Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Competition-driven selection in covalent dynamic networks and implementation in organic reactional selectivity†
P.
Kovaříček‡
 ,
A. C.
Meister§
,
K.
Flídrová
,
R.
Cabot¶
,
K.
Kovaříčková||
and
J.-M.
Lehn
*
,
A. C.
Meister§
,
K.
Flídrová
,
R.
Cabot¶
,
K.
Kovaříčková||
and
J.-M.
Lehn
*
Institut de Science et d'Ingénierie Supramoléculaires, Université de Strasbourg, 8 allée Gaspard Monge, 67000 Strasbourg, France. E-mail: lehn@unistra.fr
First published on 10th February 2016
Abstract
Competition among reagents in dynamic combinatorial libraries of increased complexity leads to reactional self-sorting (improved regioselectivity) in mixtures of aldehydes and oligoamines. High selectivity of a given library component is transferred to a different reacting component of low selectivity through a network of underlying equilibrating reactions which provide component exchange between all species. The selectivity of various carbonyl compounds in reactions with amines was also assessed towards the formation of defined sequences of residues along oligoamine chains. The approach was further exploited for defining selective dynamic protecting groups (DPGs), based on the reversible linkage between the substrate and the protecting group. They represent an intermediate approach between the conventional protecting groups and the protecting-group-free approach in organic synthesis. Removal of the protecting group is effected via dynamic exchange trapping by formation of a more stable product. The establishment of equilibrium eliminates the need for isolation and purification of the dynamically protected intermediate(s) and enables as well the selective sequential derivatisation of oligoamines. The DPG concept can be generalised to other reversible reactions and can thus represent a valuable alternative in the design of total synthesis of complex molecules.
Introduction
Dynamic covalent libraries (DCLs)1–11 are formed by reversible combinatorial linkage of molecular components generating an equilibrium mixture of constituents under thermodynamic control. The reversibility of the linkage allows for constitutional exchange giving access to all possible combinations of components. By application of a stimulus, the DCL is able to undergo constitutional adaptation whereby some species are amplified at the expense of others that are depleted. These features make dynamic covalent chemistry (DCC) attractive for application in material science,12–20 surface modification,21 synthesis of nanoarchitectures,22–25 design of receptors7 and sensors26 as well as in the search for biologically active compounds.2,27–32 In fact, only “virtual presence”1 of, i.e. access to, all combinations is required as the amplified species can be formed upon the application of the stimulus – in the terms of the “lock and key” principle, one may say that the lock assembles its key.Imines, resulting from the reversible condensation of a carbonyl component with an amine, are of particular interest for setting up DCLs, due to the ease of formation and exchange, usually under mild conditions. The heteroatomic imine bond is also satisfactorily orthogonal33–36 to many other reversible bonds and its dynamic nature can be “frozen” by reduction or other transformations.37 Simplification of the complex mixture of species in the library is achieved through adaptive sorting,38–41 for example in course of crystallisation,42–44 oxidation,45 distillation46,47 or coordination.48–52
Herewith we report the operation of a reactional self-sorting process in DCLs of imines of increasing complexity, driven by competition among the reagents in mixtures of aldehydes and oligoamines components and leading to improved selectivity in product formation and/or in site of reaction. The aldehydes in the studied DCLs are moderately selective in reactions with different amines to form various products such as imines, aminals or amino-lactones in the case of o-carboxybenzaldehyde.53,54 Competition between an increasing number of aldehydes for a given mixture of amines leads to increased selectivity of each of them. It leads to a simplification of the final composition resulting from the complexity of the DCL and its collapse into a system of reduced multiplicity (and lower entropy), amounting to a state that may be termed “simplexity” in a process of general type from chemical to biological systems.55,56 In fact, any recognition process, be it reactional or interactional (see Fig. 3 in ref. 57) results in the reduction of the complexity of a mixture, of its simplification, through the operation of competitive selection. On the supramolecular/interactional level it is expressed in the “instructed mixture paradigm”, whereby the behaviour of mixtures is driven by the instructions (molecular information) present in its members resulting in self-recognition (or self-sorting), a self-process,58 belonging to the general phenomenon of self-organization.9,59 The term “simplexity” does not carry the meaning that the system in itself is less complex, as there is in fact an increased complexity (increased number of reacting species) underlying and resulting in the simplification (increased selectivity) observed. We also propose a formal approach for the representation and comparison of DCLs (see below and ESI†) which has been used for evaluating the efficiency of selection in the self-sorting processes investigated here within the realm of dynamic covalent chemistry (DCC).1–5,28–30,57
Aldehyde–amine dynamic covalent libraries
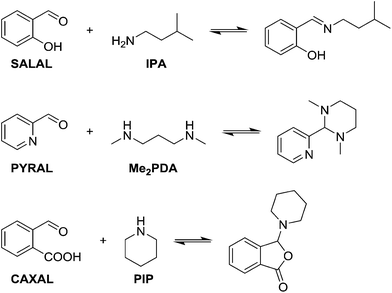
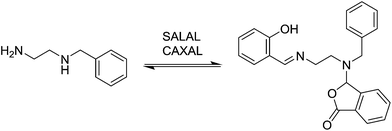
In view of the ubiquity of amines and imines in organic chemistry and in biochemistry, as well as the ability to control each partner in the reaction, condensations between aldehydes and amines were taken as model reactions in order to probe reaction selectivity in competitive dynamic reversible systems.At first, different aldehydes were reacted with different amines in order to test their differences in reactivity and the nature of the product formed. Salicylaldehyde (SALAL), pyridine-2-carboxaldehyde (PYRAL) and 2-carboxy-benzaldehyde (CAXAL) were selected as prototypical aldehydes. On the other hand, isopentylamine (IPA), N,N′-dimethyl-1,3-diaminopropane (Me2PDA) and piperidine (PIP) were selected as their amine counterparts. As solvent a mixture of d6-DMSO with 1% of D2O was used to guarantee solubility of all partners, in which the water addition maintains constant water content during the aldehyde–amine condensation. On the basis of previous studies, differences in reaction outcome was expected for the various aldehyde–amine pairs,53 anticipating that: (a) SALAL would have a preference for primary amines to form imines,53 (b) PYRAL would react with diamines to provide a cyclic aminal53 and (c) CAXAL would react with secondary amines to afford amino-lactones through trapping of the intermediary iminium by the neighbouring carboxylate group.54 These anticipated selectivities towards formation of different condensation products were verified in separate experiments: SALAL reacted with IPA to provide the expected imine, PYRAL gave the six-membered cyclic aminal with Me2PDA, and CAXAL indeed reacted with PIP to form the amino-lactone. These reactions proceeded with quantitative conversion and the resulting preferentially formed products may be considered as “matching pairs”, effecting reactional recognition, like supramolecular systems effect interactional recognition (Fig. 1).4,57
[2 × 2] aldehyde–amine mixtures
Checking first the combinations opposite to the “matching pairs” (see above) showed that both aldehydes reacted with both amines: when CAXAL was mixed with 1 eq. of IPA, equilibrium was reached almost instantaneously giving a dynamic mixture of the imine and lactone formed with IPA (details in ESI, Section 3.2†); on the other hand, SALAL reacted with PIP in approximately 30% conversion to the aminal formed from two amine molecules and one aldehyde. In subsequent experiments, the propensity towards the formation of the “matching pair” product was tested in presence of a competing amine, i.e. each aldehyde was reacted with an equimolar mixture of the two amines. SALAL showed a very high preference for imine formation reacting solely with IPA and leaving PIP unreacted in the solution. However, the conversion of the aldehyde in this case was not complete, approximately 10% remaining unreacted. In contrast, CAXAL under the same reaction conditions provided two products: the lactone on the PIP ring was formed in about 39% yield and the dynamic imine–lactone product from IPA was present in about 61% yield (details in the ESI, Section 3.2†). Finally, the full [2 × 2] library was considered: SALAL and CAXAL were mixed with both IPA and PIP in equimolar amounts and the resulting product mixture was examined using NMR spectroscopy. Equilibrium was reached after 5 hours and the mixture contained only the two “matching pair” species, the imine SALAL–IPA and the lactone CAXAL–PIP, both in quantitative conversion.
