic wall reactors
The greatest advantage of using packed bed reactors is that (deactivated) catalysts can be easily replaced. However, there are also certain disadvantages; notably, heat and mass-transfer limitations when reactions are particularly exothermic or fast (as is the case for most oxidation reactions). Another limitation is the need to employ catalyst particles of a certain size, so as not to cause an excessive pressure drop. Incorporating the catalyst into the reactor wall (Fig. 5) allows better mass/heat transfer characteristics for fast reactions.This was demonstrated in a microreactor, where a gold catalyst was immobilised onto a polysiloxane-coated capillary through cross-linking with a copolymer.67 O2, substrate, solvent (dichloroethane) and aqueous K2CO3 were pre-mixed, and the multiphasic mixture was passed through the capillary tube at 60–70 °C. The Au-based system was used for oxidation of benzylic, aliphatic, allylic, secondary benzylic alcohols to ketones, while a Au/Pd-immobilised capillary column reactor was required for the oxidation of primary benzyl alcohols. The system could be operated continuously for at least four days without loss of activity. The same concept can be applied at a larger scale using a monolith reactor, such as that described by Pollington et al. for the selective oxidation of glycerol.68 Employing a stainless steel reactor (2.5 cm I.D.) housing Au/C coated monoliths, it was designed for co-current downflow operation, liquid recirculation and continuous air feed; with gas and liquid mixed before the catalyst bed. Reaction rate was found to be an order of magnitude greater for the monolith as compared to an autoclave batch reactor.
4.5 Membrane reactors
So far in our discussion, all the reactor systems deploy mixtures of O2 and substrate as mobile phases. This may pose safety concerns, particularly if the outlet of the reactor contains gas/vapour mixtures that are within the flammable regime (see section 3.4). Another approach to improve safety is to keep the reactive O2 separate from the other reaction components by using a membrane (Fig. 6), i.e. the membrane acts as a gas distributor.45 Oxygen diffuses across the membrane into the liquid phase, which may already contain the catalyst (homogeneous), or flows into a packed bed (heterogeneous catalyst). Provided that the membrane is chemically inert to organic solvents, this approach allows oxygen to permeate along the length of the reactor, hence avoiding axial concentration gradients. In order to deliver O2 sufficiently quickly without forming a headspace in the reactor, the pressure difference between the two sides needs to be carefully controlled. This will naturally place an upper working limit on the O2 pressure and its subsequent mass transfer into the liquid phase.69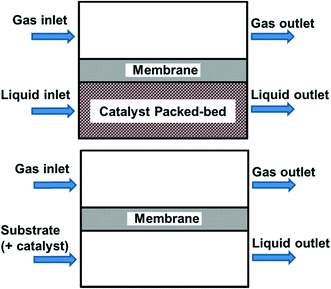 | ||
| Fig. 6 Membrane reactors separating reactive gas from the other reaction components, using a heterogeneous catalyst (top) or homogeneous/no catalyst (bottom). | ||
One such design used a ceramic membrane with a concentric configuration to minimize the radial packed bed mass transfer distance, as demonstrated by Constantinou et al.70 In this work, an inner tube created an annulus for the catalyst packed-bed through which the liquid phase flowed. The tubular membrane (7 mm I.D.), comprised of layers of alumina and a zirconia top layer with an average pore size of 50 nm and separated this solid–liquid mixture from O2, which was fed from the opposite side of the membrane in the outer shell of the reactor. Conversion of benzyl alcohol to benzaldehyde was achieved using a Pd–Au/TiO2 catalyst. In order to overcome the deficiency of oxygen in the catalyst bed area (which could lead to lower selectivity), O2 supply was increased by raising the gas pressure, diluting the substrate concentration and increasing residence time, achieving benzaldehyde selectivity 88% at 75% conversion.
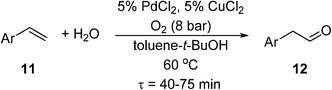
If the reaction temperature is sufficiently low, polymeric membranes can be used. A tube-in-tube reactor based on a gas-permeable Teflon AF-2400 membrane was first developed by the Ley group at Cambridge University, initially for homogeneous and heterogeneous catalytic hydrogenation reactions.71 This was subsequently deployed for an anti-Markovnikov Wacker oxidation of functionalized styrenes (11) to linear arylacetaldehydes (12) using PdCl2/CuCl2 catalysts (Scheme 8). Exploratory experiments were performed in a batch reactor, but optimisation of the reaction conditions was carried out in a tube-in-tube reactor (the annulus between the inner and the outer tubes was pressurized with O2) followed by a heated stainless steel reactor coil.72 Although the oxygen permeability of Teflon AF-2400 is high,73 long residence times were required to achieve good yields (56–80%). As O2 has an upper solubility limit in the reaction solvent, O2 depletion is a significant issue at higher reactant concentrations. This may be circumvented by double dosing the reaction stream (by inserting another tube-in-tube device between two reaction coils). This can lead to some improvement, but increasing the O2 pressure can lead to over-oxidation, hence careful adjustment of concentrations is required. In another study by Wu et al.,74 an AF-2400 membrane was used to deliver O2 along the entire length of the reactor packed with Pd-Au/TiO2 catalyst particles, for the aerobic oxidation of benzyl alcohol (known to be a very fast process). At 120 °C and 6 bar of O2, 44% conversion of the neat alcohol could be achieved at 115 gcat s galcohol−1 catalyst contact time. Under these conditions, formation of toluene was a competitive process.
