Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recyclable Cu(I)/melanin dots for cycloaddition, bioconjugation and cell labelling†
Yao
Sun
ab,
Suhyun
Hong
a,
Xiaowei
Ma
a,
Kai
Cheng
a,
Jing
Wang
a,
Zhe
Zhang
a,
Meng
Yang
c,
Yuxin
Jiang
c,
Xuechuan
Hong
*b and
Zhen
Cheng
*a
aMolecular Imaging Program at Stanford (MIPS), Bio-X Program, Department of Radiology, Canary Center at Stanford for Cancer Early Detection, Stanford University, California 94305-5344, USA. E-mail: zcheng@stanford.edu
bState Key Laboratory of Virology, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (MOE), Hubei Provincial Key Laboratory of Developmentally Originated Disease, Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China. E-mail: xhy78@whu.edu.cn
cChinese Academy of Medical Science, Peking Union Medical College Hospital, Department of Ultrasound, Beijing, 100730, China
First published on 20th May 2016
Abstract
Development of biocompatible and high-performance heterogeneous catalysts for bioconjugation and cell labeling is highly challenging. Melanin has previously been used as a target for melanoma imaging and therapy. Herein, this important biomarker was transferred into a novel catalytic platform. A biocompatible Cu(I)/melanin dot-based catalyst [Cu(I)/M-dots] was easily prepared and exhibited high catalytic activity and excellent reusability in various Cu(I)-catalyzed azide-alkyne cycloadditions (CuAAC). Furthermore, DNA bioconjugation was carried out efficiently using Cu(I)/M-dots under ligand-free and reductant-free conditions, and the Cu(I)/M-dots could easily be removed by centrifugation. Lastly, the integrin receptor (alkyne RGD targeted) of U87MG cells was effectively labelled with a fluorescent dye (Cyanine5.5 azide) in combination with Cu(I)/M-dots. These attractive properties of Cu(I)/M-dots render it a promising catalytic platform in bioconjugation and chemical biology applications.
Introduction
In recent years, tremendous efforts have been made in the development of biocompatible, biodegradable and renewable materials for various chemical and biomedical applications.1–4 Therefore, a variety of natural materials such as proteins, RNA, polysaccharides, etc., have been actively explored to serve as molecular platforms to create next generation materials for drug delivery, imaging agents and theranostic applications.5–7 Melanin, a well-known biopolymer that is widely distributed in many living organisms, is particularly attractive owing to its excellent physical and chemical properties, including strong metal ion (Fe3+, Gd3+, Cu2+, Cu+, Zn2+, etc.) trapping ability and free radical quenching ability.8,9 Melanin has traditionally been used as a tumor biomarker for melanoma imaging and therapy.10 Recently, by mimicking natural melanin, our group has prepared artificial ultrasmall size (<10 nm) melanin nanoparticles (named as melanin dots, M-dots) and further used it as a nanoplatform for tumor multimodality imaging and drug delivery.11,12 It has been demonstrated that the M-dots platform not only exhibits good biocompatibility and water solubility, but also actively chelates various metal ions via its intrinsic chelating functional groups. Encouraged by these promising properties, we propose to use M-dots directly as a support for catalytic applications, which presents the following advantages: (1) the natural nitrogen groups of M-dots can coordinate metal ions and the resulting heterogeneous catalyst can greatly accelerate the kinetics of metal-catalyzed reactions; (2) the water-solubility of M-dots conveniently performs catalytic reactions under aqueous solvents and it can easily be removed by centrifugation; (3) the high stability and biocompatibility of M-dots are attractive for biological applications; (4) M-dots are able to scavenge radical molecules produced by metals under reducing conditions, which is one major challenges of current metal-mediated bioconjugate chemistry.13 Therefore, these excellent advantages could greatly expand the applications of heterogeneous catalytic systems in bioconjugation and chemical biology.Within the last decade, bioorthogonal reactions have become a powerful tool for the selective modification of biomolecules in living systems.14,15 One hallmark of bioorthogonal chemistry is the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click reaction which was discovered by Sharpless and Meldal in early 2000s.16,17 However, the use of the CuAAC in a bioconjugation and cellular context has been limited because of the toxicity associated with Cu(I).18 For example, recent studies have shown that the Cu(I)-mediated generation of reactive oxygen species (ROS) from O2 can cause detrimental consequences to cellular metabolism.19 To address this problem, several water-soluble Cu(I) ligands have been designed as Cu(I) stabilizers to reduce the cellular toxicity of copper.20 Another approach was recently reported by the Ting and Taran groups, using highly reactive azides according to the concept of a chelation-assisted CuAAC reaction.21,22 Despite the significant progress made, it is still very difficult to remove residual Cu(I) from conjugated products. This raises concerns about protein or DNA damage/precipitation and the preservation of their structural integrity.13 Furthermore, in order to stabilize the Cu(I) oxidation state for efficient catalysis, a large amount of chelating ligands and reducing agents are usually required. It could induce further damage to cellular metabolism during a labeling reaction.23 These challenges prompted us to explore a novel recyclable Cu(I) catalyst that performs click reactions under ligand-free and reductant-free conditions, which will lead to a major step forward in bioconjugation and chemical biology.