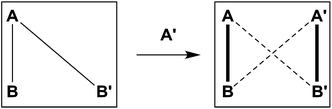
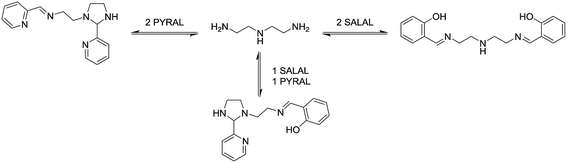
This remarkable simplicity achieved in the comparatively complex mixture, contrasts with the ability of both aldehydes to react with both amines, and vice versa each amine with the two aldehydes. It demonstrates the occurrence of a selectivity enhancement of a poor selector by competition with a strong selector. Specifically, a species reacting non-selectively with several compounds is brought to become selective towards a given product, when put in presence of a competing species of high selectivity which monopolises one of the components. The process amounts to a competitive selectivity enhancement. The formation of the matching pair products can also be seen as resulting from agonist amplification9,57 by competition via component exchange through an underlying network of equilibrating reactions (Fig. 2).
Stoichiometry is of course a crucial parameter providing the enhanced selection of the inherent self-sorting process. In the present case, the components react in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio to form the preferred product. Therefore, the initial mixture must contain the components in this ratio so that the condensation of the matching pair in quantitative conversion depletes all of the starting materials, which in turn do not interfere with the formation of the second matching pair in the complex system. This is clearly demonstrated by the fact that whereas the aforementioned mixture SALAL–CAXAL–IPA–PIP can give, in principle, 14 products (accounting for 2 imines, 4 homo- and 2 heteroaminals, 4 hemiaminals and 2 lactones), only two products are observed in the mixture containing all four species in equimolar amounts.
1 ratio to form the preferred product. Therefore, the initial mixture must contain the components in this ratio so that the condensation of the matching pair in quantitative conversion depletes all of the starting materials, which in turn do not interfere with the formation of the second matching pair in the complex system. This is clearly demonstrated by the fact that whereas the aforementioned mixture SALAL–CAXAL–IPA–PIP can give, in principle, 14 products (accounting for 2 imines, 4 homo- and 2 heteroaminals, 4 hemiaminals and 2 lactones), only two products are observed in the mixture containing all four species in equimolar amounts.
As the final concentration of species can play a role in the selection process,60 we repeated the library experiments at several concentrations: 4, 10, 20, 60 and 100 mM of the components. The equilibrium composition of the library in this range was constant showing no concentration dependency.
The library was also assessed for the effect of pH. To this end, the pre-equilibrated library was examined on addition up to 4 equivalents of acid (MeSO3H) or up to 2 eq. of base (t-BuOK). Addition of 1 eq. of acid leads to almost complete hydrolysis of the aminals of both SALAL and PYRAL, while on addition of the second equivalent of MeSO3H the imine hydrolysis reached 60%. Continued titration by acid led to complete hydrolysis of the imines and extensive hydrolysis of the CAXAL derived lactone, finally giving only the hydrolysed products, after addition of 4 equivalents of acid. In contrast, upon titration by base, disappearance of the lactone signals was observed. Interestingly, this lactone depletion was not accompanied by the emergence of the aldehyde peak of CAXAL, but rather of a new imine of CAXAL with IPA and liberation of SALAL was observed. This example clearly displays the operation of the underlying network of interconnected equilibrating reactions which leads to the complex adaptation of the dynamic library to a stimulus.
The selectivity enhancement displayed here for [n × n] dynamic libraries represents a process of competitive selection, whereby simplification results from competition within the set of equilibrating constituents undergoing component exchange. This behaviour relates also to the process of co-evolution in a dynamic library, leading to the synergistic expression of given constituents.9,61
We have developed a formal method for quantification of selection in dynamic combinatorial libraries and especially for (self)-sorting processes which involve coupled reactions. It is based on a matrix representation of the combinatorial library including the particular case of the selection induced by increased complexity of the mixture (see ESI, Section 2,† for detailed description).
Kinetics in dynamic reaction selectivity
The key aspect of the field of dynamic covalent chemistry (DCC) is that it is governed by thermodynamics and therefore relates to the equilibrium composition. However, the equilibration of the mixture proceeds via component exchanges, each with a given rate constant, and the equilibrium is thus established when the sum of all reaction rates is zero. Kinetics are therefore intimately involved in the status of the composition of a dynamic set at a given time.62 It is in particular the case when considering the vastly different rates of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond formation between different carbonyl groups and different types of amino compounds.63 Such kinetic features can be briefly illustrated on the library consisting of SALAL, CAXAL, IPA and PIP.
