Mapping students' modes of reasoning when thinking about chemical reactions used to make a desired product
M. L.
Weinrich
and
V.
Talanquer
*
Department of Chemistry and Biochemistry, University of Arizona, Tucson, AZ 85721, USA. E-mail: vicente@u.arizona.edu; Tel: +1-520-626-8169
First published on 15th February 2016
Abstract
The central goal of this study was to analyze the complexity of students' explanations about how and why chemical reactions happen in terms of the types of causal connections students built between expressed concepts and ideas. We were particularly interested in characterizing differences in the types of reasoning applied by students with different levels of training in the chemistry, from college to graduate school. Using a qualitative research approach, we identified diverse modes of reasoning expressed by students when engaged in the analysis of different sets of chemical reactions selected to produce a targeted compound. Main findings indicate that dominant modes of reasoning varied with educational level and the nature of the task. Although participants applied diverse modes of reasoning, linear causal reasoning was prevalent across educational levels and types of tasks. Many students tended to generate explanations based on the identification of a single agent that caused a sequential chain of events. Advanced undergraduate students in our sample generated the most complex explanations. The results of our study have important implications for the development of causal mechanistic reasoning in chemistry.
Introduction
Current efforts in science education across the world emphasize the need for students to more actively engage in science practices such as building explanations using models and generating arguments based on evidence (Osborne and Dillon, 2008; NRC, 2012). It is expected that students will learn how to use their scientific knowledge to build mechanistic explanations about systems and processes of relevance to them and to the societies in which they live. Educational research indicates that students have resources for productive mechanistic thinking but often struggle to explain phenomena using mechanistic accounts (Russ et al., 2008). Additionally, teachers frequently fail to pay attention to the substance of student thinking and to recognize both productive and constraining forms of reasoning, thereby missing valuable opportunities to support and guide the development of meaningful understandings (Coffey et al., 2011). Teachers' work would thus greatly benefit from a better understanding of how students reason with core concepts in a discipline and how that reasoning is likely to evolve as students progress in their studies.A central goal in chemistry education is to help students understand why and how chemical reactions happen. Educational research on students' ideas about chemical reactions is thus abundant but has mostly focused on eliciting alternative conceptions that students commonly express about these types of processes (Taber, 2002; Kind, 2004; Barke et al., 2009). Fewer studies have explored the actual structure of students' explanations of chemical phenomena seeking to characterize the nature of the explanations themselves (Taber and Watts, 2000; Kraft et al., 2010; Talanquer, 2010; Christian and Talanquer, 2012). A deeper understanding of how students connect ideas and build explanations in core areas of chemistry is needed to devise strategies that can better scaffold students' explanatory and argumentative skills.
To enrich the existing knowledge base on student reasoning in chemistry, in this study we analyzed the complexity of students' explanations about how and why chemical reactions happen in terms of the types of causal connections students built between expressed concepts and ideas. We were interested in characterizing differences in the types of reasoning applied by students with different levels of training in the discipline, from college to graduate school. Study participants were presented with diverse chemical substances and reactions used with the intention of making a desired product, and asked to engage in different tasks, from evaluating reaction feasibility to selecting proper reactants to achieve targeted goals. Hence, our results also shed light on the effect of different types of tasks on student reasoning.
We have already published in this journal a description of students' conceptual modes when analyzing the same set of chemical reactions described in the present study (Weinrich and Talanquer, 2015). In particular, we characterized different ways in which students conceptualized why chemical reactions happen (chemical causality), how these processes occur (chemical mechanism), and how they can be controlled (chemical control). In this contribution, we present an analysis of the same set of data but using a domain-general analytical framework focused on the characterization of the types of causal connections that students built as they engaged in the research tasks. The results of our two studies are complementary and interested readers may want to consult the related prior publication (Weinrich and Talanquer, 2015) to develop a more comprehensive picture of our research goals. In general, our work is part of a larger project focused on the characterization of changes in students' chemical thinking with training in the domain (Sevian and Talanquer, 2014).
Students' explanations about chemical phenomena
A significant body of research in chemistry education has focused on the analysis of secondary school students' ideas about chemical change and what happens to matter during different chemical processes (Andersson, 1990; Ahtee and Varjola, 1998; Boo and Watson, 2001). These studies have revealed that many students struggle to understand the concept of chemical substance (Stavridou and Solomonidou, 1998) and tend to rely on surface macroscopic features of reactants and products rather than on submicroscopic models of matter to predict the outcomes of chemical reactions (Hesse and Anderson, 1992). Findings from research conducted at the post-secondary level indicate that more advanced chemistry students (undergraduate and graduate) also focus on surface features when reasoning through reaction mechanisms and do not attribute meaning to the symbols used to represent changes in chemical structure during a reaction (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012).Analyses of students' explanations for different types of chemical phenomena suggest that pupils often build pseudo-explanations that tend to be vague and circular and are based on a combination of intuition with fragmented pieces of chemical knowledge (Taber and Watts, 2000). Students often talk about chemical processes as driven by active agents acting on more passive agents (Hatzinikita et al., 2005; Talanquer, 2006; Taber and García-Franco, 2010), and attribute intentionality to the behaviour of diverse chemical entities (Taber, 2013; Talanquer, 2013). Some authors have sought to characterize students' explanations of chemical reaction based on different ways of understanding and talking about these processes (Solsona et al., 2003; Weinrich and Talanquer, 2015). Results from these studies suggest that students may express different conceptualizations of chemical reactions depending on the context.
Students at all educational levels struggle with multi-variate thinking and rely on reasoning heuristics to simplify chemistry problems and reduce cognitive load (Talanquer, 2006; Bhattacharyya, 2014). They often rely on recognition, similarity, and one-reason decision making to guide their thinking (Maeyer and Talanquer, 2013; Graulich, 2015). Student reasoning when engaged in solving organic chemistry tasks such as predicting the product of chemical reactions and proposing reaction mechanisms has been categorized into three main types: rule-based, case-based, and model-based reasoning (Kraft et al., 2010; Christian and Talanquer, 2012). This classification is based on the type of knowledge (i.e., rules, cases, models) applied by an individual to propose an explanation or make a prediction. In general, students at the post-secondary level have been found to more frequently rely on rule-based reasoning than on model-based reasoning when facing academic tasks.