Considering the merits of M-dots, we envision that loading Cu(I) into M-dots can obtain a ligand-free, water-soluble, biocompatible and recyclable Cu(I) catalyst. Moreover, intrinsic nitrogen groups presented in M-dots can coordinate the metal ions and the resulting Cu(I)/M-dots can greatly accelerate the kinetics of Cu(I)-catalyzed reactions and stabilize the Cu(I) oxidation state. As a proof of concept, we prepared a novel Cu(I)/M-dot catalyst and demonstrated its excellent properties when applied in the CuAAC reaction, DNA bioconjugation and cell labeling (Fig. 1). To the best of our knowledge, reports of metal-based heterogeneous catalysis in biological applications are still scarce compared to their homogeneous counterparts.24,25
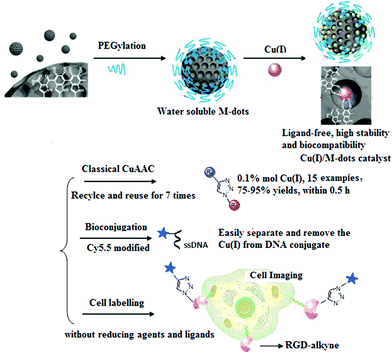 | ||
| Fig. 1 The preparation of Cu(I)/M-dots heterogeneous catalyst and its applications in classic CuAAC reactions, bioconjugation and cell labeling. | ||
Results and discussion
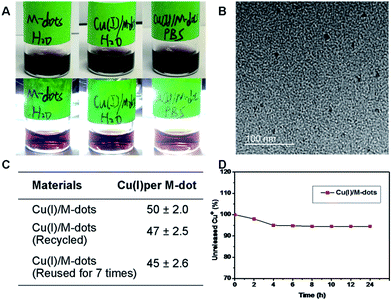
Fig. 1 schematically illustrates the procedure for preparing the Cu(I)/M-dots heterogeneous catalyst. The M-dots were synthesized from commercial melanin granules according to a previous report.12 The average sizes of M-dots were ∼7 nm measured by transmission electron microscopy (TEM) (ESI Fig. S1A†). The hydrodynamic diameters of M-dots were ∼7.45 nm by dynamic light scattering (DLS), and the zeta potential of M-dots was −2.2 mV (ESI Table S1†). The 1H-NMR of M-dots in D2O display a characteristic signal at 3.58 ppm due to the hydrogen atoms of the PEG chains (ESI Fig. S1B†). The molecular weight of the M-dots was about 86 kDa determined by MALDI-TOF MS (ESI Fig. S1C†). All these data confirm the successful preparation of M-dots. In our previous work, PEG encapsulation was demonstrated to enhance biocompatibility, metal loading and water-solubility.12Encouraged by the vast applications of CuAAC chemistry, the preparation of Cu(I)/M-dots was undertaken (see ESI†). The Cu(I)/M-dots were purified using a centrifugal filter (MWCO = 30 kDa). After purification, the fresh Cu(I)/M-dots catalyst maintained good water and phosphate buffer (PBS)-solubility without any observed precipitation (Fig. 2A). A TEM image indicates that Cu(I)/M-dots were monodispersed and homogeneous in water (Fig. 2B). The Cu(I)/M-dots exhibited good Cu(I) loading capacities. The number of freshly prepared Cu(I) per single M-dot was determined to be 50 ± 2.0 (mean ± SD, Fig. 2C) according to the inductively coupled plasma mass spectrometry (ICP-MS) results. After Cu(I)-chelation, the size of the Cu(I)/M-dots increased to ∼9 nm and its zeta-potential went from −2.2 mV to +5.1 mV due to the introduction of positive Cu(I) on the M-dots surface (ESI Table S1†). The presence of Cu(I) on the M-dots platform was confirmed by X-ray photoelectron spectroscopy (XPS) (ESI Fig. S2†). The binding energy peaks at 931.6 and 952.8 eV can be attributed to those characteristic of Cu(I).26 The possible chelating mechanisms of Cu(I) with M-dots is shown in Fig. S3.