N double bond formation between different carbonyl groups and different types of amino compounds.63 Such kinetic features can be briefly illustrated on the library consisting of SALAL, CAXAL, IPA and PIP.
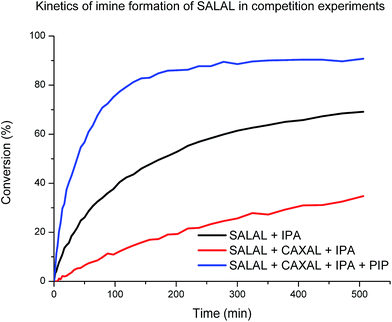
When SALAL was reacted with IPA (20 mM, buffered d6-DMSO, see ESI, Section 3,† for details) the formation of the imine followed second order kinetics with the rate constant of 0.01 M−1 s−1 and 90% overall conversion to the imine (note that in the reaction without a buffer the conversion was quantitative). When the experiment was repeated in presence of 1 eq. of CAXAL, significantly slower rate (k = 0.001 M−1 s−1) and only 77% conversion of SALAL was observed (Chart 1), showing that competition of the two aldehydes for one amine affects both the equilibrium (conversion) and the kinetics (rate of formation). Finally, when the experiment was performed in presence of 1 eq. of both CAXAL and PIP, forming the corresponding lactone, the original SALAL conversion of 90% was restored and the rate of formation was even accelerated (k = 0.03 M−1 s−1) due probably to the catalytic effect of the secondary amine.64 Thus, as expected, on the way towards the simplexity state, the evolution of the fractions of the different library constituents depends on the kinetics until equilibrium has been reached.
Reactional organization along oligoamine amine chains
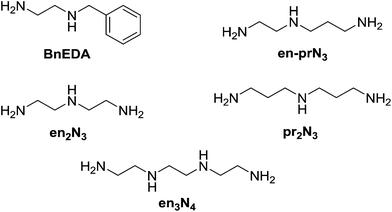
Selection in aldehyde–amine DCLs described above arises from the preference of a given aldehyde for its matching amine partner through equilibration involving reaction both with different amines and with different sites in an oligo(poly)amine. Combining different amine structural motives within one molecule opens the way to intramolecularly organise aldehyde residues along an oligo(poly)amine chain in a given sequence. Thus, using the described “matching pairs” a multivalent polyamine molecule containing both primary amine end groups and secondary amines along the chain could serve as the organisational scaffold for the positioning of aldehyde residues under functional recognition (Fig. 4).4,57,65In the simplest case of N-benzylethylenediamine (BnEDA), the chain has one primary and one secondary nitrogen, expected to represent the reactivity of both IPA and PIP respectively. When an equimolar mixture of SALAL and CAXAL was reacted with 1 eq. BnEDA the dominant species in the solution (70%) was the expected imine–lactone: the imine of SALAL formed on the primary nitrogen and the lactone of CAXAL closed on the secondary nitrogen (Fig. 5, see ESI, Section 3.5,† for details). This predominant formation of a single product is remarkable given the fact that the mixture can in principle generate a large number of other species.
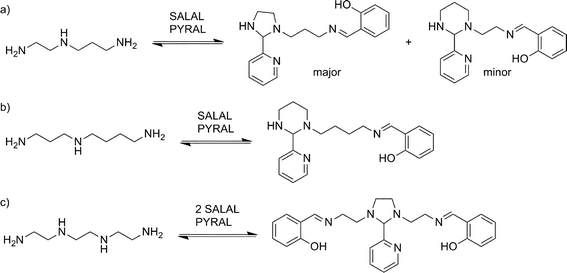
With extended chains with three nitrogen sites, such as diethylenetriamine (en2N3) or bis(3-aminopropyl)amine (pr2N3), selective formation of aminals with PYRAL can be assessed. Closure of the aminal ring bridges two nitrogen sites, terminal and central, while the third one, the other primary amine, is available for imine formation. Thus, when either of the triamines was reacted with 2 eq. of PYRAL, the imine–aminal was the major product formed. In contrast, when the triamines were reacted with 2 eq. of SALAL, the terminal bis-imine was the only product. Remarkably, reaction of the two aldehydes mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the two triamines, resulted in selective formation of the expected products, the SALAL-imine and PYRAL-aminal, with respectively 77% and 84% conversion for en2N3 and pr2N3 (Fig. 6).
1 ratio with the two triamines, resulted in selective formation of the expected products, the SALAL-imine and PYRAL-aminal, with respectively 77% and 84% conversion for en2N3 and pr2N3 (Fig. 6).
The non-symmetric triamines en-prN3 and spermidine were also examined in the reaction with the two aldehydes. When en-prN3 was reacted with 1 eq. of PYRAL and 1 eq. of SALAL the desired imine–aminal species with five membered aminal ring formed in 85% conversion, with trace amounts of six-membered isomer. When spermidine was reacted under the same conditions, only one size of the aminal ring can be formed as seven-membered rings are much more difficult to form compared to the six-membered ones, and indeed the NMR spectrum revealed that the six-membered PYRAL aminal bearing the SALAL-imine on the C4-arm was formed in overall conversion of 71% (Fig. 7a, see ESI, Section 3.5,† for details).
In a further extension to four amino sites, the reaction of triethylenetetramine (en3N4) with 2 equivalents of SALAL gave a complex mixture of products: four different imine signals (three sharp and one broad) and three aminal peaks were observed in the NMR spectrum, corresponding to all possible combinations of imine–aminal structures formed in an essentially statistical fashion. However, when 1 equivalent of PYRAL was added to the mixture, preferential formation of its aminal on the middle secondary amino groups enhanced the formation of the imines of SALAL at the extremities leading to an equilibrium conversion of about 60% (Fig. 7c).
These experiments clearly demonstrate that despite the ambiguous reactivity of both the amine sites and the aldehydes (imine–aminal-lactone equilibria), the system of higher complexity involving all components led to a pronounced competition-enhanced selectivity towards a preferred species, as compared to less complex mixtures. These results provide a remarkable illustration of selectivity amplification, i.e. simplification induced by an increase in complexity.