In a search for more cost-effective membranes, cheaper polymeric materials were evaluated by the MadOx group, who found that PTFE exhibited an acceptable combination of low cost, chemical stability and gas diffusion properties. A tube-in-shell reactor was duly constructed with a Teflon coil (1.6 mm I.D.), contained within an oven (tube-in-shell).75 The use of this reactor was demonstrated in the aerobic oxidation reactions of alcohols facilitated by both homogeneous (Cu/TEMPO and Cu/ABNO) or heterogeneous (Ru(OH)x/Al2O3) catalysts. Complete conversion of benzyl alcohol to benzaldehyde (0.5–1 M) can be obtained using the Ru catalyst, but required a residence time of nearly 1 h (9.2 bar and 80 °C). Conversely, homogeneous Cu/TEMPO and Cu/ABNO catalysts were mixed with substrates and flowed within the inner tube, while O2 was pressurised in the outer tube. Near quantitative product yields in a residence time of 1 min were achieved for various benzylic alcohols at 24 bar O2 pressure.
4.6 Catalytic membrane reactors
A catalytic membrane reactor essentially combines features of a membrane reactor and a catalytic with a catalyst wall reactor. In this design, the catalyst is embedded within a nanoporous membrane, thus positioning it between the liquid–gas boundary, so as to maximise mass transfer whilst also keeping gaseous oxidant and liquid hydrocarbons separated and thus improve safety. Typically, ceramic membranes resembling common catalyst supports (e.g. alumina, silicates) are impregnated with the catalyst. To date, catalytic membrane reactors have been largely employed for gas-phase reactions, and none has yet been reported for aerobic oxidation reactions in the liquid phase. In related work, several membranes were evaluated for direct synthesis of hydrogen peroxide,76 with a Pd catalyst deposited into the finest porous layer on the inner side of the membranes. Oxygen was fed from the outer side of the membrane, while hydrogen was dissolved in methanol solvent at high pressure and fed through the inner side of the membrane. As might be expected, the diffusive transport of the reactants to the catalytically active zone, located on the inner walls of the membrane channel, was found to be crucial. Radial mixing was increased by filling the membrane with small glass beads and led to higher productivity.5. Current industrial processes for the production of fine chemicals and APIs by aerobic oxidation
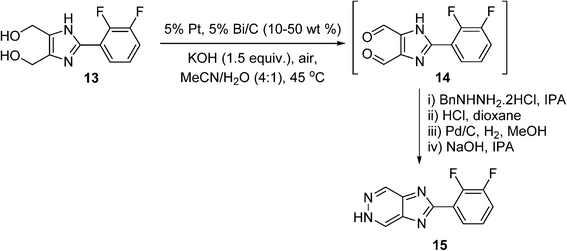
There has been quite a number of aerobic oxidation reactions reported in the organic process development literature in recent years. Case studies selected from the fine chemicals/pharmaceutical industry are presented below. These examples include uncatalyzed reactions, as well as those enabled by homogeneous and heterogeneous catalysts. In some cases, multi-kilograms of products can be obtained, although commercial production using these processes has yet to be realised. Most of these processes are performed initially in batch reactors, although continuous flow systems have also been described in some cases.It is often more practical to adopt heterogeneous catalysis in reaction scale up, as it is generally more amenable to process intensification (e.g. continuous flow), and the catalyst can be easily separated from the reaction mixture. However, in a recent review of heterogeneously-catalysed alcohol oxidation for the fine chemical industry,77 none of the cited work has translated into commercial processes. A rare example of a heterogeneously-catalysed aerobic oxidation reaction can be found in a process developed by GlaxoSmithKline (GSK) for the synthesis of an hepatitis C virus (HCV) replicase inhibitor.78 The synthetic route requires the double oxidation of a diol (13) into a dialdehyde (14), which condenses with a protected hydrazine to produce a 5H-imidazo[4,5-d]pyridazine (15) (Scheme 9). In the early stages of the program, the double oxidation was carried out on a 600 g scale using an excess of MnO2 as the oxidant, requiring 5.7 kg of the oxidant to reach completion, giving a 69% yield of 15. A screen for heterogeneous catalysts capable of effecting the aerobic oxidation of diol 13 was subsequently performed, including 106 combinations of catalysts (Ru, Pt, Pd, and Bi additives), loading, solvent and basic additives. This led to the identification of 5% Pt/Bi on C in acetonitrile with added potassium hydroxide as the best performer. The reaction was carried out on 2 g scale by vigorously stirring the reaction mixture in open air at 45 °C; the dialdehyde (14) formed contained some impurities but was telescoped through a hydrazine condensation process, forming the desired product 15 in 70% overall yield. Unfortunately, the cancellation of this project stopped further investigation into the oxidation; however, the catalytic process is unquestionably more atom-economical and has greater potential for scale-up.
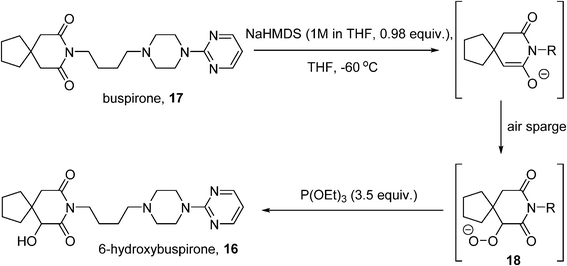
6-Hydroxybuspirone (16) is a metabolite of the anxiolytic drug buspirone (17) which shows good affinity for the same 5-HT1A receptor as the parent drug.79 Challenged with the production of 100 kg quantities of 16 to support toxicological and clinical studies, Bristol-Myers Squibb developed a route based on the Gardner modification80 of a Barton enolate oxidation81 whereby the enolate of buspirone is treated with O2 to generate a hydroperoxide (18), which is reduced in situ by a phosphite to 16 (Scheme 10). Initial studies were performed as a batch process,82 where the enolate was generated using sodium hexamethyldisilazide (NaHMDS) at −70 °C, in the presence of an excess of triethylphosphite. Although small-scale lab experiments had shown that the rate of the oxidation was enhanced by the use of pure O2, safety considerations dictated that the O2 concentration in the reactor headspace be kept below 6% on the larger scale process, achieved by use of an air sparge coupled with a nitrogen sweep (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volumetric ratio of nitrogen to air), with an oxygen monitor to maintain the O2 level at ≤5.5%. Careful control of the enolate formation is required to avoid residual starting material or side-reactions caused by excess base, which was monitored using in-process FT-IR. The resulting process was implemented on a 10 kg batch scale successfully, delivering >7 kg of 16 (71% yield). However, further scale-up of the procedure was deemed not to be feasible on the basis of several considerations, including the extended times required for the aerobic oxidation caused by mass transfer issues, the expense and difficulty of operating in the cryogenic regime (−60 °C), and increased risk associated with reliably purging the headspace at larger scale.