† A stability assay of Cu(I)/M-dots in PBS indicates the high stability of the chelating platform (Fig. 2D). Moreover, we investigated the biocompatibility of the Cu(I)/M-dots compared to the classic Cu(I)/THPTA complex (abbreviation THPTA: [tris(3-hydroxypropyltriazolylmethyl)amine], a widely used and water-soluble ligand for CuAAC) on two cell lines: mouse embryonic fibroblast cell line (NIH3T3) and human glioblastoma cell line (U87MG) with different Cu(I) concentrations (50, 100, 200 µM) and different incubation times (1, 12, 24, 48 h). For a short incubation time (1 h), both Cu(I)/M-dots and Cu(I)/THPTA systems were non-toxic to two cell lines. However, at longer incubation times (12, 24, and 48 h) an increased cytotoxicity was observed at all copper concentrations (50 µM to 200 µM) for Cu(I)/THPTA. In contrast, no toxicity of Cu(I)/M-dots was observed according to dimethylthiazolyl-diphenyltetrazolium (MTT) assays for all time points (Fig. S4 and S5†). These results clearly demonstrate that Cu(I)/M-dots show high biocompatibility and are promising for further biological applications.
Ascorbate-driven, Cu(I)-induced hydroxyl radical production is a major issue in CuAAC bioconjugate chemistry.13 Therefore, we investigated the free radical quenching ability of M-dots in a model reaction with 3-coumarin carboxylic acid (3-CCA, hydroxyl radical detector) in the presence of Cu(II)/ascorbate.27 3-CCA was stable in the presence of CuSO4 or ascorbate alone in PBS, but the combination of the two induced the oxidation of approximately 50% of 3-CCA to 7-OH CCA after 20 h (ESI Fig. S6†). Therefore, M-dots not only functioned as a traditional support for a heterogeneous catalyst, but also scavenged radical molecules to reduce the toxicity induced by Cu(I), which can greatly expand its biological application.
The relative reactivity of M-dots-based heterogeneous catalysts, Cu(I)/M-dots and Cu(II)/M-dots with or without ascorbate was evaluated using a coumarin-based fluorogenic probe which enabled us to monitor the reaction by simple fluorescence measurements of the corresponding triazole product (ESI Fig. S7,† ex = 320 nm; em = 400 nm).28 As expected, Cu(I)/M-dots afforded high yields, and the kinetics was improved by increasing the amount of Cu(I)/M-dots. A significantly lower yield (<5%) was observed under Cu(II)/M-dots treatment. However, a high yield was obtained by the addition of ascorbate to the Cu(II)/M-dots system. Thus, the function of M-dots appears to be crucial not only for maintaining the stability of the Cu(I) catalyst, but also for greatly accelerating the reaction.