Selective dynamic protection of amino groups by reaction with aldehydes
Specific reversible reaction of different carbonyl reagents with different amino groups may offer a strategy that can be exploited for the selective dynamic protection of amines. The synthesis of complicated multifunctional molecules often requires to perform a selective reaction with a given functional group in presence of other similar groups. Such specific addressing of a given group in complex molecules is enabled by the use of protecting groups (PG).66,67 However, introduction and removal of a PG necessitates two more synthetic steps accompanied by purification procedures. It is therefore of much interest to develop “protecting-group-free” synthetic methodologies.68,69 Selective dynamic protecting groups (DPGs) could in principle offer an attractive intermediate approach whereby the desired functional group would be protected directly in the reaction mixture under thermodynamic control without isolation and purification, yet the dynamic nature provides reversibility for comparatively easy dynamic deprotection, for instance via transimination. Selective DPGs can be fully complementary to traditional PGs in organic synthesis66 as there are several types of dynamic linkages which have been shown to be orthogonal to each other,33–36,70 thus providing a pool of reagents for different functional groups.71,72 Together with dynamic kinetic resolution,45 the present work demonstrates the contribution that the implementation of Dynamic Covalent Chemistry (DCC) can make to the field of organic synthesis.Amine protection with carbonyl compounds enables C-alkylations,73–76 O-alkylations77,78 or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond dihydroxylation.79 As protecting aldehydes, salicylaldehyde80–83 and formaldehyde84–86 have been used for selective imine or aminal formation respectively followed by the typical acidic hydrolysis work up.73–76 Deprotection can be effected by transimination on application of hydrazides87 or hydroxylamine derivatives.82,88
C double bond dihydroxylation.79 As protecting aldehydes, salicylaldehyde80–83 and formaldehyde84–86 have been used for selective imine or aminal formation respectively followed by the typical acidic hydrolysis work up.73–76 Deprotection can be effected by transimination on application of hydrazides87 or hydroxylamine derivatives.82,88
Selective derivatisation of amines
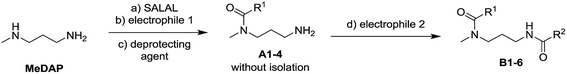
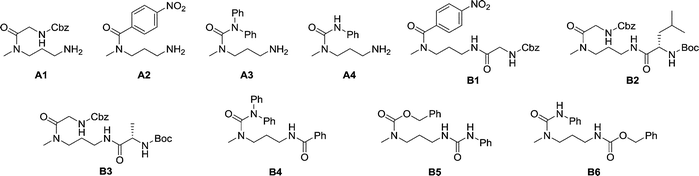
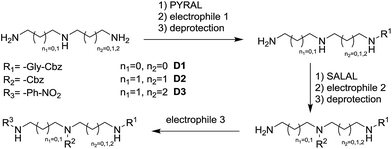
The dynamic nature of the reversible imine bond forming reaction gives the opportunity for facile protecting group removal in conditions which do not affect acylated amines or similar derivatives. Imine hydrolysis under acidic conditions can be replaced by much milder thermodynamically driven imine exchange. To this end, addition of a hydrazine, a hydrazide or an alkoxyamine leads to cleavage of the imine to give a hydrazone, an acylhydrazone or an oxime (respectively) of the carbonyl partner in quantitative conversion with liberation of the free primary amino group. The double implementation of the features of DCC gives the advantage to perform the three-step reaction sequence, protection–derivatisation–deprotection, in a “one pot” fashion without the need for isolation and purification of the intermediates (Fig. 8). A number of such processes have been explored (Fig. 8; Table 1). To be successful, this selective DPG approach requires of course high imine formation, high transimination on deprotection, as well as stability of the imine in the conditions used for the derivatization. Comprehensive method optimisation, synthetic details and full characterisation of all products are provided in the ESI, Section 4.† In the following text, the reported yields represent isolated amount of pure products.
| Ref. | Electrophile 1 | Electrophile 2 | Solvent | Deprotecting agent | Yield |
|---|---|---|---|---|---|
| A1 | Cbz-GlyONp | — | CH3CN | BnONH3Cl | 82% |
| A2 | pNO2PhCOCl | — | CH3CN | BnONH3Cl | 69% |
| A3 | Ph2NCOCl | — | CH3CN | PhCONHNH2 | 70% |
| A4 | PhNCO | — | CH3CN | BnONH3Cl | 86% |
| B1 | pNO2PhCOCl | Cbz-GlyONp | CH3CN | PhCONHNH2 | 78% |
| B2 | Cbz-GlyONp | Boc-LeuOSu | CH3CN | PhCONHNH2 | 83% |
| B3 | Cbz-GlyONp | Boc-AlaOSu | CH3CN | PhCONHNH2 | 82% |
| B4 | Ph2NCOCl | PhCOCl | EtOH | BnONH3Cl | 75% |
| B5 | CbzCl | PhNCO | CH3CN | PhCONHNH2 | 71% |
| B6 | PhNCO | CbzCl | EtOH | BnONH3Cl | 82% |
In the reaction of MeDAP with the p-nitrophenyl activated ester of protected glycine (Cbz-GlyONp) using dynamic protection by 1 eq. SALAL, the N-Cbz-glycyl substituent was introduced on the secondary amine, giving isolated yield of 82% for the full three-step one-pot reaction sequence. Importantly, when the reaction was performed without the addition of SALAL, it afforded 85% isolated yield of a product consisting of a mixture of regioisomers due to unsufficient difference in reactivity of the two nitrogen atoms. The regioisomers were present in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in favour of the product of acylation on the secondary amine. The product of double acylation of MeDAP was isolated as well in about 6% yield. We explored the versatility of the protocol by varying the reagents, the solvent and also the nature of the deprotecting agent. The results are summarized in Table 1 (entries A1–A4). Good isolated yields in the range of 69–86% of the desired products were obtained after the complete three-step reaction sequence.
1 ratio in favour of the product of acylation on the secondary amine. The product of double acylation of MeDAP was isolated as well in about 6% yield. We explored the versatility of the protocol by varying the reagents, the solvent and also the nature of the deprotecting agent. The results are summarized in Table 1 (entries A1–A4). Good isolated yields in the range of 69–86% of the desired products were obtained after the complete three-step reaction sequence.