Theoretical framework
Various authors have sought to characterize different levels of complexity in students' understanding of a subject as manifested in students' performance in diverse tasks. For example, Biggs and Collis (1982) proposed a hierarchy of stages in student understanding in many different fields based on the number of knowledge elements used and on their level of integration (Structure of the Observed Learning Outcome, SOLO taxonomy). The SOLO taxonomy includes five major stages in student understanding, from a pre-structural level in which knowledge is highly fragmented to an extended abstract stage in which multiple connections are built between concepts and ideas can be generalized and transferred across domains. The SOLO taxonomy has guided the development of other frameworks for the analysis of both student understanding in science (Claesgens et al., 2009; Brown et al., 2010) and the types of tasks used to assess student knowledge (Bernholt and Parchmann, 2011).For example, Claesgens et al. (2009) proposed a conceptual framework to characterize how high school students learn to reason like chemists as they develop explanatory models in chemistry. Judgments of levels of performance in this framework, from pre-structural to generative, were based on the analysis of both conceptual understanding and type of reasoning demonstrated in a student's answers. On the other hand, Brown et al. (2010) developed a construct map to assess student's conceptual depth in science domains by analyzing the number of causal elements in students' responses and the relationships between them. These authors' construct map included different kinds of cognition such as acausal, causal unjustified, causal justified, multiple, and emergent. These causal models share many similarities with those proposed by Grotzer (2003) in their analysis of the complexity of interactions in different types of causal explanations.
Recently (Sevian and Talanquer, 2014), we have suggested that student understanding in different areas in chemistry can be assessed based on both the nature of the underlying assumptions students make about the structure and properties of chemical entities and phenomena (conceptual modes), and the complexity of student reasoning in terms of their ability to connect ideas, build justifications, make decisions, and construct complex explanations (modes of reasoning). In that work, we proposed a generic set of potential modes of reasoning derived from the research described in the paragraphs above (Biggs and Collis, 1982; Grotzer, 2003; Brown et al., 2010). These modes of reasoning were labelled as descriptive, relational, linear causal, and multi-component. The detailed definitions of each of these modes of reasoning can be found in the work by Sevian and Talanquer (2014). Our exploration of student reasoning about chemical reactions used to generate a desired product was guided by this analytical framework, which we adapted and modified (see Table 2 in the Findings section) to better represent the modes of reasoning expressed by our study participants.
Goals and research questions
The central goal of this study was to characterize the modes of reasoning expressed by students with different levels of training in chemistry when analyzing various chemical reactions. In particular, our study was guided by the following research questions:• What modes of reasoning are more commonly present in students' explanations of the feasibility of chemical reactions selected to generate a targeted product?
• How do these modes of reasoning vary depending on students' level of training in the domain and the type of task that students confront?
Research design
Context and participants
This research was conducted at a large research-intensive public university in the United States, where approximately 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 undergraduate and graduate students were in attendance at the time of the study. The student body was 52% female and 48% male. The ethnic diversity was 56% Caucasian, 20% Hispanic, 22% other minorities, and 2% unknown. A range of educational levels were recruited in order to capture diverse modes of reasoning using semi-structured interviews. The participants (N = 71) included undergraduate students in the first semester of general chemistry (N = 16; labelled from I-A to I-P), in the second semester of organic chemistry (N = 15; labelled O-A to O-O), and advanced students in the last semesters of their undergraduate chemistry studies (N = 9; labelled U-A to U-I). We also interviewed first-year graduate students in chemistry and biochemistry (N = 15; labelled G-A to G-O) and PhD candidates in their third to sixth year of graduate school (N = 16; labelled C-A to C-P). PhD candidates represented 13 different research groups (3 Analytical, 2 Biochemistry, 8 Organic, 3 Physical) and 11 of these students performed synthesis in their research. Undergraduate student participants were recruited through announcements in their chemistry courses; PhD candidates were recruited via email. All participants volunteered and consented to participate in the study without any type of reward.
000 undergraduate and graduate students were in attendance at the time of the study. The student body was 52% female and 48% male. The ethnic diversity was 56% Caucasian, 20% Hispanic, 22% other minorities, and 2% unknown. A range of educational levels were recruited in order to capture diverse modes of reasoning using semi-structured interviews. The participants (N = 71) included undergraduate students in the first semester of general chemistry (N = 16; labelled from I-A to I-P), in the second semester of organic chemistry (N = 15; labelled O-A to O-O), and advanced students in the last semesters of their undergraduate chemistry studies (N = 9; labelled U-A to U-I). We also interviewed first-year graduate students in chemistry and biochemistry (N = 15; labelled G-A to G-O) and PhD candidates in their third to sixth year of graduate school (N = 16; labelled C-A to C-P). PhD candidates represented 13 different research groups (3 Analytical, 2 Biochemistry, 8 Organic, 3 Physical) and 11 of these students performed synthesis in their research. Undergraduate student participants were recruited through announcements in their chemistry courses; PhD candidates were recruited via email. All participants volunteered and consented to participate in the study without any type of reward.
Instrument and data collection
Individual semi-structured interviews were used to explore students' reasoning. The interview began by asking students to generally define what is important in a chemical synthesis. We used the term “chemical synthesis” in our research instrument to represent chemical reactions used to make a desired product. Then, the participants were asked three different types of problems: (a) compare the easiness of chemical synthesis (4 questions); (b) design the synthesis of a compound (3 questions), and (c) evaluate the feasibility of a proposed reaction (1 question). The specific question prompts are shown in Table 1. Throughout the interview we used relatively simpler looking compounds or structures in order to assess introductory students' ways of thinking. We designed these qualitative tasks to involve science practices such as explaining, predicting, evaluating, and designing which are major aspects of chemists' work (Sevian and Talanquer, 2014). Additionally, we created open-ended questions with different levels of difficulty that could be approached with different complexity of reasoning. For example, question two asked students to compare the easiness of three different processes. These were presented in a format that students may encounter in a general chemistry course but are also very complex reactions and have an extensive research history and importance in the fuel industry (Davis and Occelli, 2007). Thus, the selected reactions created opportunities to uncover different types of reasoning. We anticipated that novices might make intuitive associations and focus their attention on surface features of the representations such as different stoichiometric coefficients or might focus on their familiarity with certain substances such as methane (Maeyer and Talanquer, 2013; Graulich, 2015). We considered that more complex forms of reasoning might incorporate multiple different factors into an analysis such as bond strength, rate, enthalpy and entropy of reaction. Pilot interviews with an introductory student, a graduate student, and a professor were used to test questions for readability, understandability, and appropriateness. Their feedback was used to create the final version used in this study.Before the interviews began we emphasized to the students that we were not interested in whether they provided right or wrong answers but instead how they were thinking through the problems. Students were provided with a periodic table and paper to write on, and asked to think aloud. During the interview we posed additional questions to explicitly explore participants' thinking about the feasibility of the chemical reaction they chose or proposed, how the reaction might proceed to form the products, and why the chemical reaction could or could not happen. Each interview lasted approximately 20–60 minutes and was audio recorded and transcribed. Artifacts, such as participant's drawings, were also collected. This research project received approval from the Human Subjects Protection Program at our institution.
