Open Access Article
Open Access ArticleDual stimuli-induced formation of a μ-hydroxido bridged [Zn9L5(μ-OH)6]12+ half-pipe†
Christopher S. Wood,
Tanya K. Ronson,
Anna J. McConnell,
Derrick A. Roberts and
Jonathan R. Nitschke *
*
Department of Chemistry, University of Cambridge, Lensfield Road, CB2 1EW, UK. E-mail: jrn34@cam.ac.uk
First published on 17th November 2015
Abstract
Low-symmetry metal–organic architectures that feature unusual binding motifs are useful for exploring new modes of guest recognition. Such structures remain difficult to create using current rational design principles. One approach to constructing such architectures is to employ ligands with coordination vectors oriented to preclude the formation of simple, low nuclearity molecular assemblies upon complexation to metal ions. Here we report two new supramolecular assemblies generated from such a ligand: a simple metastable [Zn3L3]6+ assembly, which was observed to convert to a more complex [Zn9L5(μ-OH)6]12+ twisted half-pipe architecture. Two chemically distinct stimuli—an anionic template and a base—must be applied for the conversion to occur. Perchlorate, perrhenate, trifluoromethanesulfonate and 2-naphthalenesulfonate were found to act as competent templates for the [Zn9L5(μ-OH)6]12+ structure.
Introduction
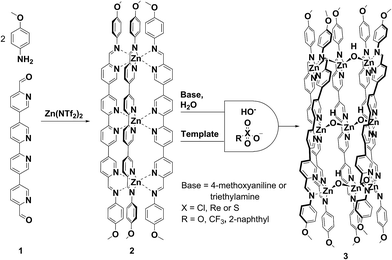
Complex, responsive molecular networks constitute living systems and are thus subjects of intense study.1–4 Recently, the development of synthetic chemical networks that respond in complex ways to stimuli has provided a platform to study and emulate the fundamental processes of natural systems.5–8 A promising strategy is to exploit the dynamic nature of organic9–14 and metal–organic self-assembled complexes15–19 to generate systems that undergo rearrangement upon application of a chemical signal. Previously reported systems have registered a single response to a single signal, e.g., a template,20–23 ligand24–27 or light.28–35 More complex behaviour can be designed when systems exhibit differential responses to multiple signals, acting as logic gates.36–44 In the present case of an AND gate,45,46 two distinct stimuli must be present together in order to elicit a functional response.Subcomponent self-assembly47–54 has proved to be a powerful tool for generating metallosupramolecular assemblies.55–62 Many of these assemblies, by virtue of the dynamic interactions that hold them together, exhibit complex responses to external stimuli.63,64 Increasing the number and type of ligands that comprise the system can increase the complexity of its response, as different components respond to stimuli in distinct ways.
We have recently developed a strategy to favour the formation of heteroleptic assemblies containing multitopic pyridyl-imine ligands.65,66 Quaterpyridine dialdehyde subcomponent 1 (Scheme 1) was designed to form ligands with three rigidly parallel coordination vectors following metal-templated Schiff-base formation. The orientations of these vectors precluded the formation of discrete structures upon complexation with iron(II) salts, instead favouring the formation of mixed-ligand structures when other complimentary ligands were employed in the self-assembly reaction.65 With zinc(II), the geometry of 1, together with in-situ-generated μ-hydroxide complimentary ligands and specific anionic templates, gave rise to a singular twisted ‘half-pipe’ architecture, 3.