The operation of DCC under thermodynamic control has been exploited here for the protection and deprotection steps. The establishment of equilibrium offers the possibility to perform sequences of reactions in systems of increasing complexity. Thus, the equilibrium mixtures in the previous experiments before isolation of products consist of the oxime or acylhydrazone of SALAL together with a MeDAP derivative displaying a free primary amino group (complete conversion in deprotection). We have therefore investigated the possibility to perform a controlled sequential derivatisation in a “one pot” fashion (Fig. 8), in which the imine formation by SALAL directs the sequence of derivatization to give products bearing the residue introduced first on the secondary amine group and the second one on the primary amine site. The reaction was repeated for various combinations of electrophiles, in two solvents and with two deprotecting agents giving yields of the desired products in the range of 71–83% (92–95% per step, Table 1 entries B1–B6). In contrast, when the reaction was performed in absence of the SALAL-imine protecting group using N,N-diphenylcarbamoyl chloride and benzoyl chloride as electrophiles, a mixture of all four possible products was obtained in yields from 16 to 34% (details in the ESI, Section 4.1.1.9†).
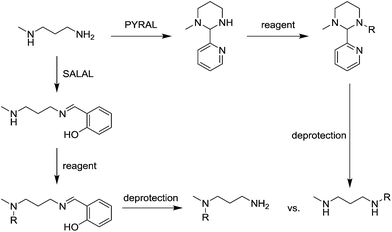
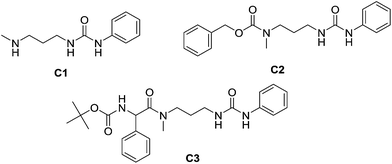
When PYRAL was mixed with MeDAP, efficient formation of the aminal within 2 hours was confirmed by NMR. The equilibrated solution was then treated with phenyl isocyanate forming the urea moiety and the protecting group was removed by the exchange reaction with O-benzylhydroxylamine which converts the aminal of PYRAL completely to the corresponding oxime. The resulting product C1 was isolated in 74% yield, accompanied with 2% of the opposite regioisomer (Table 2). It is thus possible to perform a double derivatisation of the diamine in a “one pot” fashion by addition of a second electrophile after deprotection. To this end, we have used phenyl isocyanate as the first electrophile and the activated ester of N-protected phenylalanine or benzyl chloroformate in the second acylation (isolated yields 76 and 74%, respectively), demonstrating the potential of the approach implementing dynamic protecting groups and mild deprotection procedures compared to conventional protecting groups used in peptide synthesis.
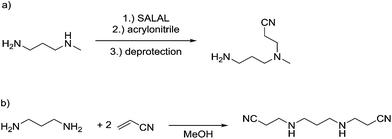
The reaction employing PYRAL protection via aminal formation was studied with several different electrophiles used in the first acylation, but the isolated product always consisted of the diamine derivatised on the secondary amine (due to aminal–imine equilibrium, see above). Even after extensive optimisation of reaction conditions (details in the ESI, Section 4.1.3†) and replacement of PYRAL with formaldehyde, reported in the literature as aminal forming reagent,84,85 the terminal regioisomer was only obtained with phenyl isocyanate. Literature reports describe the use of aminal forming aldehyde to drive the selectivity of the Michael addition of acrylonitrile to polyamines.85,90 In this vein, MeDAP was reacted first with 1 eq. of PYRAL (or SALAL) and then 1 eq. of acrylonitrile was added. Formation of the product of Michael addition (Fig. 10a) was followed by NMR (at r.t. in d4-methanol) revealing that the reaction time in presence of PYRAL was much longer (35% conversion after 24 hours) than in the case of SALAL protected version (quantitative in 24 hours). Moreover, while SALAL drove the reaction with full selectivity for the addition on the secondary nitrogen, the reaction mixture with PYRAL consisted of both regioisomers in ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 in favour of the same product as with SALAL (due to the aminal–imine dynamic interconversion). When the acrylonitrile Michael addition was repeated without any protecting aldehyde, again a mixture of products was obtained, but in this case favouring the addition on the primary amine. This finding indicates kinetic resolution between primary and secondary amines in the acrylonitrile Michael addition, which was supported by the reaction of 1,3-diaminopropane with 2 eq. of acrylonitrile providing a quantitative yield of the N,N′-bis(2-cyanoethyl)-1,3-diaminopropane (Fig. 10b, details in the ESI, Section 4.4†).91 In conclusion, the PYRAL protection is a suitable approach in the case of highly reactive isocyanate species, presumably because it reacts faster than is the rate of intramolecular aminal–imine interconversion. In the case of acyl chlorides this equilibrium, although strongly shifted towards the aminal, leads to non-selective derivatisation of both amino groups. Acrylonitrile, reacts preferentially with primary amines and SALAL protection is needed to achieve the selective reaction on the secondary nitrogen.
2.5 in favour of the same product as with SALAL (due to the aminal–imine dynamic interconversion). When the acrylonitrile Michael addition was repeated without any protecting aldehyde, again a mixture of products was obtained, but in this case favouring the addition on the primary amine. This finding indicates kinetic resolution between primary and secondary amines in the acrylonitrile Michael addition, which was supported by the reaction of 1,3-diaminopropane with 2 eq. of acrylonitrile providing a quantitative yield of the N,N′-bis(2-cyanoethyl)-1,3-diaminopropane (Fig. 10b, details in the ESI, Section 4.4†).91 In conclusion, the PYRAL protection is a suitable approach in the case of highly reactive isocyanate species, presumably because it reacts faster than is the rate of intramolecular aminal–imine interconversion. In the case of acyl chlorides this equilibrium, although strongly shifted towards the aminal, leads to non-selective derivatisation of both amino groups. Acrylonitrile, reacts preferentially with primary amines and SALAL protection is needed to achieve the selective reaction on the secondary nitrogen.