Data analysis
Transcripts of the interviews were carefully read and tentatively coded to identify major themes. We used the analytical framework proposed by Sevian and Talanquer (2014) to begin our identification of different modes of reasoning. An iterative process was employed where code categories and themes were constantly revisited, compared, and modified as the coding process occurred (Charmaz, 2006). Constant discussion and reflection involving two researchers were used to ensure reliability in the data analysis. One researcher analyzed all transcripts and the second researcher separately analyzed a randomly selected set of student answers (100 of the 639 responses to prompts, 16%). There was 88% agreement in the coding of these two researchers for the selected responses. In most cases, differences in coding were associated with initial divergences in how each researcher interpreted some codes that were resolved through discussion. Differences also occurred when students' expressed ideas that were difficult to understand. Only questions two through nine in our interview protocol were analyzed as part of this study, since question one was a general question about chemical synthesis. A single code was assigned to a student's response to each of these questions based on the dominant mode of reasoning applied in generating the answer.Iterative analysis of students' responses led to the identification of the different modes of reasoning described in Table 2. We kept track of the frequency with which elicited modes of reasoning manifested in each of the responses provided by all study participants. Different modes of reasoning applied across an individual interview were analyzed to identify the number of prompts where a student's overall mode of reasoning was the same, determine how many different overall modes of reasoning were applied across an interview for individual students, and characterize a student's interview as either using one main mode, split between two main modes, or spread across many different types of modes.
| Modes of reasoning | Description | Frequency (%) |
|---|---|---|
| Descriptive | Phenomena are re-described by asserting that things are as they are without referring to causes. Focus mainly on explicit salient features of a system. Strong influence of surface similarity and recognition on judgment and decision making. | 4 |
| Relational | Correlations between properties and behaviors are established but not explained or justified. Explicit and implicit features of a system are noticed. | |
| Uni-relational: explanation based on a single relation | 18 | |
| Multi-relational: explanation based on multiple relations | 8 | |
| Linear-causal | Although the influence of many factors may be recognized, phenomena tend to be reduced to the result of the actions of a single agent on other entities; proposed mechanisms involve linear cause–effect relations and sequential chains of events. Explicit and implicit features of a system are noticed. | |
| Linear chain: simple causal chains are used in explanations | 33 | |
| Linear chain multi-relational: a combination of simple causal chains and unjustified correlations are used in explanations. | 16 | |
| Multi-component | Phenomena are seen as the result of the static or dynamic interplay of more than one factor and the direct interactions of several components. Causal stories are built. Explicit and implicit features of a system are noticed. | |
| Isolated: effects of several variables are considered and weighed separately. | 18 | |
| Integrated: explanations as interconnected stories of how different variables affect the entities involved. | 3 | |
Findings
The analysis of students' explanations and justifications led us to characterize the different modes of reasoning described in Table 2. They included the four major groups: descriptive reasoning, relational reasoning, linear causal reasoning, and multicomponent reasoning, with additional subcategories to better characterize the data.The presentation of the modes of reasoning in Table 2 is not intended to imply a hierarchy from less to more desirable ways of thinking in all circumstances; instead it describes a hierarchy of complexity in reasoning. Depending of the task, one type of reasoning may be more productive than other. For example, not all questions in chemistry demand paying attention to the effects of multiple variables and their interactions. Similarly, simplified relations are often used by experts when thinking about complex problems. We also included in Table 2 the relative frequency of each mode of reasoning across all interviews. In general, linear causal reasoning was most commonly expressed by students in our sample. However, the relative frequency of a given mode of reasoning varied with the educational level of the students (Fig. 1a) and the nature of the prompt (Fig. 1b). The percentages of responses represented in Fig. 1 are a measure of the frequency with which elicited modes of reasoning manifested in all answers provided by students at each level (Fig. 1a) and in the answers from all students to each question (Fig. 1b). In the following sections we summarize major characteristics of the different types of modes of reasoning identified in our study.
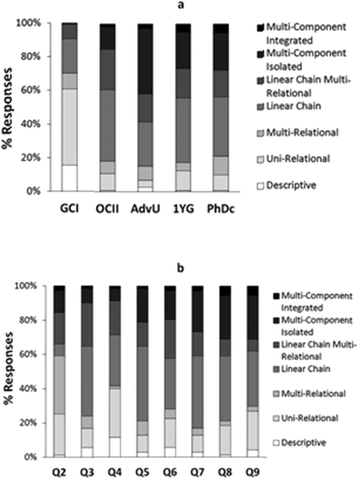 | ||
| Fig. 1 Relative percentage of study participants' responses expressing a given mode of reasoning by (a) educational level (GCI – General Chemistry I, OCII – Organic Chemistry II, AdvU – Advanced Undergraduate, 1YG – First Year Graduate, PhDc – Advanced Graduate), and (b) question prompt (see Table 1). | ||
Descriptive
This mode of reasoning was identified in 4% of overall responses to the interview questions (Table 1). Students who expressed this type of reasoning answered questions by simply describing explicit salient features of chemical substances and reactions, and tended to rely on recognition or recall of a familiar substance (or part of a substance) while making decisions or justifying choices. The following excerpt from student I-B's response to Question 4 illustrates this mode of reasoning:I-B Q4: I want to guess the hydrogen and chlorine […] I'm trying to think of why I feel that way uhh… Because! That's my reason, because. Because… […] ok um because… I'm guessing that's why, that's why, because I'm guessing and I just like hydrogen and chlorine […]
Interviewer: do you know why you like HCl more?