Results and discussion
When 1 (1 equiv.), 4-methoxyaniline (2 equiv.) and zinc(II) bis(trifluoromethylsulfonyl)imide (triflimide, 2 equiv.) were mixed in acetonitrile, assembly 2 was observed to form. Its 1H NMR spectrum was consistent with a symmetric structure possessing a single ligand environment (ESI, Fig. S1†) and the ESI mass spectrum was consistent with a [Zn3L3]6+ formulation (ESI, Fig. S5†). By contrast, no well-defined species were observed to form in the analogous reaction involving iron(II) in place of zinc(II).65 We hypothesize that 2 forms with zinc(II) due to its larger ionic radius (compared to FeII) and its lack of a stereoelectronic preference for a regular octahedral coordination environment.67 The ground-state geometry of 2 may be either helical68 (D3 point symmetry) or a ‘mesocate’69–72 (D3h symmetry).Upon addition of a suitable template anion and a source of hydroxide, 2 reorganised to give the [Zn9L5(μ-OH)6]12+ twisted half-pipe architecture 3, featuring a longitudinal groove into which template anions were observed to bind (Scheme 1). The conversion of 2 into 3 was monitored by 1H NMR spectroscopy following addition of tetrabutylammonium perchlorate (4 equiv.) to an acetonitrile solution of 2, 4-methoxyaniline (6 equiv.) and H2O (ca. 50 equiv.) at room temperature. After two minutes the resonances associated with 2 decreased in intensity and were replaced with a complicated series of broad resonances. Over a period of 12 h at 70 °C the resonances associated with 3 appeared and grew in intensity (ESI, Section 1.7†). ESI-MS of the resulting solution revealed signals with m/z values corresponding to [Zn9L5(μ-OH)6]12+ with both perchlorate and triflimide anions. After 12 h at 70 °C there were no further changes to the 1H NMR spectrum of the reaction mixture. Resonances corresponding to 2 could, however, still be observed, which is attributed to the stoichiometric mismatch between the M3L3 and M9L5 architectures.
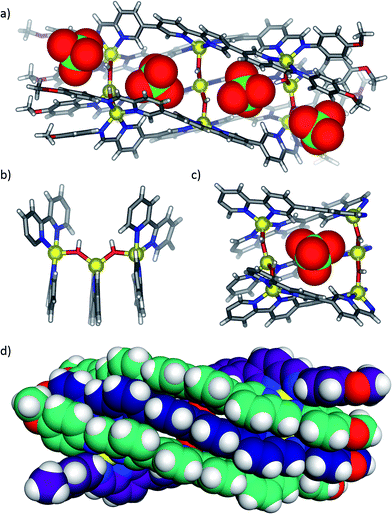
Half-pipe 3 could also be prepared directly from its precursors. When zinc(II) perchlorate hexahydrate (6 equiv.), 1 (5 equiv.) and 4-methoxyaniline (16 equiv.) were mixed in acetonitrile for 12 h at 70 °C, a turbid yellow suspension resulted. Following filtration and precipitation with diethyl ether the resulting product possessed a 1H NMR spectrum corresponding to a species with five distinct ligand environments (ESI, Fig. S9 and S10†). Slow diffusion of diethyl ether into an acetonitrile solution of the product afforded X-ray quality crystals. X-ray crystallography revealed the structure of complex 3, which contains nine zinc(II) centres linked by five ligands (Fig. 1). Three ligands pack tightly side-by-side with their coordination vectors parallel and aromatic stacking interactions occurring between neighbouring ligands. The remaining two ligands lie opposite, with their coordination vectors antiparallel to the first three. This arrangement produces a longitudinal groove between the two outermost ligands, within which four perchlorate anions bind (Fig. 1a and c). These anions hydrogen bond to six bridging hydroxide anions which line the base of the groove, each of which bridges two zinc centres bound by adjacent ligands (Fig. 1b). Thus, the three inner zinc(II) centres adopt a tetrahedral coordination sphere defined by one bidentate and two hydroxide ligands, and the six outer zinc(II) centres are five-coordinate, each bound by two bidentate ligands and a single hydroxide. Both ESI-MS and NMR spectroscopy (ESI, Section 1.3†) provided results consistent with the observed solid-state structure.
The hydroxide (μ-OH) bridges in 3 are inferred to form when 4-methoxyaniline deprotonates water present in the reaction mixture. Indeed, we found that the cleanest formation of 3 could be observed when six extra equivalents of either 4-methoxyaniline or triethylamine were included in a reaction mixture of the correct stoichiometry. However, the direct addition of six equivalents of tetramethylammonium hydroxide caused the formation of intractable precipitates in the presence of perchlorate, possibly due to the kinetic precipitation of zinc oxide or hydroxide in the presence of free HO−. The perchlorate anions, which were observed to bind in the groove of the structure, are also necessary but not sufficient to cause the transformation of 2 into 3. Complex 2 is stable for 12 h at 70 °C in the presence of either four equivalents of tetrabutylammonium perchlorate or six equivalents of 4-methoxyaniline and water. In order to convert complex 2 into 3 both a templating anion and a source of hydroxide were thus necessary.