1 volumetric ratio of nitrogen to air), with an oxygen monitor to maintain the O2 level at ≤5.5%. Careful control of the enolate formation is required to avoid residual starting material or side-reactions caused by excess base, which was monitored using in-process FT-IR. The resulting process was implemented on a 10 kg batch scale successfully, delivering >7 kg of 16 (71% yield). However, further scale-up of the procedure was deemed not to be feasible on the basis of several considerations, including the extended times required for the aerobic oxidation caused by mass transfer issues, the expense and difficulty of operating in the cryogenic regime (−60 °C), and increased risk associated with reliably purging the headspace at larger scale.
The decision was therefore taken to investigate continuous processing methods.45 Initial small-scale studies were performed in segmented flow using a microreactor (CPC CYTOS®) with a pre-formed solution of the enolate and oxygen gas as the input streams. A conversion of 85–92% was obtained with a residence time of 5–6 minutes; the short reaction time allowed the reaction temperature to be raised. A higher output can be obtained using a trickle-bed reactor to achieve better gas–liquid mixing. A solution of the enolate was flowed through a 1 inch Pro-Pak-filled column with a counter-current flow of O2 at −37 °C. Operating in continuous mode also allowed for the enolization step to be adapted: an initial mixing of base and 17 at −17 °C and a longer residence time at −35 °C, allowed for complete enolate formation without requiring cryogenic conditions. Further scale-out of the oxidation step was eventually accomplished with four parallel tubular reactors combined within a single cooling jacket, fed by a single oxygen inlet. Under steady-state conditions, the Quadreactor could produce over 100 kg of hydroxybuspirone 16.
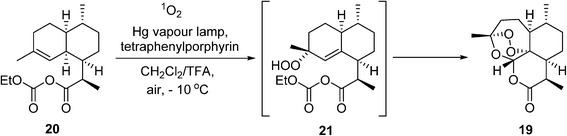
In 2015, the Nobel Prize in Medicine was awarded to Youyou Tu for her isolation of the powerful anti-malaria drug artemisinin (19) (‘qinghao su’). Currently, artemisinin and its derivatives are recommended by WHO as the first-line treatment for P. falciparum malaria. Artemisinin is a naturally-occurring compound found in the leaves of the plant Artemisia annua, but the supply from this natural plant is neither sufficient nor reliable enough to meet global demands. To address this issue, a semisynthetic route for producing artemisinin has been implemented on a process scale by Sanofi.83,84 This work has gathered much attention in recent years, not only for its innovation in combining biotechnology with a photochemical reaction, but also serving as an inspiring example of how academia (UC Berkeley), industry (Sanofi, Amyris), non-profit organizations (NRC, Canada, OneWorld Health), as well as funding agencies (WHO, Bill & Melinda Gates Foundation), can form a powerful alliance to deliver innovative solutions to global challenges. The major breakthrough in this remarkable story began with the genetic engineering of Baker's yeast to produce artemisinic acid, a precursor to the mixed anhydride 20. This is followed by another key transformation, whereby 20 reacts with singlet oxygen (1O2) to produce the hydroperoxide intermediate 21, which undergoes rearrangement spontaneously to the artemisinin 19 (Scheme 11). Singlet oxygen is produced photochemically under UV irradiation using tetraphenylporphyrin as a sensitizer. This presents a double challenge for process development: not only does the hazard of handling oxygen present itself, but photochemical reactions are notoriously difficult to scale due to issues with light transmission. In the event, a bespoke semi-batch reactor was designed, wherein a solution of the precursor 20 in dichloromethane and trifluoroacetic acid is circulated with the photocatalyst (ca. 0.05 wt%) at around −10 °C through the photoreactor chamber with air bubbling. The use of air dictates the choice of the chlorinated solvent. The process delivers ca. 370 kg of artemesinin per 600 kg batch of artemesinic acid, and delivered 60 tonnes of 19 in 2014 (a third of the global demand).
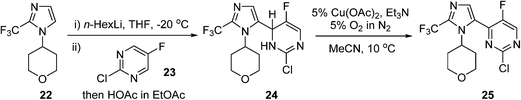
An AstraZeneca team developed a scale-up process for the synthesis of a GSK3β inhibitor used for the treatment of CNS (central nervous system) disorders (Scheme 12). The synthesis route initiates with the lithiation of an imidazole precursor 22, and the addition of the resultant organometallic reactant to the pyrimidine 23. In the following step, the intermediate dihydropyrimidine 24 has to re-aromatise to the desired product 25; this is best achieved by an oxidative dehydrogenation, using air in the presence of a copper catalyst.85 To avoid the risk of organic peroxide formation, a solvent-swap from the THF to acetonitrile following the lithiation/addition sequence is necessary. The aerobic oxidation was subsequently carried out using 5% O2 in N2 to ensure safe operation under batch conditions, delivering ca. 120 g of 25 in a single batch.