I-B Q4: because I'm used to it, like I don't see fluoride as much or whatever it is, I see hydrogen and chlorine more often, that's why I like them
When asked to compare synthetic processes, this student focused on an explicit salient feature of the problem (names of atoms: hydrogen and chlorine) and justified the choice based on familiarity with the substances involved. Similarly, other students judged the likelihood of a chemical process based on the familiarity with reactants and products, reasoning that a known substance will be more stable and thus more likely to be formed (or less likely to react). Overreliance on recognition and familiarity in student judgment and decision making in chemistry has been highlighted in prior studies (Maeyer and Talanquer, 2013; Graulich, 2015).
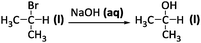
The descriptive mode of reasoning occurred relatively infrequently in the explanations generated by all groups of students in our sample, with the largest percentage corresponding to general chemistry students (Fig. 1a). This mode of reasoning was cued most often by question four (Fig. 1b) which involved substances common in daily life (NaCl) and, as shown in the following excerpt, judged by some students as easier to synthesize:
Interviewer: Here are three reactions using a similar process which one of these three compounds, sodium fluoride, chloride, or bromide would be easier to synthesize?
I-E Q4: I want to say, the sodium chloride only because I'm familiar with sodium chloride
Interviewer: ok so the sodium chloride reaction being easiest, is there anything other than its familiarity, you've seen it much more, that would make that reaction easier?
I-E Q4: not that I know of
Relational
Close to 26% of all responses to our interview questions were categorized as expressing relational reasoning in which students built simple associations to explain or justify their decisions. In this relational mode students paid attention to either explicit (e.g. symbolic representation of substances, HF) or implicit (e.g. electronegativity) salient features of chemical entities while building simple correlations. Although students who expressed this mode of reasoning often relied on familiarity with the substances involved in a reaction to guide their reasoning, they also referred to their properties to make claims about chemical reactivity. While applying this mode of reasoning, some students reduced the number of variables they paid attention to and based their justifications on a single entity or property. The following excerpt illustrates this type of uni-relational reasoning:Interviewer: how likely do you think this reaction is?
I-J Q2: very likely compared to these other two
Interviewer: ok, because?
I-J Q2: because it's like CH4instead of like, I feel like I see this a lot more in chemistry than any of these other molecules […]
Interviewer: ok cool, is there anything else that makes this reaction easier than the other two reactions?
I-J Q2: because, or actually does it have anything to do with like… are these balanced already?
Interviewer: they're already balanced
I-J Q2: oh ok, because there's less of it so there's not as much concentration I guess that you have to deal with, like when you have to do with like 3 H2and one CO it's not as much as having to like completely synthesize like 7 H2and 3 CO
Initially, this student relied on familiarity with CH4 to make a choice. When prompted, this study participant related the amounts of reactants represented in the chemical equation with the easiness of the process by building the intuitive association “the fewer the amounts, the easier the process.” Other students relied on overgeneralized rules learned in their chemistry courses to make choices and justify decisions. For example, they would express that certain features of a substance would make it more stable (such as the presence of double bonds) or more reactive (such as the presence of electronegative atoms), and use the presence or absence of these features to judge the likelihood of a reaction. It is important to point out that this type of reasoning is not necessarily wrong or unproductive when thinking about chemical reactions. Consider, for example, the following interview excerpt:
G-G Q6: it looks like he's going just nucleophilic substitution and if it's SN2 it's sort of iffy on a secondary, so it could work I think
Interviewer: ok why would it be iffy on a secondary?
G-G Q6: just some sort of steric reasons, I don't remember, if it's tertiary it wouldn't work by SN2, secondary I'm not sure, primary it probably would work
In this case, the student successfully reasoned through the problem by using a set of productive rules used in chemistry to relate molecular structure with chemical reactivity for the specific type of reaction under analysis.
A small fraction (8%) of the responses categorized as expressing relational reasoning corresponded to cases in which students' explanations invoked more than one association or rule. The following excerpt is representative of this type of multi-relational reasoning:
Interviewer: Here are three reactions, using a similar process, which one of these three compounds, methane, propane, or hexane would be easier to synthesize?
I-O Q2: um not sure let's see… I guess to a liquid to me would maybe be a little bit more difficult because you would have to, I think, lower pressure or raise pressure […] I would just say a gas to a gas might be a little bit easier you wouldn't have to worry as much about changing pressure or temperature for it […]
Interviewer: ok and you talked about the gases versus liquids, is there anything else that makes this reaction easier than the other two reactions?
I-O Q2: […] I guess if it's carbon and hydrogen and it's going to react the same way that carbon and hydrogen do I guess the fewer molecules that you have the faster it would form, I just don't know why they make different compounds or how that happens yet so
This student built two relations while answering this problem. First, the study participant related the state of matter with the easiness of making a product (gases are easier to synthesize than liquids). Then, when prompted the student correlated the number of molecules of reactants with the speed of the process (the fewer the number of molecules, the faster the process).
Relational modes of reasoning occurred less often than linear causal reasoning (Table 2) but were the second most frequent modes elicited from our data. Relational reasoning was the most prominent type of reasoning expressed by general chemistry students (Fig. 1a) and decreased in relative frequency for the other educational levels. Across different prompts (Fig. 1b) relational reasoning was the most prominent mode of reasoning only in question two. This may have been due to different factors. On the one hand, most students may have been unfamiliar with the mechanism for this particular set of reactions. On the other hand, student reasoning could have become more complex as a participant progressed through the interview and constant questioning triggered additional chemical knowledge.
Linear causal
A majority of students' explanations (49%) relied on linear causal reasoning in which chemical phenomena were described as sequential chains of events with causes and effects. Students who expressed this type of reasoning identified entities, explicit or implicit, that had particular properties and were engaged in particular activities or interactions. In general, these study participants tended to focus on a main substance or entity as if it were the major agent driving the process. The actions of this agent and its interactions were described as a sequence of events unfolded (“A causes B causes C”) in the reaction (linear chain). Often, this active agent was described as acting with a particular purpose or intention (teleological reasoning). When engaged in this mode of reasoning, students often referred to various salient features but reduced the number of factors they paid attention to in making their decision. The following interview excerpt illustrates this linear chain mode of reasoning:C-H Q7: the electrons from the nitrogen would attack the carbonyl carbon displacing the electrons on the carbon oxygen bond and then the um… electrons from the oxygen would kick back down, and the chlorine would leave um leaving this NH3amide species and then the chlorine atom comes in and takes the extra hydrogen off of the nitrogen and you're left with just the amide
In this case, the student described a sequence of events where an agent (electrons) was involved in a series of activities (attacking, displacing, kicking). The student described interactions between substances that resulted in a particular effect (synthesis of the amide product).