Perrhenate, trifluoromethanesulfonate (triflate) and 2-naphthalenesulfonate anions were also found to serve as competent templates for the [Zn9L5(μ-OH)6] motif. Mixtures of zinc(II) triflimide (9 equiv.), 1 (5 equiv.), 4-methoxyaniline (16 equiv.) and a suitable salt of the template anion (4 equiv.) were heated in acetonitrile at 70 °C for 12 h. The resulting turbid yellow suspensions were filtered, and the products isolated by precipitation with diethyl ether. 1H NMR spectroscopy and ESI-MS analyses confirmed that both 2 and 3 were present in solution in all three cases. The proportion of 3 to 2 could be increased by slow crystallization and subsequent isolation of the crystalline solid by filtration. However, attempts to increase the concentration of 3 relative to 2 by increasing the concentration of the templating anion were found to increase the formation of insoluble precipitates. The solubility of the Zn(II) salts of these templating anions may be limiting the degree to which the equilibrium between these two species can be influenced through template addition.
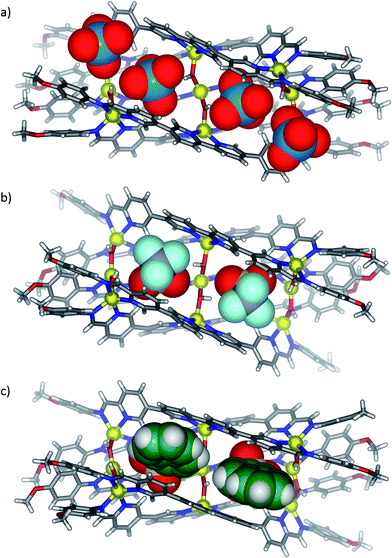
X-ray quality crystals were obtained for all three anion-templated structures by slow diffusion of diethyl ether into acetonitrile solutions. Solution and solid-state analyses indicated all of these assemblies share the same overall 3 architecture. In the case of the perrhenate anion, which is a close structural analogue of perchlorate, four anions were observed to bind within the groove in similar positions as perchlorate (Fig. 2a); the outer two perrhenates were, however, disordered and modelled with partial occupancy. By contrast, triflate and 2-naphthalenesulfonate were observed to occupy only the two inner binding sites of the groove (Fig. 2b and c). In each case, the SO3− group was oriented into the grove while the CF3 and naphthalene groups pointed away from the groove between the two outer ligands. The planar aromatic naphthalene moieties underwent π-stacking interactions with the two outer ligands, with centroid–centroid distances of 3.7–4.0 Å (Fig. 3).
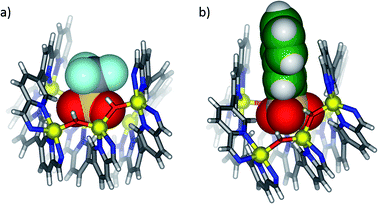 | ||
| Fig. 3 View down the length of the groove in cutaway X-ray crystal structures of 3 templated by (a) triflate and (b) 2-naphthalenesulfonate. The templating anions are shown in space-filling mode. | ||
These additional π-stacking interactions are postulated to engender a greater binding affinity for 2-naphthalenesulfonate than was observed for the other templating anions.73 Two equivalents of 2-naphthalenesulfonate sufficed to displace the perchlorate, perrhenate or triflate anions from 3; complex mixtures of products were observed when only one equivalent of 2-naphthalenesulfonate was added (ESI, Section 1.12†). A more comprehensive hierarchy of binding affinities could not be determined due to the similarity and complexity of the spectra obtained in other anion displacement experiments (ESI Section 1.11†).
Conclusions
In conclusion, we demonstrate that the combination of a metal template without stereoelectronic coordination preferences – zinc(II) – with a subcomponent designed to disfavour the formation of simple low nuclearity structures – 1 – produces an assembly that is susceptible to multi-stimuli-induced structural transformations. Here a simple [Zn3L3]6+ assembly was found to rearrange to a more complex [Zn9L5(μ-OH)6]12+ twisted half-pipe assembly only in the presence of both a base, necessary for the generation of the secondary hydroxide ligands, and a templating anion. The cationic, chiral groove of 3, and other congeners of this new structure type, may prove useful in recognising and binding more complex anionic substrates, perhaps inducing new chemical transformations within its highly-charged, half-encapsulated inner environment.Acknowledgements
This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC). DAR acknowledges the Gates Cambridge Scholarship for PhD funding. We thank Diamond Light Source (UK) for synchrotron beam time on I19 (MT8464).Notes and references
- E. Schrodinger, What is Life?: With Mind and Matter and Autobiographical Sketches, Cambridge University Press, Cambridge, 2012 Search PubMed.