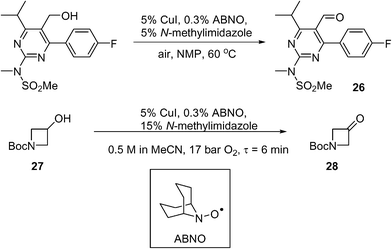
Scale-up of aerobic oxidation of alcohols to aldehydes, mediated by copper(I) salts and nitroxyl radicals, was investigated by the MadOx team.86 Economic (catalytic loadings, additive) and safety (avoiding solvent flashpoint) considerations were taken into account in this work, leading to an optimised batch process that has been carried out on 250 mmol scale on simple test substrates. An application to the synthesis of a pyrimidine intermediate 26 (used for the production of a statin, rosuvastatin) was demonstrated on a 10 mmol scale (Scheme 13). A continuous flow method was subsequently developed using the tube-in-shell membrane reactor described previously (see section 4.5 above). The use of CuI as a catalyst precursor was found to corrode stainless steel components. This prompted the modification of the reactor, where the stainless steel catalyst injection loop was replaced by PTFE. The amount of additive was increased (from 5 to 15 mol%) to prevent precipitation of insoluble Cu species. Using the modified system, the conversion of 1-Boc-3-hydroxyazetidine (27) to the corresponding ketone (28) was demonstrated (Scheme 13): a steady-state yield of ≥98% can be achieved, however, the productivity was not high; only 9 mmol of product was obtained in 3 h.
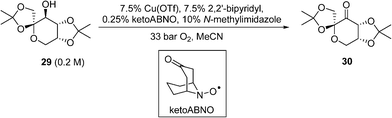
A similar copper(I)/nitroxyl system for continuous catalytic alcohol oxidation reactions has also been described recently by DSM, to convert the fructose-derived epoxol 29 into epoxone 30 (Scheme 14).87 In this process, the catalytic chemistry was implemented in a bespoke 3-D printed ‘zig-zag’ continuous reactor to ensure turbulent flow. Although the reaction rate is independent of O2 pressure, 33 bar of O2 was employed to achieve a homogeneous phase. Under these conditions, full conversions of 29 (0.20 M) to 30 can be achieved within 17 min at 100 °C. Economic comparisons between the Cu(OTf) and CuI procedures were highlighted in this work.
6. Future directions?
Having surveyed the current landscape, the remaining challenges and some future opportunities for realising aerobic oxidation reactions for the production of speciality chemicals are discussed in this section. At smaller scale and higher margin, capital expenditure (Capex) is usually dwarfed by the operating expenditure (Opex) in the pharma and fine chemicals industry. Ideally, there needs to be certain flexibility in the design of the chemical plant, such that a variety of different chemical reactions can be implemented at the same site, i.e. highly modular units of operation, each with a small footprint, is preferred over dedicated facilities. Greater emphasis on the development and adaptation of continuous flow technology in the fine chemicals and pharmaceutical sectors bodes well for future applications of aerobic oxidation reactions, as it can overcome the explosive hazards currently hampering the wider implementation of this synthetic methodology. Conversely, it has been shown (as in the case of Amoco's TA production) that it is possible to execute large-scale aerobic oxidation reactions in a homogeneous solution of flammable solvents, as long as the amount of O2 in the headspace can be managed by a good understanding of the underlying kinetics and mass transfer parameters.As to future aspects, these may be broadly divided into Engineering and Chemistry challenges (listed in the following sections), although the issues tend to be highly interrelated. Thus, a coordinated and interdisciplinary approach is necessary to deliver truly innovative solutions.
6.1 Engineering challenges
1. Flammability of solvents and explosive hazards remain at the top of the agenda, which can be addressed by better control of reaction and process parameters. This will require a detailed study of the reaction kinetics involved, combined with a clear understanding of the fluid mechanics in the reaction vessel, allowing for the design of the appropriate residence time and holdup volume of the gas phase. This in turn causes, by design, the oxygen concentration to be below the flammability limit upon release into the head space of the vessel. With no excess oxidant in the system, the reaction will need to be able to operate under an O2-lean regime. Selectivity of the reaction must not be compromised, and any catalyst designed for such processes must not deactivate under these conditions.2. Equally, heat release is effectively controlled by operating at the boiling point of the solvent. This could be tuned by adjustment of the reactor pressure, however, this will also directly affect oxygen solubility, autoignition temperatures and flammability limits (as discussed in section 3.2).
3. O2 delivery is critical to maintain process efficiency and safety. Ideally, the O2 is delivered at a rate that is comparable to the oxidation process. The use of membrane reactors can eliminate accumulation of excess O2 in the headspace, but this is currently limited by the availability of suitable materials that can maintain both a high flux of O2 and pressure differential (between the gas and mobile phases) at the same time.
4. Synthetic routes in the fine chemicals and pharmaceutical industry tend to be multistep. Hence it is desirable to operate the individual steps in tandem to minimise the need to isolate intermediates. This is best achieved by a continuous process with attendant continuous separation stage, for example: organic solvent nanofiltration,88 continuous distillation,89 or continuous crystallization.90–92 The implementation of reliable online/inline process analytics and effective feedback control is critical for quality assurance – this is still a considerable challenge.
5. Handling and maintaining multiple phases in flow reactors is still somewhat difficult and should be avoided where possible. Particularly, if the reaction system results in the formation of solids, a batch reactor system may still be the better option (e.g. Amoco's TA process), particularly if long residence times are involved.