Selective derivatisation of oligoamines
Selective dynamic protection of amino groups was further explored with challenging polyamine substrates. To demonstrate the principle and to draw a comparison with known alternative protocols, we have examined the monoderivatisation of diethylenetriamine en2N3 at the central secondary amine group. The traditional protection/reaction/deprotection approach89 employs trifluoroacetyl protection of the terminal NH2 functions, followed by reaction with Boc2O and removal of the trifluoroacetate groups by reflux in ammonia solution with overall 63% yield including at least two chromatographic purification steps. In the present case, SALAL is used as the protecting agent (2 eq.) and the reaction is performed at room temperature, in “one pot” fashion, with a single isolation and purification step giving the desired product in an overall yield of 80% (see ESI for the synthetic protocol†). Selective DPGs offer large versatility which is not easily available with conventional protecting groups. If pr2N3 is protected by 1 eq. of PYRAL forming the aminal and then reacted with an electrophile, selective monoderivatisation of one of the termini is achieved in good yields (70%, Fig. 11, see ESI, Section 4.5,† for synthetic details). In this case, the PYRAL protection is crucial since reproducing the reaction without the protecting aldehyde led to a complex mixture of all possible products in essentially statistic ratio.Finally, the sequential derivatisation shown above for diamines was also investigated with the biologically relevant triamine spermidine. In this case, the reaction sequence started with protection by 1 eq. of PYRAL forming the aminal and thus leaving only one primary amino group available for the subsequent reaction with the activated ester of an amino acid (Cbz-GlyONp). The protecting group was removed by exchange reaction with benzyloxyamine and without isolation, the subsequent protection by 1 eq. of SALAL was introduced. The selective imine formation at the other terminal NH2 group of the starting polyamine left the central secondary amine free, thus allowing for selective derivatisation in the middle of the chain by benzyl chloroformate. Deprotection was again performed by imine exchange with benzyloxyamine and the last remaining nitrogen atom was thereafter derivatized using p-nitrobenzoyl chloride (Fig. 12). This 7-step, one-pot reaction sequence performed at r.t. under ambient atmosphere afforded after a single purification step the desired triply derivatized product in 31% yield (85% calc. per step), demonstrating the potential of dynamic selective protecting groups in organic synthesis.
The presented strategy of selective DPGs showed remarkable versatility when SALAL was used as the protecting agent. On the other hand, inverted sequence of derivatisation by employing PYRAL was limited to cases in which the acylating reagent reacts faster than the rate of equilibration of the protecting group between several species. These two approaches are thus complementary and when combined, provide a powerful tool to perform selective and/or sequential functionalization of polyamines.
Conclusions
Dynamic covalent chemistry (DCC) operates at thermodynamic equilibrium achieved by component exchange through reversible covalent reactions. The condensation of carbonyl compounds with amines is of special interest as it can result in the formation of N–C–N aminals in addition to the usual C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imines, depending on the nature of the carbonyl component. It offers thus a richer palette of constituents that may be exploited towards the design of selective reaction pathways of interest for organic synthetic strategies. It allows for substrate selectivity in mixtures of amines as well as for the programming of reaction sequences towards the control of regioselective positioning of carbonyl residues along an oligo(poly)amine chain. These features lead to the concept of selective dynamic protecting groups, whereby primary and secondary amine functional groups may be reversibly derivatized in processes presenting selectivity between different amine compounds as well as between different amino sites within a polyamine via a network of underlying equilibrating reactions.
N imines, depending on the nature of the carbonyl component. It offers thus a richer palette of constituents that may be exploited towards the design of selective reaction pathways of interest for organic synthetic strategies. It allows for substrate selectivity in mixtures of amines as well as for the programming of reaction sequences towards the control of regioselective positioning of carbonyl residues along an oligo(poly)amine chain. These features lead to the concept of selective dynamic protecting groups, whereby primary and secondary amine functional groups may be reversibly derivatized in processes presenting selectivity between different amine compounds as well as between different amino sites within a polyamine via a network of underlying equilibrating reactions.
The selective DPG concept has been exploited towards the regioselective derivatization of various amines with different reagents ranging from isocyanates to activated esters of amino acids. The establishment of equilibrium in the reaction of the substrate with the protecting group as well as in the deprotection step eliminates the need for isolation and purification of intermediates and allows to run reactions in “one-pot” fashion. Furthermore, the thermodynamic control over the reversible condensations opens the possibility to perform sequences or networks of reactions in systems of increasing complexity, as it was shown in the case of selective sequential derivatisation of polyamines. Exploitation of other reversibly formed species, such as acetals or boroxines, can provide useful alternatives in total synthesis of complex products. Altogether the present work represents an extension of the thermodynamically driven DCC, implementing dynamic organic reactivity, into the traditionally kinetically governed realm of organic synthesis and provides ground for further exploration of its potential.
Finally, in a broad perspective, the data presented provide a remarkable illustration of simplification within a given constitutional dynamic library, induced by an increase in complexity, which leads to enhanced competition within the set of equilibrating constituents undergoing component exchange via a network of interconnected reactions engaged in agonistic and antagonistic relationships with feedback loops. In general terms, higher complexity results in simplification through competition.
Acknowledgements
We thank the ERC Advanced grant “SUPRADAPT” for financial support. A. C. M. thanks the French Embassy in Berlin for a post-doctoral fellowship. P. K. thanks the Université de Strasbourg for a doctoral fellowship. K. K. thanks the Erasmus exchange programme for an international internship.Notes and references
- J.-M. Lehn, Chem.–Eur. J., 1999, 5, 2455–2463 CrossRef CAS.
- O. Ramstrom and J.-M. Lehn, Nat. Rev. Drug Discovery, 2002, 1, 26–36 CrossRef CAS PubMed.
- P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS PubMed.
- J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151–160 RSC.
- S. Ladame, Org. Biomol. Chem., 2008, 6, 219–226 CAS.
- R. A. R. Hunt and S. Otto, Chem. Commun., 2011, 47, 847–858 RSC.
- S. R. Beeren and J. K. M. Sanders, J. Am. Chem. Soc., 2011, 133, 3804–3807 CrossRef CAS PubMed.
- S. Hamieh, V. Saggiomo, P. Nowak, E. Mattia, R. F. Ludlow and S. Otto, Angew. Chem., Int. Ed., 2013, 52, 12368–12372 CrossRef CAS PubMed.
- J.-M. Lehn, Angew. Chem., Int. Ed., 2013, 52, 2836–2850 CrossRef CAS PubMed.
- S. P. Black, J. K. M. Sanders and A. R. Stefankiewicz, Chem. Soc. Rev., 2014, 43, 1861–1872 RSC.
- J.-M. Lehn, Angew. Chem., Int. Ed., 2015, 54, 3276–3289 CrossRef CAS PubMed.
- C. Wang, G. Wang, Z. Wang and X. Zhang, Chem.–Eur. J., 2011, 17, 3322–3325 CrossRef CAS PubMed.
- D. N. Bunck and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 14952–14955 CrossRef CAS PubMed.
- J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814–831 CrossRef CAS.
- E. Moulin, G. Cormos and N. Giuseppone, Chem. Soc. Rev., 2012, 41, 1031–1049 RSC.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- J. Li, J. M. A. Carnall, M. C. A. Stuart and S. Otto, Angew. Chem., Int. Ed., 2011, 50, 8384–8386 CrossRef CAS PubMed.