- D. Bray, Science, 2003, 301, 1864–1865 CrossRef CAS PubMed.
- V. Spirin and L. A. Mirny, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 12123–12128 CrossRef CAS PubMed.
- L. H. Hartwell, J. J. Hopfield, S. Leibler and A. W. Murray, Nature, 1999, 402, C47–C52 CrossRef CAS PubMed.
- B. Lewandowski, G. de Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D'Souza, A. E. Fernandes and D. A. Leigh, Science, 2013, 339, 189–193 CrossRef CAS PubMed.
- S. N. Semenov, A. S. Y. Wong, R. M. van der Made, S. G. J. Postma, J. Groen, H. W. H. van Roekel, T. F. A. de Greef and W. T. S. Huck, Nat. Chem., 2015, 7, 160–165 CrossRef CAS PubMed.
- T. le Saux, R. Plasson and L. Jullien, Chem. Commun., 2014, 50, 6189–6195 RSC.
- M. G. Cowan, J. Olguin, S. Narayanaswamy, J. L. Tallon and S. Brooker, J. Am. Chem. Soc., 2012, 134, 2892–2894 CrossRef CAS PubMed.
- P. Mukhopadhyay, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2006, 128, 14093–14102 CrossRef CAS PubMed.
- S. Otto, Acc. Chem. Res., 2012, 45, 2200–2210 CrossRef CAS PubMed.
- R. B. P. Elmes, N. Busschaert, D. D. Czech, P. A. Gale and K. A. Jolliffe, Chem. Commun., 2015, 51, 10107–10110 RSC.
- J. Kim, K. Baek, D. Shetty, N. Selvapalam, G. Yun, N. H. Kim, Y. H. Ko, K. M. Park, I. Hwang and K. Kim, Angew. Chem., Int. Ed., 2015, 54, 2693–2697 CrossRef CAS PubMed.
- R. T. Woodward, L. A. Stevens, R. Dawson, M. Vijayaraghavan, T. Hasell, I. P. Silverwood, A. V. Ewing, T. Ratvijitvech, J. D. Exley, S. Y. Chong, F. Blanc, D. J. Adams, S. G. Kazarian, C. E. Snape, T. C. Drage and A. I. Cooper, J. Am. Chem. Soc., 2014, 136, 9028–9035 CrossRef CAS PubMed.
- G. Zhang and M. Mastalerz, Chem. Soc. Rev., 2014, 43, 1934–1947 RSC.
- J. T. Foy, D. Ray and I. Aprahamian, Chem. Sci., 2015, 6, 209–213 RSC.
- A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 CrossRef CAS PubMed.
- S. Tashiro and M. Shionoya, Chem. Lett., 2013, 42, 456–462 CrossRef CAS.
- J. B. Beck and S. J. Rowan, J. Am. Chem. Soc., 2003, 125, 13922–13923 CrossRef CAS PubMed.
- W. Cullen, C. A. Hunter and M. D. Ward, Inorg. Chem., 2015, 54, 2626–2637 CrossRef CAS PubMed.
- M. Scherer, D. L. Caulder, D. W. Johnson and K. N. Raymond, Angew. Chem., Int. Ed., 1999, 38, 1587–1592 CrossRef.
- S. Freye, R. Michel, D. Stalke, M. Pawliczek, H. Frauendorf and G. H. Clever, J. Am. Chem. Soc., 2013, 135, 8476–8479 CrossRef CAS PubMed.
- K. Hagiwara, M. Otsuki, M. Akita and M. Yoshizawa, Chem. Commun., 2015, 51, 10451–10454 RSC.
- B. Hasenknopf, J.-M. Lehn, N. Boumediene, A. Dupont-Gervais, A. van Dorsselaer, B. Kneisel and D. Fenske, J. Am. Chem. Soc., 1997, 119, 10956–10962 CrossRef CAS.
- C. G. Oliveri, P. A. Ulmann, M. J. Wiester and C. A. Mirkin, Acc. Chem. Res., 2008, 41, 1618–1629 CrossRef CAS PubMed.