6. Removal of homogeneous catalysts from the reaction mixture has always been a problem in process chemistry, which also hampers the development of tandem processes. Thus, development and adoption of heterogeneous catalysts for the synthesis of complex molecules needs to be further exemplified, particularly for more robust catalysts that can be incorporated into an appropriate flow reactor without losing efficiency. That said, catalyst leaching from heterogeneous catalysts (leading to contamination of the final product) is also an important issue seldom addressed in academic papers.
6.2. Chemistry challenges
1. There is a need to expand the scope of aerobic oxidation reactions with high selectivities. Currently, much of the research effort has been focussed on the oxidation of alcohols to aldehydes and ketones, typically benzyl alcohols to benzaldehydes. There needs to be more studies on the reaction scope of new catalysts, especially towards substrates with multiple functional groups. Conversely, other aerobic oxidation reactions (particularly type II) are also under-developed. An example is the epoxidation of alkenes, which is an important process for the fine chemicals/pharmaceutical sector. With the notable exception of silver-catalysed epoxidation of ethylene (achieved on an industrial scale in the gas phase at 220–280 °C),93 direct oxidation of alkenes by O2 (in the liquid phase) is unknown on industrial scale.2. Certain aerobic oxidation reactions proceed via free-radical intermediates (e.g. oxidation of benzylic and allylic C–H bonds). Currently, these require high temperatures, and are thus generally difficult to control and often lead to low selectivity. This can be addressed by broadening the chemistry of singlet O2/photocatalysis94–100 (the artemisinin project showed that it is possible to scale-up such processes). Electrochemistry is another interesting possibility, e.g. O2 can be converted into effective oxidants using redox mediators.101–104 Photo- and electro-chemistry are generally performed at (sub-) ambient conditions therefore have great potential to deliver unique chemistry.
3. Ionic liquids (ILE's)105,106 and CO2,107–109 have been employed in aerobic oxidation reactions, in order to circumvent the need for flammable solvents. However, the use of such unconventional reaction media may require specialised equipment to contain sc-CO2 and to recycle ILE, which may be too energy-intensive. In the context of pharmaceutical intermediates, there may be certain applications where the use of such reaction media, particularly sc-CO2 and perfluorinated solvents, may be uniquely advantageous: pure oxygen can be used with these non-flammable materials, and post-reaction separation can be easily achieved by a simple pressure reduction (employed in a continuous process the higher pressure required would not be as critical as in a batch process). The disadvantage here would be the formation of a multiphase system, which is more difficult to control (see point 5 in section 6.1).
Acknowledgement
The work was performed as part of a collaborative project sponsored by an EPSRC award ‘Accelerating Physical Sciences Grand Challenges towards Innovative Manufacturing’, grant number: EP/L003279/1 (Sustainable manufacturing in multiphase continuous reactors: Aerobic oxidations). We are grateful to Dr. Gill Smith for help in the preparation of this manuscript, and our industrial collaborators (AstraZeneca, GlaxoSmithKline, Johnson Matthey, Syngenta) for their participation and valuable inputs to this project.References
- D. Knoefler, L. I. O. Leicher, M. Thamsen, C. M. Cremers, D. Reichmann, M. J. Gray, W.-Y. Wholey and U. Jakob, Biochem. Soc. Trans., 2014, 42, 917–921 CrossRef CAS PubMed.
- T. V. A. Murray, A. Ahmad and A. C. Brewer, Trends Cardiovasc. Med., 2014, 24, 113–120 CrossRef CAS PubMed.
- K. Wang, T. Zhang, Q. Dong, E. C. Nice, C. Huang and Y. Wei, Cell Death Dis., 2013, 4, e537 CrossRef CAS PubMed.
- A. Bindoli and M. P. Rigobello, Antioxid. Redox Signaling, 2013, 18, 1557–1593 CrossRef CAS PubMed.
- M. G. Bonini, M. E. L. Consolaro, P. C. Hart, M. Mao, A. L. Pimenta de Abreu and A. M. Master, IUBMB Life, 2014, 66, 167–181 CrossRef CAS PubMed.
- Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang and Y. Yang, Chem. Soc. Rev., 2014, 43, 3480–3524 RSC.
- T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329–2363 CrossRef CAS PubMed.
- J. Piera and J. E. Backvall, Angew. Chem., Int. Ed., 2008, 47, 3506–3523 CrossRef CAS PubMed.
- Z. Shi, C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381–3430 RSC.
- J. Chen, J. Cen, X. Xu and X. Li, Catal. Sci. Technol., 2016, 6, 349–362 CAS.
- H. P. L. Gemoets, Y. Su, M. Shang, V. Hessel, R. Luque and T. Noel, Chem. Soc. Rev., 2016, 45, 83–117 RSC.
- E. Roduner, W. Kaim, B. Sarkar, V. B. Urlacher, J. Pleiss, R. Glaeser, W. D. Einicke, G. A. Sprenger, U. Beifuss, E. Klemm, C. Liebner, H. Hieronymus, S. F. Hsu, B. Plietker and S. Laschat, ChemCatChem, 2013, 5, 82–112 CrossRef CAS.
- E. G. Chepaikin, J. Mol. Catal. A: Chem., 2014, 385, 160–174 CrossRef CAS.
- K. Chen, P. Zhang, Y. Wang and H. Li, Green Chem., 2014, 16, 2344–2374 RSC.
- I. Efimov, J. Basran, S. J. Thackray, S. Handa, C. G. Mowat and E. L. Raven, in Advances in Inorganic Chemistry, ed. R. Van Eldik and I. Ivanović-Burmazović, 2012, vol. 64, pp. 33–51 Search PubMed.