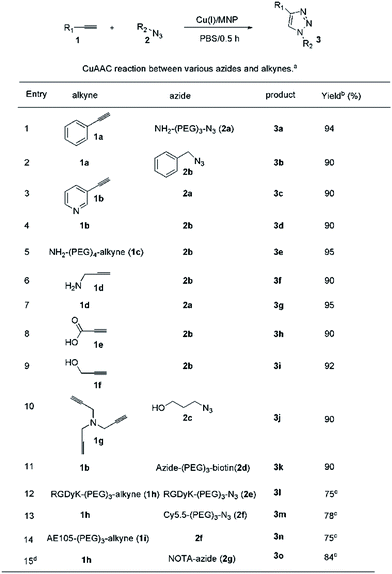
Based on these promising results, the scope of the CuAAC was examined using various substrates (Fig. 3). To our delight, the Cu(I)/M-dots system was efficient for each substrate (only 0.1% mol Cu+), leading to yields of about 90% in the absence of ascorbate and ligands (entries 1–10). These result confirm that Cu(I) was stabilized within the M-dots thus avoiding the use of a reducing agent. Moreover, the construction of biomolecules of medical interest was also explored (entries 11–15). Derivatives of biotin 3k and dimeric RGD 3l were obtained in good yields (entry 11–12). The imaging precursors/probes (Cy5.5-RGD (abbreviation Cy5.5: Cyanine5.5, 3m), Cy5.5-AE105 (3n) and NOTA-RGD (abbreviation NOTA: 1,4,7-triazacyclononane-triacetic acid, 3o)) were also prepared in 78%, 75% and 84% yields, respectively (entries 13–15). 3o was further efficiently labelled with a PET radionuclide, 64Cu, in 100% radiolabelling yield (ESI Fig. S8†). This indicates that no free Cu(I) escaped from the M-dots, which further confirms the stability of the Cu(I)/M-dots catalyst under CuAAC conditions.
Further experiments showed that the Cu(I)/M-dots was a robust and recyclable catalyst. It was recycled by centrifugation and used for seven cycles without loss of catalytic activity (ESI Table S2,† entries 1–7). Quantitative analysis of the product from each cycle by ICP-MS revealed no detectable leaching of Cu(I). TEM and photo images of reused Cu(I)/M-dots reveal that the structure of the catalyst remained intact and stable (ESI Fig. S9†). Moreover, the Cu(I)/M-dots system also efficiently drives the three-component click reaction of alkyl halides, sodium azide and alkynes with good recyclability (ESI Table S3 and Fig. S10†).
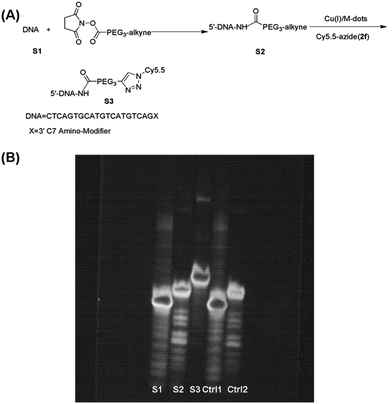
The utility of the Cu(I)/M-dots for bioconjugation was tested on an alkyne derivatised DNA strand with an azide fluorophore in low concentrations. The 3′-position modified oligonucleotide (S1, 5 nmol) in 20 µL of water was reacted with an excess of NHS ester V1 (50 equivalents) in 30 µL of water at room temperature for 12 h. Unreacted V1 was removed by a centrifugal filter (3 kDa), and then lyophilized to obtain alkyne-labelled DNA S2. A click reaction of S2 (20 µM) was then performed with Cy5.5-azide (2f, 80 µM) mediated by 2 µM Cu(I)/M-dots (containing about 100 µM Cu+) in 0.1 M PBS at pH 7 for 2 h (Fig. 4A). Unreacted Cy5.5 dye was removed by centrifugal filter (MWCO = 3 kDa). Then, the resulting fluorescent DNA S3 was separated from the catalyst by centrifugal filter (MWCO = 10 kDa). S1, S2, S3 and two control groups were loaded on a 16% denaturing PAGE gel for electrophoresis (15 mA, 3 h and 1× TBE buffer). Then, the DNA PAGE gel was imaged under 365 nm (UVP, UV imager) revealing that DNA bioconjugation was efficiently carried out only in the presence of Cu(I)/M-dots (Fig. 4B). In addition, MALDI-TOF MS showed an expected molecular weight for the corresponding cycloadduct (ESI Fig. S11†). Unlike traditional homogeneous Cu(I)/ligand/NaAsc systems, the Cu(I)/M-dots did not require a reducing agent, which is known to cause undesirable side effects such as covalent modification and potential aggregation of proteins.29,30 Moreover, the Cu(I)/M-dots system can easily be separated from biomolecules and can thus solve the problem of contamination from the residual Cu(I) catalyst.