- J.-R. Li and H.-C. Zhou, Nat. Chem., 2010, 2, 893–898 CrossRef CAS PubMed.
- Q.-Q. Wang, R. Ara Begum, V. W. Day and K. Bowman-James, Inorg. Chem., 2012, 51, 760–762 CrossRef CAS PubMed.
- D. Samanta and P. S. Mukherjee, Chem.–Eur. J., 2014, 20, 12483–12492 CrossRef CAS PubMed.
- M. Han, R. Michel, B. He, Y.-S. Chen, D. Stalke, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 1319–1323 CrossRef CAS PubMed.
- S.-S. Sun, J. A. Anspach and A. J. Lees, Inorg. Chem., 2002, 41, 1862–1869 CrossRef CAS PubMed.
- T. Murase, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2007, 46, 5133–5136 CrossRef CAS PubMed.
- P. Mobian, J.-M. Kern and J.-P. Sauvage, Angew. Chem., Int. Ed., 2004, 43, 2392–2395 CrossRef CAS PubMed.
- J. Baram, E. Shirman, N. Ben-Shitrit, A. Ustinov, H. Weissman, I. Pinkas, S. G. Wolf and B. Rybtchinski, J. Am. Chem. Soc., 2008, 130, 14966–14967 CrossRef CAS PubMed.
- L.-Y. Yao and V. W.-W. Yam, J. Am. Chem. Soc., 2015, 137, 3506–3509 CrossRef CAS PubMed.
- Y.-F. Han, G.-X. Jin and F. E. Hahn, J. Am. Chem. Soc., 2013, 135, 9263–9266 CrossRef CAS PubMed.
- Y.-F. Han, G.-X. Jin, C. G. Daniliuc and F. E. Hahn, Angew. Chem., Int. Ed., 2015, 54, 4958–4962 CrossRef CAS PubMed.
- A. P. de Silva and N. D. McClenaghan, Chem.–Eur. J., 2004, 10, 574–586 CrossRef PubMed.
- H. Li, J.-N. Zhang, W. Zhou, H. Zhang, Q. Zhang, D.-H. Qu and H. Tian, Org. Lett., 2013, 15, 3070–3073 CrossRef CAS PubMed.
- A. Credi, V. Balzani, S. J. Langford and J. F. Stoddart, J. Am. Chem. Soc., 1997, 119, 2679–2681 CrossRef CAS.
- G. McSkimming, J. H. R. Tucker, H. Bouas-Laurent and J.-P. Desvergne, Angew. Chem., Int. Ed., 2000, 39, 2167–2169 CrossRef CAS.
- G. de Ruiter and M. E. van der Boom, Angew. Chem., Int. Ed., 2012, 51, 8598–8601 CrossRef CAS PubMed.
- Y. Liu, A. Offenhäusser and D. Mayer, Angew. Chem., Int. Ed., 2010, 49, 2595–2598 CrossRef CAS PubMed.
- T. Gunnlaugsson, D. A. Mac Donail and D. Parker, Chem. Commun., 2000, 93–94 RSC.
- Z. Qi, P. Malo de Molina, W. Jiang, Q. Wang, K. Nowosinski, A. Schulz, M. Gradzielski and C. A. Schalley, Chem. Sci., 2012, 3, 2073–2082 RSC.
- M. A. Saeed, H. T. M. Le and O. S. Miljanic, Acc. Chem. Res., 2014, 47, 2074–2083 CrossRef CAS PubMed.
- A. P. de Silva, N. H. Q. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42–44 CrossRef.
- A. Romieu, Org. Biomol. Chem., 2015, 13, 1294–1306 CAS.
- H. Bunzen, Nonappa, E. Kalenius, S. Hietala and E. Kolehmainen, Chem.–Eur. J., 2013, 19, 12978–12981 CrossRef CAS PubMed.
- V. E. Campbell, R. Guillot, E. Riviere, P.-T. Brun, W. Wernsdorfer and T. Mallah, Inorg. Chem., 2013, 52, 5194–5200 CrossRef CAS PubMed.
- Y. Wu, X.-P. Zhou, J.-R. Yang and D. Li, Chem. Commun., 2013, 49, 3413–3415 RSC.