- W. Nam, Y. M. Lee and S. Fukuzumi, Acc. Chem. Res., 2014, 47, 1146–1154 CrossRef CAS PubMed.
- M. Sallmann and C. Limberg, Acc. Chem. Res., 2015, 48, 2734–2743 CrossRef CAS PubMed.
- J. B. Hendrickson, J. Am. Chem. Soc., 1975, 97, 5784–5800 CrossRef CAS.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 CAS.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC.
- J. H. Teles, I. Hermans, G. Franz and R. A. Sheldon, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley & Sons, 2011 Search PubMed.
- BASF, DE092952709, 1981.
- O. A. Kholdeeva, O. V. Zalomaeva, A. B. Sorokin, I. D. Ivanchikova, C. Della Pina and M. Rossi, Catal. Today, 2007, 121, 58–64 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- D. Cespi, E. S. Beach, T. E. Swarr, F. Passarini, I. Vassura, P. J. Dunn and P. T. Anastas, Green Chem., 2015, 17, 3390–3400 RSC.
- S. Caron, R. W. Dugger, S. G. Ruggeri, J. A. Ragan and D. H. B. Ripin, Chem. Rev., 2006, 106, 2943–2989 CrossRef CAS PubMed.
- R. J. Sheehan, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed.
- Market Research Report: Purified Terephthalic Acid (PTA) Market: Global Industry Analysis and Opportunity Assessment 2015–2025, http://www.futuremarketinsights.com/reports/purified-terephthalic-acid-pta-market, accessed 30 August 2016.
- R. A. F. Tomas, J. C. M. Bordado and J. F. P. Gomes, Chem. Rev., 2013, 113, 7421–7469 CrossRef CAS PubMed.
- P. M. Osterberg, J. K. Niemeier, C. J. Welch, J. M. Hawkins, J. R. Martinelli, T. E. Johnson, T. W. Root and S. S. Stahl, Org. Process Res. Dev., 2015, 19, 1537–1543 CrossRef CAS PubMed.
- F. Cavani, J. Chem. Technol. Biotechnol., 2010, 85, 1175–1183 CrossRef CAS.
- N. Zotova, K. Hellgardt, G. H. Kelsall, A. S. Jessiman and K. K. Hii, Green Chem., 2010, 12, 2157–2163 RSC.
- N. N. Semenov, Z. Phys., 1928, 48, 571–582 CrossRef CAS.
- D. A. Frank-Kamenetskii, in Diffusion and Heat Exchange in Chemical Kinetics (translation edited by J. P. Appleton), Plenum Press, New York, 1969 Search PubMed.
- R. H. Perry and D. W. Green, in Perry's Chemical Eengineers' Handbook, McGraw-Hill, New York, 2008 Search PubMed.
- NFPA 69: Standard on Explosion Prevention Systems, accessible at: http://www.nfpa.org/codes-and-standards/document-information-pages?mode=code&code=69, accessed 30 August 2016.
- C. V. Mashuga and D. A. Crowl, Process Saf. Prog., 1998, 17, 176–183 CrossRef CAS.
- A more extensive collection of relevant data for 500 combustible gases, liquids and solids, including autoignition temperature, flammability limits etc. can be found in J. M. Kuchta, Investigation of fire and explosion accidents in the chemical, mining and fuel-related industries, a manual, Bulletin 680, US Bureau of Mines, 1985 Search PubMed.
- R. Bounaceur, P. A. Glaude, B. Sirjean, R. Fournet, P. Montagne, M. Vierling and M. Molière, J. Eng. Gas Turbines Power, 2015, 138, 021505 CrossRef.
- E. Brandes, W. Hirsch and T. Stolz, Autoignition temperatures for mixtures of flammable liquids in closed vessels, European Combustion Meeting 2005.
- M. G. Zabetakis, Flammability characteristics of combustible gases and vapours, U.S. Department of Mines, Bulletin 627, 1965 Search PubMed.
- M. Ryng, Bezpieczenstwo techniczne w przemysle chemicznym – poradnik, Wydawnictwo Naukowo Techniczne, Warsaw, 1985 Search PubMed.
- A. Li, S. Tang, P. Tan, C. Liu and B. Liang, J. Chem. Eng. Data, 2007, 52, 2339–2344 CrossRef CAS.
- H. L. Clever, R. Battino, H. Miyamoto, Y. Yampolski and C. L. Young, J. Phys. Chem. Ref. Data, 2014, 43, 033102 CrossRef.
- T. L. LaPorte, M. Hamedi, J. S. DePue, L. F. Shen, D. Watson and D. Hsieh, Org. Process Res. Dev., 2008, 12, 956–966 CrossRef CAS.
- D. S. Mannel, S. S. Stahl and T. W. Root, Org. Process Res. Dev., 2014, 18, 1503–1508 CrossRef CAS PubMed.
- B. N. Zope and R. J. Davis, Top. Catal., 2009, 52, 269–277 CrossRef CAS.
- K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406–446 CrossRef PubMed.
- J. Yue, G. Chen, Q. Yuan, L. Luo and Y. Gonthier, Chem. Eng. Sci., 2007, 62, 2096–2108 CrossRef CAS.
- J. Yue, L. Luo, Y. Gonthier, G. Chen and Q. Yuan, Chem. Eng. Sci., 2009, 64, 3697–3708 CrossRef CAS.
- X. A. Ye, M. D. Johnson, T. N. Diao, M. H. Yates and S. S. Stahl, Green Chem., 2010, 12, 1180–1186 RSC.
- J. F. Greene, J. M. Hoover, D. S. Mannel, T. W. Root and S. S. Stahl, Org. Process Res. Dev., 2013, 17, 1247–1251 CrossRef CAS.