- T. Ono, S. Fujii, T. Nobori and J.-M. Lehn, Chem. Commun., 2007, 4360–4362 RSC.
- T. Ono, S. Fujii, T. Nobori and J.-M. Lehn, Chem. Commun., 2007, 46–48 RSC.
- C. B. Minkenberg, F. Li, P. van Rijn, L. Florusse, J. Boekhoven, M. C. A. Stuart, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2011, 50, 3421–3424 CrossRef CAS PubMed.
- A. Ciesielski, M. El Garah, S. Haar, P. Kovaříček, J.-M. Lehn and P. Samorì, Nat. Chem., 2014, 6, 1017–1023 CrossRef CAS PubMed.
- A. Granzhan, T. Riis-Johannessen, R. Scopelliti and K. Severin, Angew. Chem., Int. Ed., 2010, 49, 5515–5518 CrossRef CAS PubMed.
- K. Acharyya, S. Mukherjee and P. S. Mukherjee, J. Am. Chem. Soc., 2013, 135, 554–557 CrossRef CAS PubMed.
- F. B. L. Cougnon, N. Ponnuswamy, N. A. Jenkins, G. D. Pantoş and J. K. M. Sanders, J. Am. Chem. Soc., 2012, 134, 19129–19135 CrossRef CAS PubMed.
- S. J. Rowan and J. F. Stoddart, Org. Lett., 1999, 1, 1913–1916 CrossRef CAS.
- G. J. Mohr, C. Demuth and U. E. Spichiger-Keller, Anal. Chem., 1998, 70, 3868–3873 CrossRef CAS.
- T. Hotchkiss, H. B. Kramer, K. J. Doores, D. P. Gamblin, N. J. Oldham and B. G. Davis, Chem. Commun., 2005, 4264–4266 RSC.
- Dynamic combinatorial chemistry in drug discovery, bioorganic chemistry, and materials science, ed. B. L. Miller, Wiley, Hoboken, N.J, 2010 Search PubMed.
- M. Hochgürtel and J.-M. Lehn, in Fragment-based Approaches in Drug Discovery, ed. W. Jahnke and D. A. Erlanson, Wiley-VCH Verlag GmbH & Co. KGaA, 2006, pp. 341–364 Search PubMed.
- A. Herrmann, Chem. Soc. Rev., 2014, 43, 1899–1933 RSC.
- S. Duan, W. Yuan, F. Wu and T. Jin, Angew. Chem., Int. Ed., 2012, 51, 7938–7941 CrossRef CAS PubMed.
- J. D. Cheeseman, A. D. Corbett, J. L. Gleason and R. J. Kazlauskas, Chem.–Eur. J., 2005, 11, 1708–1716 CrossRef CAS PubMed.
- V. Goral, M. I. Nelen, A. V. Eliseev and J.-M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 1347–1352 CrossRef CAS PubMed.
- M. Schmittel and K. Mahata, Angew. Chem., Int. Ed., 2008, 47, 5284–5286 CrossRef CAS PubMed.
- A. Wilson, G. Gasparini and S. Matile, Chem. Soc. Rev., 2014, 43, 1948–1962 RSC.
- M. L. Saha, S. De, S. Pramanik and M. Schmittel, Chem. Soc. Rev., 2013, 42, 6860–6909 RSC.
- L. A. Wessjohann, D. G. Rivera and F. León, Org. Lett., 2007, 9, 4733–4736 CrossRef CAS PubMed.
- J.-B. Lin, X.-N. Xu, X.-K. Jiang and Z.-T. Li, J. Org. Chem., 2008, 73, 9403–9410 CrossRef CAS PubMed.
- Q. Ji, R. C. Lirag and O. Š. Miljanić, Chem. Soc. Rev., 2014, 43, 1873–1884 RSC.
- Y.-M. Legrand, A. van der Lee and M. Barboiu, Inorg. Chem., 2007, 46, 9540–9547 CrossRef CAS PubMed.
- M. L. Saha and M. Schmittel, Org. Biomol. Chem., 2012, 10, 4651–4684 Search PubMed.
- C.-F. Chow, S. Fujii and J.-M. Lehn, Chem. Commun., 2007, 4363–4365 RSC.
- P. N. W. Baxter, J.-M. Lehn and K. Rissanen, Chem. Commun., 1997, 1323–1324 RSC.
- M. Hutin, C. J. Cramer, L. Gagliardi, A. R. M. Shahi, G. Bernardinelli, R. Cerny and J. R. Nitschke, J. Am. Chem. Soc., 2007, 129, 8774–8780 CrossRef CAS PubMed.
- K. Osowska and O. S. Miljanić, J. Am. Chem. Soc., 2011, 133, 724–727 CrossRef CAS PubMed.
- K. Osowska and O. Š. Miljanić, Angew. Chem., Int. Ed., 2011, 50, 8345–8349 CrossRef CAS PubMed.
- Q. Ji and O. Š. Miljanić, J. Org. Chem., 2013, 78, 12710–12716 CrossRef CAS PubMed.
- S. De, K. Mahata and M. Schmittel, Chem. Soc. Rev., 2010, 39, 1555–1575 RSC.
- K. Mahata and M. Schmittel, J. Am. Chem. Soc., 2009, 131, 16544–16554 CrossRef CAS PubMed.
- M. Schmittel and K. Mahata, Chem. Commun., 2008, 2550–2552 RSC.
- I. Kocsis, D. Dumitrescu, Y.-M. Legrand, A. van der Lee, I. Grosu and M. Barboiu, Chem. Commun., 2014, 50, 2621–2623 RSC.
- J. R. Nitschke and J.-M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 11970–11974 CrossRef CAS PubMed.
- P. Kovaříček and J.-M. Lehn, J. Am. Chem. Soc., 2012, 134, 9446–9455 CrossRef PubMed.
- P. Kovaříček and J.-M. Lehn, Chem.–Eur. J., 2015, 21, 9380–9384 CrossRef PubMed.
- (a) For general considerations on the notion of “simplexity” in particular in a biological context, see A. Berthoz, La simplexité, Odile Jacob, 2009; (b) For an elaboration in the context of organic synthesis, see: Ph. Compain, L'actualité Chimique, 2003, april-may issue, 129–134.