- D. H. Ren, D. Qiu, C. Y. Pang, Z. Li and Z. G. Gu, Chem. Commun., 2015, 51, 788–791 RSC.
- X. Wu, N. Xu, Z. Zhu, Y. Cai, Y. Zhao and D. Wang, Polym. Chem., 2014, 5, 1202–1209 RSC.
- P. D. Frischmann, V. Kunz and F. Würthner, Angew. Chem., Int. Ed., 2015, 54, 7285–7289 CrossRef CAS PubMed.
- D. Lewing, H. Koppetz and F. E. Hahn, Inorg. Chem., 2015, 54, 7653–7659 CrossRef CAS PubMed.
- T. K. Ronson, S. Zarra, S. P. Black and J. R. Nitschke, Chem. Commun., 2013, 49, 2476–2490 RSC.
- P. D. Frischmann and M. J. MacLachlan, Chem. Soc. Rev., 2013, 42, 871–890 RSC.
- J. Dömer, J. C. Slootweg, F. Hupka, K. Lammertsma and F. E. Hahn, Angew. Chem., Int. Ed., 2010, 49, 6430–6433 CrossRef PubMed.
- A. E. Kaifer, S. Yi, V. Brega and B. Captain, Chem. Commun., 2012, 48, 10295–10297 RSC.
- X. P. Zhou, J. Liu, S. Z. Zhan, J. R. Yang, D. Li, K. M. Ng, R. W. Y. Sun and C. M. Che, J. Am. Chem. Soc., 2012, 134, 8042–8045 CrossRef CAS PubMed.
- K.-C. Sham, S.-M. Yiu and H.-L. Kwong, Inorg. Chem., 2013, 52, 5648–5650 CrossRef CAS PubMed.
- X.-P. Zhou, Y. Wu and D. Li, J. Am. Chem. Soc., 2013, 135, 16062–16065 CrossRef CAS PubMed.
- P. D. Frischmann, V. Kunz, V. Stepanenko and F. Würthner, Chem.–Eur. J., 2015, 21, 2766–2769 CrossRef CAS PubMed.
- D.-H. Ren, D. Qiu, C.-Y. Pang, Z. Li and Z.-G. Gu, Chem. Commun., 2015, 51, 788–791 RSC.
- D. M. Wood, W. Meng, T. K. Ronson, A. R. Stefankiewicz, J. K. M. Sanders and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 3988–3992 CrossRef CAS PubMed.
- V. E. Campbell, X. de Hatten, N. Delsuc, B. Kauffmann, I. Huc and J. R. Nitschke, Nat. Chem., 2010, 2, 684–687 CrossRef CAS PubMed.
- C. S. Wood, T. K. Ronson, A. M. Belenguer, J. J. Holstein and J. R. Nitschke, Nat. Chem., 2015, 7, 354–358 CrossRef CAS PubMed.
- C. Browne, W. J. Ramsay, T. K. Ronson, J. Medley-Hallam and J. R. Nitschke, Angew. Chem., Int. Ed., 2015, 54, 11122–11127 CrossRef CAS PubMed.
- I. A. Riddell, Y. R. Hristova, J. K. Clegg, C. S. Wood, B. Breiner and J. R. Nitschke, J. Am. Chem. Soc., 2013, 135, 2723–2733 CrossRef CAS PubMed.
- R. Krämer, J. M. Lehn and A. Marquis-Rigault, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 5394–5398 CrossRef.
- J. Xu, T. N. Parac and K. N. Raymond, Angew. Chem., Int. Ed., 1999, 38, 2878–2882 CrossRef CAS.
- S. E. Howson, A. Bolhuis, V. Brabec, G. J. Clarkson, J. Malina, A. Rodger and P. Scott, Nat. Chem., 2012, 4, 31–36 CrossRef CAS PubMed.
- C. R. K. Glasson, G. V. Meehan, J. K. Clegg, L. F. Lindoy, J. A. Smith, F. R. Keene and C. Motti, Chem.–Eur. J., 2008, 14, 10535–10538 CrossRef CAS PubMed.
- M. Albrecht, Chem. Rev., 2001, 101, 3457–3498 CrossRef CAS PubMed.
- R. Custelcean, Chem. Soc. Rev., 2014, 43, 1813–1824 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1410155–1410158. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc03926f |
| This journal is © The Royal Society of Chemistry 2016 |