- B. Pieber and C. O. Kappe, Green Chem., 2013, 15, 320–324 RSC.
- T. Baumeister, H. Kitzler, K. Obermaier, S. Zikeli and T. Roder, Org. Process Res. Dev., 2015, 19, 1576–1579 CrossRef CAS.
- L. Vanoye, J. D. Wang, M. Pablos, R. Philippe, C. de Bellefon and A. Fayre-Reguillon, Org. Process Res. Dev., 2016, 20, 90–94 CrossRef CAS.
- H. Alex, N. Steinfeldt, K. Jahnisch, M. Bauer and S. Hubner, Nanotechnol. Rev., 2014, 3, 99–110 CAS.
- C. Aellig, D. Scholz, S. Conrad and I. Hermans, Green Chem., 2013, 15, 1975–1980 RSC.
- N. Asao, N. Hatakeyama, Menggenbateer, T. Minato, E. Ito, M. Hara, Y. Kim, Y. Yamamoto, M. W. Chen, W. Zhang and A. Inouei, Chem. Commun., 2012, 48, 4540–4542 RSC.
- A. L. Tarasov, L. M. Kustov, A. A. Bogolyubov, A. S. Kiselyov and V. V. Semenov, Appl. Catal., A, 2009, 366, 227–231 CrossRef CAS.
- K. Kaizuka, K. Y. Lee, H. Miyamura and S. Kobayashi, J. Flow Chem., 2012, 2, 1–4 CrossRef CAS.
- A. Muzen, M. S. Fraguio, M. C. Cassanello, M. A. Ayude, P. M. Haure and O. M. Martinez, Ind. Eng. Chem. Res., 2005, 44, 5275–5284 CrossRef CAS.
- T. Homma and T. Kitaoka, Appl. Catal., A, 2014, 486, 201–209 CrossRef CAS.
- D. V. Bavykin, A. A. Lapkin, S. T. Kolaczkowski and P. K. Plucinski, Appl. Catal., A, 2005, 288, 175–184 CrossRef CAS.
- T. Osako, K. Torii and Y. Uozumi, RSC Adv., 2015, 5, 2647–2654 RSC.
- D. Obermayer, A. M. Balu, A. A. Romero, W. Goessler, R. Luque and C. O. Kappe, Green Chem., 2013, 15, 1530–1537 RSC.
- S. R. Chaudhuri, J. Hartwig, L. Kupracz, T. Kodanek, J. Wegner and A. Kirschning, Adv. Synth. Catal., 2014, 356, 3530–3538 CrossRef CAS.
- N. W. Wang, T. Matsumoto, M. Ueno, H. Miyamura and S. Kobayashi, Angew. Chem., Int. Ed., 2009, 48, 4744–4746 CrossRef CAS PubMed.
- S. D. Pollington, D. I. Enache, P. Landon, S. Meenakshisundaram, N. Dimitratos, A. Wagland, G. J. Hutchings and E. H. Stitt, Catal. Today, 2009, 145, 169–175 CrossRef CAS.
- A. A. Lapkin, B. Bozkaya and P. K. Plucinski, Ind. Eng. Chem. Res., 2006, 45, 2220–2228 CrossRef CAS.
- A. Constantinou, G. W. Wu, A. Corredera, P. Ellis, D. Bethell, G. J. Hutchings, S. Kuhn and A. Gavriilidis, Org. Process Res. Dev., 2015, 19, 1973–1979 CrossRef CAS.
- M. O'Brien, N. Taylor, A. Polyzos, I. R. Baxendale and S. V. Ley, Chem. Sci., 2011, 2, 1250–1257 RSC.
- S. L. Bourne and S. V. Ley, Adv. Synth. Catal., 2013, 355, 1905–1910 CrossRef CAS.
- H. Zhang and S. G. Weber, Top. Curr. Chem., 2012, 308, 307–337 CrossRef CAS PubMed.
- G. W. Wu, A. Constantinou, E. H. Cao, S. Kuhn, M. Morad, M. Sankar, D. Bethell, G. J. Hutchings and A. Gavriilidis, Ind. Eng. Chem. Res., 2015, 54, 4183–4189 CrossRef CAS.
- J. F. Greene, Y. Preger, S. S. Stahl and T. W. Root, Org. Process Res. Dev., 2015, 19, 858–864 CrossRef CAS.
- A. Pashkova, R. Dittmeyer, N. Kaltenborn and H. Richter, Chem. Eng. J., 2010, 165, 924–933 CrossRef CAS.
- R. Ciriminna, V. Pandarus, F. Beland, Y. J. Xu and M. Pagliaro, Org. Process Res. Dev., 2015, 19, 1554–1558 CrossRef CAS.
- R. K. Bowman, A. D. Brown, J. H. Cobb, J. F. Eaddy, M. A. Hatcher, M. R. Leivers, J. F. Miller, M. B. Mitchell, D. E. Patterson, M. A. Toczko and S. P. Xie, J. Org. Chem., 2013, 78, 11680–11690 CrossRef CAS PubMed.
- H. Wong, R. C. Dockens, L. Pajor, S. Yeola, J. E. Grace, A. D. Stark, R. A. Taub, F. D. Yocca, R. C. Zaczek and Y. W. Li, Drug Metab. Dispos., 2007, 35, 1387–1392 CrossRef CAS PubMed.
- J. N. Gardner, F. E. Carlon and O. Gnoj, J. Org. Chem., 1968, 33, 3294–3297 CrossRef CAS PubMed.
- E. J. Bailey, D. H. R. Barton, J. Elks and J. F. Templeton, J. Chem. Soc., 1962, 1578–1591 RSC.