Some students' explanations (16%) were characterized as expressing linear chaining combined with multiple instances of relational reasoning. The following quotation illustrates this linear chain multi-relational mode of reasoning:
O-J Q7: Acyl chloride, right right this is an acyl transfer reaction or something like that, which was also on the last test, and then NH3, because NH3will form a base [amide] and that's more stable than the acyl chloride is
Interviewer: ok so the NH3will form a base um can you describe to me what that base is that you're talking about?
O-J Q7: I don't know what it is called… an amide
Interviewer: […] what happens to form the product?
O-J Q7: So NH3adds and then the double bond on the oxygen goes up and makes um oxygen anion and then the Cl is still attached so it's a tetrahedral intermediate and then it goes based on stability… so because Cl is the weaker link it's the one that when the electron pair wants to go back down and make a double bond again, Cl will get kicked out because NH3is much more stable
Interviewer: ok so the reaction proceeds because the Cl is the weaker link you said
O-J Q7: yea
Interviewer: and so the NH3is more stable
O-J Q7: right because the Cl is the weaker base
This student began the explanation by recognizing a type of reaction (acyl transfer reaction) and then built upon this recognition by using a rule (an amide is more stable than an acyl chloride) to justify their choice of reagents. When prompted, the student then described the actions of an agent (NH3) which initiated a sequence of events on a submicroscopic level. During this sequence of events different parts of the substances interacted resulting in a particular effect (synthesis of the amide product). The student described one of the entities in the sequence of events as acting intentionally or with a particular purpose (the electron pair wants to go back down). Additionally the student used a rule to justify what happened in the sequential story (Cl is the weaker link because it is a weaker base).
Linear causal reasoning was the most frequent mode of reasoning used by our study participants (Table 2). Across different educational levels (Fig. 1a), this mode of reasoning was demonstrated infrequently by general chemistry students, but was the most frequent mode of reasoning for all other groups. Across different prompts (Fig. 1b) linear causal reasoning was the most prominent in every question except for question Q2 where relational reasoning was more dominant. We observed a slight increase in the manifestation of this mode of reasoning in questions Q3, Q5, and Q7 which asked students to design and evaluate a synthesis rather than to compare and evaluate different synthetic processes.
Multicomponent
Close to one fifth of students' explanations (21%) invoked multiple causal elements that affected and drove chemical processes. Similarly to the linear causal mode of reasoning, students who expressed this type of thinking described events as involving causes and effects. However, they explored and weighed the effects of multiple salient factors. These causes and effects were described either as somewhat isolated linear-chains of events or as networks of events. We characterized these two forms of reasoning as multicomponent isolated and multicomponent integrated, respectively.Students who applied multicomponent isolated reasoning reasoned through the problems by considering the effects of multiple salient entities that acted in a rather independent manner. The outcome of a chemical processes thus appeared to be the additive result of the properties and actions of these multiple agents. Consider, for example, the following interview excerpt:
U-B Q8: I think the first would be easiest to synthesize, the third reaction I could see that first compound being really reactive because it's got the I don't know why I can't, the three membered ring um which to me is really really unstable because you have this really tight bond angles so I could see that being really reactive but not in the way that's shown there where is keeps that three membered ring, I'd think that you would have something where the water would attack to break open that ring or somehow it would split open […]
Interviewer: Is there anything else that makes the top reaction easier than the bottom two?
U-B Q8: I don't know how big of an effect they are but carbons are somewhat electron donating so you may have the, it would make the carbonyl carbon less electropositive, and less vulnerable to attack from the water than the upper compound where you don't have any real electron with, or donating effects
This student referred to various casual elements but discussed them separately. The explanation began with a discussion of the potential high reactivity of one of the substrates due to its molecular structure (three membered ring, tight bond angles). Then, the student separately discussed the effects of a different factor (electron donating carbons) and described how this electronic feature would affect the interaction between reactants.
In very few instances (3%) students reasoned through problems by building a network of multiple interconnected causal elements with particular properties. The outcome of a chemical reactions emerged from these multiple interactions. The following except is representative of this type of multicomponent integrated mode of reasoning:
G-M Q2: I would, and this might be completely wrong, but I would probably say that the first one would be easiest to synthesize since you're decreasing entropy the least since you are going from four gas molecules to four gas molecules as opposed to the others which are going from a lot more gas molecules, the last one you're going to two liquids but that results in even less entropy than a gas and a liquid, so yea I would go with the first one
Interviewer: ok can you tell me a little bit more about entropy?
G-M Q2: sure, I don't remember exactly which law it is which is kinda sad uh the disorder in a system never decreases, it increases or stays the same so if you're going to decrease the entropy of a system you have to increase the entropy of somewhere else and so generally when I'm looking at these reaction systems I think either there is going to be, likely be some heat released in these reactions which is of course going to increase the entropy of the atmosphere still balancing it out but I still would probably say the first one is going to be the easiest to do
Interviewer: ok can you tell me a little bit more about the heat changing the entropy of the atmosphere?
G-M Q2: sure just in terms of if you're adding more heat to the atmosphere you are increasing the kinetic energy of the molecules you're moving around more, they're having more collisions in general creating more disorder, I would like to go into the actual statistical meaning of disorder but I can't remember it right now
Interviewer: ok and how do you know that this reaction will give off heat?
G-M Q2: um so what I'm comparing it to in my mind is the reaction of hydrogen with oxygen to produce water, you know generally you're going to have to give it some sort of ignition source to get it started but when that reaction goes it's violent and releases energy in all types of forms, explosion, so some light, and some heat, some sound, so that's kind of what I am basing it off of
Interviewer: and this looks similar as
G-M Q2: as producing water from hydrogen and oxygen
Interviewer: cool, what else makes this reaction easier than the other two reactions?
G-M Q2: hmm… let's see… I'm not sure
Interviewer: how likely do you think this reaction would be?