- (a) For an early case, see the competitive selection in the generation of double and triple helicates in an interconverting set of ligands and metal ions: see R. Krämer, J.-M. Lehn and A. Marquis-Rigault, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 5394–5398 CrossRef CAS PubMed; (b) For the effect of increased complexity in self-sorting, see: K. Mahata and M. Schmittel, Beilstein J. Org. Chem., 2011, 7, 1555–1561 Search PubMed.
- J.-M. Lehn, in Constitutional Dynamic Chemistry, ed. M. Barboiu, Springer, Berlin Heidelberg, 2012, pp. 1–32 Search PubMed.
- J.-M. Lehn, Supramolecular chemistry: concepts and perspectives, VCH, Weinheim, 1995 Search PubMed.
- J.-M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4763–4768 CrossRef CAS PubMed.
- I. Saur and K. Severin, Chem. Commun., 2005, 1471–1473 RSC.
- S. Ulrich and J.-M. Lehn, J. Am. Chem. Soc., 2009, 131, 5546–5559 CrossRef CAS PubMed.
- C. Bohne, Chem. Soc. Rev., 2014, 43, 4037–4050 RSC.
- M. Chaur, S. Kulchat, J.-M. Lehn, and work in progress.
- M. Ciaccia, R. Cacciapaglia, P. Mencarelli, L. Mandolini and S. Di Stefano, Chem. Sci., 2013, 4, 2253–2261 RSC.
- C. Fasting, C. A. Schalley, M. Weber, O. Seitz, S. Hecht, B. Koksch, J. Dernedde, C. Graf, E.-W. Knapp and R. Haag, Angew. Chem., Int. Ed., 2012, 51, 10472–10498 CrossRef CAS PubMed.
- P. G. M. Wuts and T. W. Greene, Greene's protective groups in organic synthesis, Wiley-Interscience, Hoboken, N.J, 4th edn, 2007 Search PubMed.
- K. C. Nicolaou, Classics in total synthesis: targets, strategies, methods, VCH, Weinheim, New York, 1996 Search PubMed.
- P. S. Baran, T. J. Maimone and J. M. Richter, Nature, 2007, 446, 404–408 CrossRef CAS PubMed.
- K. C. Nicolaou, J. Org. Chem., 2009, 74, 951–972 CrossRef CAS PubMed.
- C.-H. Wong and S. C. Zimmerman, Chem. Commun., 2013, 49, 1679–1695 RSC.
- Y. Li and X. Liu, Chem. Commun., 2014, 50, 3155–3158 RSC.
- S. Mundinger, U. Jakob and W. Bannwarth, Chem.–Eur. J., 2014, 20, 1258–1262 CrossRef CAS PubMed.
- P. Bey and J. P. Vevert, Tetrahedron Lett., 1977, 18, 1455–1458 CrossRef.
- B. W. Metcalf and P. Casara, Tetrahedron Lett., 1975, 16, 3337–3340 CrossRef.
- R. Polt and M. A. Peterson, Tetrahedron Lett., 1990, 31, 4985–4986 CrossRef CAS.
- J. M. Hornback and B. Murugaverl, Tetrahedron Lett., 1989, 30, 5853–5856 CrossRef CAS.
- M. Bergmann and L. Zervas, Ber. Dtsch. Chem. Ges. B, 1931, 64, 975–980 CrossRef.
- M. A. Peterson and R. Polt, J. Org. Chem., 1993, 58, 4309–4314 CrossRef CAS.
- E. J. Corey, A. Guzman-Perez and M. C. Noe, J. Am. Chem. Soc., 1995, 117, 10805–10816 CrossRef CAS.
- J. N. Williams Jr and R. M. Jacobs, Biochem. Biophys. Res. Commun., 1966, 22, 695–699 CrossRef CAS.
- J. C. Sheehan and V. J. Grenda, J. Am. Chem. Soc., 1962, 84, 2417–2420 CrossRef.
- A. R. Khomutov, A. S. Shvetsov, J. J. Vepsäläinen and A. M. Kritzyn, Tetrahedron Lett., 2001, 42, 2887–2889 CrossRef CAS.
- J. D. Prugh, L. A. Birchenough and M. S. Egbertson, Synth. Commun., 1992, 22, 2357–2360 CrossRef CAS.
- J. S. McManis and B. Ganem, J. Org. Chem., 1980, 45, 2041–2042 CrossRef CAS.
- B. Ganem, Acc. Chem. Res., 1982, 15, 290–298 CrossRef CAS.
- J. C. Bradley, J. P. Vigneron and J. M. Lehn, Synth. Commun., 1997, 27, 2833–2845 CrossRef CAS.
- D. R. Mootoo and B. Fraser-Reid, Tetrahedron Lett., 1989, 30, 2363–2366 CrossRef CAS.
- K.-J. Fasth, G. Antoni and B. Langström, J. Chem. Soc., Perkin Trans. 1, 1988, 3081–3084 RSC.
- S. Srinivasachari, Y. Liu, G. Zhang, L. Prevette and T. M. Reineke, J. Am. Chem. Soc., 2006, 128, 8176–8184 CrossRef CAS PubMed.
- B. Frydman, S. Bhattacharya, A. Sarkar, K. Drandarov, S. Chesnov, A. Guggisberg, K. Popaj, S. Sergeyev, A. Yurdakul, M. Hesse, H. S. Basu and L. J. Marton, J. Med. Chem., 2004, 47, 1051–1059 CrossRef CAS PubMed.
- S. K. Sharma, Y. Wu, N. Steinbergs, M. L. Crowley, A. S. Hanson, R. A. Casero and P. M. Woster, J. Med. Chem., 2010, 53, 5197–5212 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, NMR spectra, synthetic protocols and mathematical model for formal dynamic combinatorial library description. See DOI: 10.1039/c5sc04924e |
| ‡ Current address: Department of Low-dimensional Systems, J. Heyrovsky Institute of Physical Chemistry, Dolejškova 2155/3, 18223 Prague 8, Czech Republic. |
| § Current address: Merck KGaA, Frankfurter Straße 250, 64293 Darmstadt, Germany. |
| ¶ Current address: Department of Chemistry, University of Cambridge, Lensfield Road, CB2 1EW Cambridge, United Kingdom. |
| || Current address: Institute of Chemical Technology Prague, Department of Research and Development, Technická 5, 160 00 Prague, Czech Republic. |
| This journal is © The Royal Society of Chemistry 2016 |