- D. J. Watson, E. D. Dowdy, J. S. DePue, A. S. Kotnis, S. Leung and B. C. O'Reilly, Org. Process Res. Dev., 2004, 8, 616–623 CrossRef CAS.
- J. Turconi, F. Griolet, R. Guevel, G. Oddon, R. Villa, A. Geatti, M. Hvala, K. Rossen, R. Goller and A. Burgard, Org. Process Res. Dev., 2014, 18, 831 CrossRef CAS.
- J. Turconi, F. Griolet, R. Guevel, G. Oddon, R. Villa, A. Geatti, M. Hvala, K. Rossen, R. Göller and A. Burgard, Org. Process Res. Dev., 2014, 18, 417–422 CrossRef CAS.
- A. Witt, P. Teodorovic, M. Linderberg, P. Johansson and A. Minidis, Org. Process Res. Dev., 2013, 17, 672–678 CrossRef CAS.
- J. E. Steves, Y. Preger, J. R. Martinelli, C. J. Welch, T. W. Root, J. M. Hawkins and S. S. Stahl, Org. Process Res. Dev., 2015, 19, 1548–1553 CrossRef CAS.
- P. Alsters, conference presentation Aerobic (alcohol) oxidation in flow, Organic Process Research and Development, Barcelona, September 2015, https://scientificupdate.co.uk/images/stories/presentations/OPRD_Sep2015_PDF_2.pdf.
- P. Marchetti, M. F. J. Solomon, G. Szekely and A. G. Livingston, Chem. Rev., 2014, 114, 10735–10806 CrossRef CAS PubMed.
- V. Aneesh, R. Antony, G. Paramasivan and N. Selvaraju, Chem. Eng. Process., 2016, 104, 219–242 CrossRef CAS.
- C. Rougeot and J. E. Hein, Org. Process Res. Dev., 2015, 19, 1809–1819 CrossRef CAS.
- T. McGlone, N. E. B. Briggs, C. A. Clark, C. J. Brown, J. Sefcik and A. J. Florence, Org. Process Res. Dev., 2015, 19, 1186–1202 CrossRef CAS.
- E. Chabanon, D. Mangin and C. Charcosset, J. Membr. Sci., 2016, 509, 57–67 CrossRef CAS.
- W. M. H. Sachtler, C. Backx and R. A. Van Santen, Catal. Rev.: Sci. Eng., 1981, 23, 127–149 CAS.
- J. An, Y. Q. Zou, Q. Q. Yang, Q. Wang and W. J. Xiao, Adv. Synth. Catal., 2013, 355, 1483–1489 CrossRef CAS.
- W. Ding, Q. Q. Zhou, J. Xuan, T. R. Li, L. Q. Lu and W. J. Xiao, Tetrahedron Lett., 2014, 55, 4648–4652 CrossRef CAS.
- C. Gambarotti, L. Melone, T. Caronna and C. Punta, Curr. Org. Chem., 2013, 17, 2406–2419 CrossRef CAS.
- N. Iqbal, S. Choi, Y. You and E. J. Cho, Tetrahedron Lett., 2013, 54, 6222–6225 CrossRef CAS.
- K. Marui, A. Nomoto, H. Akashi and A. Ogawa, Synthesis, 2016, 48, 31–42 CAS.
- T. Nevesely, E. Svobodova, J. Chudoba, M. Sikorski and R. Cibulka, Adv. Synth. Catal., 2016, 358, 1654–1663 CrossRef CAS.
- C. K. Wu, T. J. Liou, H. Y. Wei, P. S. Tsai and D. Y. Yang, Tetrahedron, 2014, 70, 8219–8225 CrossRef CAS.
- M. Shibuya and Y. Iwabuchi, J. Synth. Org. Chem., Jpn., 2013, 71, 515–525 CrossRef CAS.
- W. C. Li, C. C. Zeng, L. M. Hu, H. Y. Tian and R. D. Little, Adv. Synth. Catal., 2013, 355, 2884–2890 CrossRef CAS.
- C. Christopher, S. Lawrence, M. A. Kulandainathan, K. Kulangiappar, M. E. Raja, N. Xavier and S. Raja, Tetrahedron Lett., 2012, 53, 2802–2804 CrossRef CAS.
- A. J. Bosco, S. Lawrence, C. Christopher, S. Radhakrishnan, A. A. J. Rosario, S. Raja and D. Vasudevan, J. Phys. Org. Chem., 2015, 28, 591–595 CrossRef CAS.
- N. Gunasekaran, Adv. Synth. Catal., 2015, 357, 1990–2010 CrossRef CAS.
- D. Betz, P. Altmann, M. Cokoja, W. A. Herrmann and F. E. Kuehn, Coord. Chem. Rev., 2011, 255, 1518–1540 CrossRef CAS.
- T. Seki and A. Baiker, Chem. Rev., 2009, 109, 2409–2454 CrossRef CAS PubMed.
- R. Ciriminna, M. L. Carraro, S. Campestrini and M. Pagliaro, Adv. Synth. Catal., 2008, 350, 221–226 CrossRef CAS.
- A. O. Chapman, G. R. Akien, N. J. Arrowsmith, P. Licence and M. Poliakoff, Green Chem., 2010, 12, 310–315 RSC.
- N. Z. Burns, P. S. Baran and R. W. Hoffmann, Angew. Chem., Int. Ed., 2009, 48, 2854–2867 CrossRef CAS PubMed.
Footnote |
| † Current affiliation: Division of Chemical and Petroleum Engineering, School of Engineering, London South Bank University, 103, Borough Road, London SE1 0AA, UK. |
| This journal is © The Royal Society of Chemistry 2016 |