G-M Q2: spontaneously just by itself? I don't think it would be too likely, similar to having hydrogen and oxygen floating around in the air I wouldn't expect those things to react unless they were in really concentrated amounts and probably had some source of energy input whether that be a flame or something like that
Interviewer: ok, so there… under certain conditions it wouldn't be possible but under other conditions it would be possible… so some sort of flame
G-M Q2: some source of ignition combined with higher concentration
Interviewer: ok and then can you tell me anything about why those things would make this reaction
G-M Q2: go?
Interviewer: yea more feasible?
G-M Q2: so um as I look at those, you have carbon oxygen bond in carbon monoxide, triple bond and so you will have to overcome the strength of those bonds the stability of them to break them and form new bonds, the fact that the reaction would go, those new bonds that are produced are in the end will be lower in energy but you have to add some energy to the system at first to essential break whatever bonds are there and then reform the more stable ones
Through this interview this student built an integrated description of how multiple salient entities affected the various events and outcome of the chemical process under analysis. With constant prompting, this study participant described a variety of interconnected phenomena (released heat affecting entropy, ignition source to start the reaction, formation of lowered energy stable bonds) to reason through the problem.
The relative frequency of multicomponent modes of reasoning in students' explanations was lower among the general chemistry and organic chemistry students in our sample (Fig. 1a). On average, the relative frequency of these forms of reasoning tended to increase across the interview (Fig. 1b). This may have been due to the nature of the interview where students were continually asked to tell more about what they were thinking, or to the sequence of questions in our interview protocol which became more representative of the types of organic chemistry problems often discussed in mechanistic ways in post-secondary chemistry courses.
Modes of reasoning for individual participants
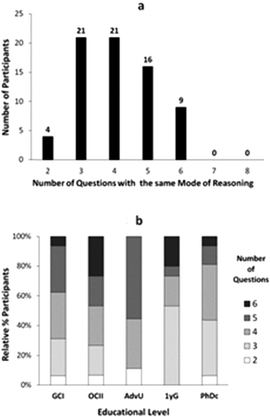
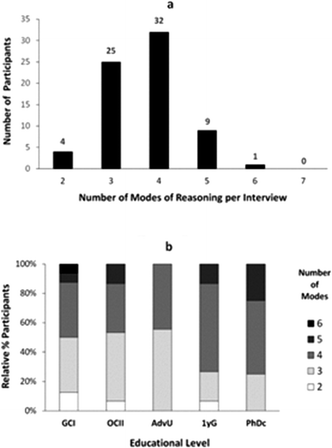
As part of our analysis, we sought to characterize how different modes of reasoning manifested throughout the individual interviews conducted as part of our study. Let us first describe the number of questions, of eight total, that were answered by our study participants using the same mode of reasoning (Fig. 2).As shown in Fig. 2a, there were no students in our sample who used the same mode of reasoning in their responses to all the questions, and only a few of them (nine) used the same mode of reasoning in three-quarters of the questions (6 out of 8 questions). Nevertheless, more than half of the participants (46/71) applied the same mode of reasoning in answering at least half of the questions (4 or more). Across different educational levels (Fig. 2b), the percentage of undergraduate students who applied the same mode of reasoning to different questions was larger with more training in the discipline. This trend changed, however, for the graduate students who on average applied the same mode of reasoning in fewer cases.
The analysis of the number of different modes of reasoning used by study participants in their interview revealed that most students applied three to four different modes (Fig. 3a). Only four students were highly consistent and expressed only two modes of reasoning, while there were ten students who applied most of the modes of reasoning identified in our study. Across different educational levels (Fig. 3b), we observed a decrease in the number of different modes of reasoning applied by the more advanced undergraduates compared to both lower undergraduate and graduate students.
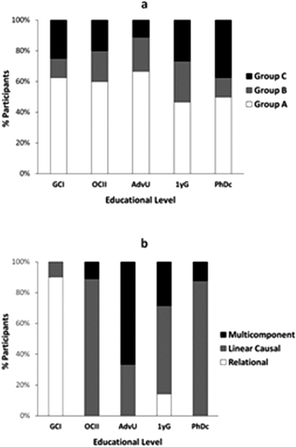
In general, we found that individual participants' interviews could be classified into three major groups:
• Group A: expressing one main mode of reasoning (one mode of reasoning was applied to at least 4 of the 8 prompts; 40 students)
• Group B: expressing two main modes of reasoning (two different modes were applied in 3-4 prompts each; 13 students)
• Group C: expressing many different types of modes of reasoning (18 students)
The distribution of students into these different groups was similar across educational levels (Fig. 4a), with the advanced undergraduate students showing the least spread and the advanced graduate students demonstrating the largest spread between different modes of reasoning. Analysis of the dominant modes applied by students in Group A elicited major switches in reasoning for participants with different levels of training in chemistry (Fig. 4b): from mainly relational reasoning at the general chemistry level to mainly linear causal reasoning at the organic chemistry level followed by a gradual increase of multicomponent reasoning with advanced undergraduates and a regain in prominence of linear causal reasoning at the graduate level.
Conclusions
The central goal of our investigation was to explore the different modes of reasoning expressed by students with different levels of training in chemistry when thinking about chemical reactions selected to produce a targeted product. We uncovered the major modes of reasoning summarized in Table 2 which were expressed by most of our study participants to some extent. More than half of the students in our sample applied the same mode of reasoning when answering at least half of the eight interview questions, but none of the participants applied the same mode of reasoning across the whole interview. In general, the expression of different types of reasoning was affected by both the educational level of the students and the nature of the question they confronted. Our findings complement the results of a previous study (Weinrich and Talanquer, 2015) in which we analyzed the conceptual modes expressed by same group of students (i.e., the nature of the chemistry concepts and ideas that were used to think about the same set of chemical reactions).Linear causal reasoning was the major mode of reasoning applied by our study participants across educational levels, but was particularly dominant in the thinking of undergraduate students who were completing the second semester of organic chemistry and among graduate students. The more novice students enrolled in the first semester of general chemistry were more likely to apply relational reasoning when thinking about chemical reactions, while advanced undergraduate students expressed multicomponent reasoning at a higher frequency than other groups. In our sample, there was an increase in the level of sophistication and a decrease in the diversity of modes of reasoning expressed by undergraduate students with more training in chemistry. Both trends were reversed, however, in moving from advanced undergraduates, to first-year graduate students to advanced graduate students. Given the qualitative nature of our investigations one has to be cautious with generalizations. Our study participants may not be representative of their respective populations. However, the sharp differences observed in the reasoning expressed by those students who more consistently applied the same mode (Group A in our sample) suggest that training in chemistry actually affect the type and frequency of the mode of reasoning that is most commonly deployed.
Based on our findings, one could speculate that novice chemistry students with limited knowledge would be more likely to base their explanations on simple associations with weak causal links (relational reasoning). Completion of introductory college chemistry courses seems to have a major effect on student reasoning, increasing the prevalence of mechanistic explanations for chemical reactions based on linear chains of events triggered by active agents and their interactions (purposeful or not). Further training in the discipline seems to help students enrich their explanations by the consideration of a variety of factors affecting chemical reactivity (multi-component reasoning). However, graduate students in our sample did not express the highest level of complexity in their explanations, nor the highest consistency in the application of particular modes. This result suggests that more advanced knowledge may lead individuals to build less sophisticated but more targeted and productive explanations. Expert chemists are known to rely on simple correlations and causal links to make sense of chemical processes, particularly when thinking about well-known classes of reactions. Although graduate students in our sample applied multi-component reasoning less frequently than advanced undergraduates, they expressed a wider diversity of modes which may suggest higher ability to adapt explanations to particular types of problems (Chi, 2011). The construction of valid and productive chemical explanations and predictions does not always require the highest level of complexity in mode of reasoning but an adaptive response based on the task at hand.
The types of questions that were asked in the interview also seemed to influence the modes of reasoning used by our study participant. For example, the question involving common acid-base reactions (Question 4) tended to cue more descriptive reasoning than other problems. The presence of well-known substances in the reactants and products (e.g., NaCl) seemed to trigger the application of a recognition heuristic by several students (Maeyer and Talanquer, 2013; Graulich, 2015). On the other hand, Question 2 tended to cue more relational reasoning. In contrast to questions 5 through 8 in the interview protocol, which involved organic substances and reactions with which many of our study participants could be expected to be familiar, Question 2 presented a set of reactions whose mechanism was likely unknown to the students. This lack of familiarity may be responsible for the increased lack of sophistication in student reasoning. We also observed a slight increase of linear causal reasoning in questions 3, 5, and 7 compared to other problems. These questions asked students to the design and evaluate a synthetic route to generate a targeted product. Although further investigations are needed to explore this effect, it is possible that the prompt may have led students to build causal links to justify their selection of reactants but constrained the application of more complex reasoning given the goals of the task (i.e., design a process rather than compare different alternatives). The application of different modes of reasoning when engaged in tasks based on an engineering model (design) versus a scientific model (explain) has been discussed by other authors (Schauble et al., 1991).
Implications
The planning, instructional, and assessment efforts of most science teachers and instructors at all educational levels are often focused on the development of students' understanding of concepts and ideas deemed important in a discipline. Much less attention has been paid to the analysis of the modes of reasoning that we would like students to develop and apply in different contexts, and to the implementation of instructional and assessment strategies to scaffold such type of learning. Research has shown that although teachers and instructors may consider important that students develop the ability to generate causal mechanistic accounts of diverse phenomena, their instructional and assessment practices are often not aligned with their expressed learning objectives (Coffey et al., 2011; Windschitl et al., 2012).Our results suggest that students entering college in the United States are not necessarily prepared to use their chemical knowledge to build mechanistic explanations of chemical phenomena. A majority of general chemistry students in our sample relied on relational reasoning to build their explanations. Recent reform efforts in pre-college science education in the US are directed at addressing this problem (NRC, 2012, 2013), but their success will strongly depend on the professional development of teachers to direct their attention to both the substance and structure of student reasoning. Confronting this problem at the college level may be even more complex, given the common resistance among science faculty to rethink curricular, instructional, and assessment practices.
College students in our sample demonstrated the ability to apply different types of reasoning when thinking about chemical reactions. However, we found a prevalence of one reason decision making in which explanations were frequently reduced to the effect of a single agent acting purposely to achieve some goal. Several authors have discussed students' struggles with multi-variate thinking in the analysis of chemical systems (Kraft et al., 2010; Christian and Talanquer, 2012; Bhattacharyya, 2014). Addressing this problem may require drastic changes in the college classroom (NRC, 2015). Students need more opportunities to apply their knowledge in different contexts, actively engage in the construction of diverse explanatory accounts, and individually and collectively reflect on the scope and limitations of different types of explanations. The results of our study suggest that it may be beneficial for students to not only recognize the existence of different modes of reasoning in chemistry, but also to reflect on the types of situations in which each of these modes of reasoning may be necessary or more productive to apply.
Existing research findings show that engaging students in constructive and interactive learning tasks (Chi and Wylie, 2014) fosters the development of more sophisticated modes of reasoning. In these types of tasks, students are often asked to collaboratively analyze data, develop reasonable models to explain major patterns in the data, and evaluate the scope and limitations of the models proposed by different learners. Unfortunately, common curricula and approaches to teaching in chemistry often fail to consistently create learning opportunities in which students engage in building and evaluating models while teachers provide regular formative feedback.
Fostering and scaffolding the ability to generate mechanistic accounts of chemical phenomena demands that teachers and instructors at all educational levels learn to notice student reasoning, can recognize differences and productive elements in students' thinking, and know how to best respond to students' ideas (Coffey et al., 2011). The findings of our investigation should thus be of interest to teacher educators who seek to help prospective and in-service teachers implement more responsive ways of teaching (Robertson et al., 2016). Our results provide a map of the different modes of reasoning that chemistry instructors will likely see expressed by their students when thinking about chemical reactions.
In general, we are convinced that chemistry education around the world needs to advance by more carefully reflecting on the types of reasoning that are used in the discipline (i.e., the nature of chemical thought), the actual ways of thinking that are modelled and expected in the classroom (i.e., the focus of school chemistry), and the conceptual modes and modes of reasoning that students are likely to apply when facing chemistry tasks (i.e., the nature of student reasoning in chemistry). Our study provides valuable insights in this latter area.
Acknowledgements
The authors wish to acknowledge the funding source, US NSF award DRL-1221494, that support our work. We also thank all study participants for willingly participating in the study.References
- Ahtee M. and Varjola I., (1998), Students' understanding of chemical reaction, Int. J. Sci. Educ., 20, 305–316.
- Andersson B., (1990), Pupils' conceptions of matter and its transformations (age 12–16), Stud. Sci. Educ., 18 (1), 53–85.
- Barke H. D., Hazari A. and Yitbarek S., (2009), Misconceptions in chemistry: addressing perceptions in chemical education, Berlin: Springer-Verlag.
- Bernholt S. and Parchmann I., (2011), Assessing the complexity of students' knowledge in chemistry, Chem. Educ. Res. Pract., 12(2), 167–173.
- Bhattacharyya G., (2014), Trials and tribulations: student approaches and difficulties with proposing mechanisms using the electron-pushing formalism, Chem. Educ. Res. Pract., 15(4), 594–609.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: how students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Biggs J. B. and Collis K. F., (1982), Evaluating the quality of learning: the SOLO taxonomy (structure of the observed learning outcome), New York: Academic Press.
- Boo H. K. and Watson J. R., (2001), Progression in high school students' (aged 16-18) conceptualizations about chemical reactions in solution, Sci. Educ., 85, 568–585.
- Brown N. J. S., Nagashima S. O., Fu A., Timms M., and Wilson M., (2010), A framework for analyzing scientific reasoning in assessments, Educational Assessment, 15(3), 142–174.
- Charmaz K., (2006), Constructing grounded theory: a practical guide through qualitative analysis, Thousand Oaks: Sage.
- Chi M. T. H., (2011), Theoretical perspectives, methodological approaches, and trends in the study of expertise, in Li G. an Kaiser G. (ed.) Expertise in Mathematics Instruction An International Perspective, New York: Springer, pp. 17–39.
- Chi M. T. H. and Wylie R., (2014), The ICAP framework: linking cognitive engagement to active learning outcomes, Educ. Psychol., 49(4), 219–243.
- Christian K. and Talanquer V., (2012), Modes of reasoning in self-initiated study groups in chemistry, Chem. Educ. Res. Pract., 13(3), 286–295.
- Claesgens J., Scalise K., Wilson M., and Stacy A., (2009), Mapping student understanding in chemistry: the perspectives of chemists, Sci. Educ., 93(1), 56–85.
- Coffey J. E., Hammer D., Levin D. M., and Grant T., (2011), The missing disciplinary substance of formative assessment, J. Res. Sci. Teach., 48(10), 1109–1136.
- Davis B. H. and Occelli M. L., (2007), Fischer-Tropsch synthesis, catalyst and catalysis, Boston: Elsevier.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9(2), 102–113.
- Graulich N., (2015), Intuitive judgments govern students' answering patterns in multiple-choice exercises in organic chemistry, J. Chem. Educ., 92(2), 205–211.
- Grotzer T. A., (2003), Learning to understand the forms of causality implicit in scientifically accepted explanations, Stud. Sci. Educ., 39(1), 1–74.
- Grove N. P., Cooper M. M., and Rush K. M., (2012), Decorating with arrows: toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Hatzinikita V., Koulaidis V., and Hatzinikitas A., (2005), Modeling pupils' understanding and explanations concerning changes in matter, Res. Sci. Ed., 35, 471–495.
- Hesse J. and Anderson C. W., (1992), Students conceptions of chemical change, J. Res. Sci. Teach., 29(3), 277–299.
- Kind V., (2004), Beyond appearances: students' misconceptions about basic chemical ideas, 2nd edn, London: Royal Society of Chemistry.
- Kraft A., Strickland A. M., and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11(4), 281–292.
- Maeyer J. and Talanquer V., (2013), Making predictions about chemical reactivity: assumptions and heuristics, J. Res. Sci. Teach., 50(6), 748–767.
- National Research Council (NRC), (2012), A framework for K-12 science education: practices, crosscutting concepts, and core ideas, Washington, D.C.: National Academies Press.
- National Research Council (NRC), (2013), The Next Generation Science Standards, Washington, D.C.: National Academies Press.
- National Research Council (NRC), (2015), Reaching students: what research says about effective instruction in undergraduate science and engineering, Washington, D.C.: National Academies Press.
- Osborne J. F. and Dillon J., (2008), Science education in Europe, London: Nuffield Foundation.
- Robertson A. D., Scherr R. E., and Hammer D., (2016), Responsive teaching in science and mathematics, New York: Routledge.
- Russ R. S., Scherr R. E., Hammer D., and Mikeska J., (2008), Recognizing mechanistic reasoning in student scientific inquiry: a framework for discourse analysis developed from philosophy of science, Sci. Educ., 92(3), 499–525.
- Schauble L., Klopfer L. E., and Raghavan K., (1991), Students' transition from an engineering model to a science model of experimentation, J. Res. Sci. Teach., 28(9), 859–882.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15(1), 10–23.
- Solsona N. J., Izquierdo M., and De Jong O., (2003), Exploring the development of students' conceptual profiles of chemical change, Int. J. Sci. Educ., 25(1), 3–12.
- Stavridou H. and Solomonidou C., (1998), Conceptual reorganization and the construction of the chemical reaction concept during secondary education, Int. J. Sci. Educ., 20(2), 205–221.
- Taber K. S., (2002), Chemical misconceptions—Prevention, diagnosis and cure. Vol. I: theoretical background, London: Royal Society of Chemistry.
- Taber K. S., (2013), A common core to chemical conceptions: learners' conceptions of chemical stability, change and bonding, in Tsaparlis G. and Sevian H. (ed.) Concepts of Matter in Science Education, Dordrecht: Springer, pp. 391–418.
- Taber K. S. and García-Franco A., (2010), Learning processes in chemistry: drawing upon cognitive resources to learn about the particulate structure of matter, J. Learn. Sci., 19(1), 99–142.
- Taber K. S. and Watts M., (2000), Learners' explanations for chemical phenomena, Chem. Educ. Res. Pract., 1(3), 329–353.
- Talanquer V., (2006), Commonsense chemistry: a model for understanding students' alternative conceptions, J. Chem. Educ., 83(5), 811–816.
- Talanquer V. (2010), Exploring dominant types of explanations built by general chemistry students, Int. J. Sci. Educ., 32(18), 2393–2412.
- Talanquer V. (2013), When atoms want, J. Chem. Educ., 90 (11), 1419–1424.
- Weinrich M. L. and Talanquer V., (2015), Mapping students' conceptual modes when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 16, 561–677.
- Windschitl M., Thompson J., Braaten M., and Stroupe D., (2012), Proposing a core set of instructional practices and tools for teachers of science, Sci. Educ., 96, 878–903.
| This journal is © The Royal Society of Chemistry 2016 |