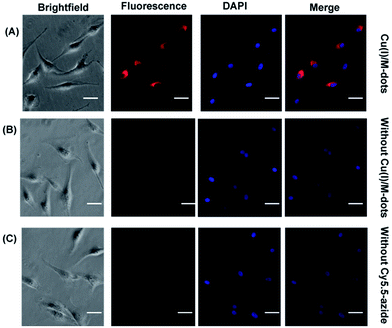
To assess the capability of the Cu(I)/M-dots for cell labelling, we used Cy5.5 azide and RGD-alkyne in combination with Cu(I)/M-dots for imaging of the integrin αVβ3 receptor. Human glioblastoma U87MG cells were selected because of their high level of integrin αVβ3 expression.31 The U87MG cells were first labelled with RGD-alkyne (1h, 10 µM) to cap the alkyne functional group on the integrin protein due to the strong binding ability of the RGD peptide with the αVβ3 integrin receptor.32 After several washing steps to remove any unbound alkyne, Cy5.5 azide (2f, 30 µM) and 4 µM Cu(I)/M-dots (containing about 200 µM Cu+) were added to the cell culture medium for 60 min at 37 °C. After the click reaction for labelling live cells and several PBS washing steps to remove Cy5.5 azide and Cu(I)/M-dots, the U87MG cells were fixed with 4% paraformaldehyde (PFA) and further stained with 4′,6-diamidino-2-phenylindole (DAPI) to reveal the location of the nuclei. The cells were then imaged using fluorescence microscopy (Axiovert 200M fluorescence microscope). Control studies were performed in the absence of Cy5.5 azide or Cu(I)/M-dots. The recorded microscopy images reveal a strong fluorescent signal of the U87MG cells incubated with RGD-alkyne, Cy5.5 azide and Cu(I)/M-dots (Fig. 5A), whereas much weaker signals are observed for the control groups (Fig. 5B and C). The results clearly indicate that the Cu(I)/M-dots enable efficient performing of the CuAAC on the cell membrane.
Conclusions
In conclusion, we reported M-dots as an effective nanoplatform for preparing a ligand-free and water-soluble heterogeneous catalyst, Cu(I)/M-dots. Cu(I)/M-dots is proven to be a high performance, biocompatible and recyclable catalytic system for CuAAC and bioconjugation without any Cu(I) toxicity. M-dots can serve as an novel metal-loading recyclable catalyst for various applications in bioconjugation and chemical biology.Acknowledgements
This work was partially supported by the Office of Science (BER), U.S. Department of Energy (DE-SC0008397) (ZC), International S&T Cooperation Program of China (2015DFA30440, 2014DFB30020), NSFC (No. 81373254 and 21390402), NSFHP (2014CFB704), the Applied Basic Research Program of Wuhan Municipal Bureau of Science and Technology (2015060101010031), the Fundamental Research Funds for the Central Universities, and the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2012ZX10004801-003-011).Notes and references
- K. Cheng, M. Yang, R. Zhang, C. Qin, X. Su and Z. Cheng, ACS Nano, 2014, 8, 9884–9896 CrossRef CAS PubMed.
- K. Yang, H. Xu, L. Cheng, C. Sun, J. Wang and Z. Liu, Adv. Mater., 2012, 24, 5586–5592 CrossRef CAS PubMed.
- E. Huynh, B. Y. Leung, B. L. Helfield, M. Shakiba, J. A. Gandier, C. S. Jin, E. R. Master, B. C. Wilson, D. E. Goertz and G. Zheng, Nat. Nanotechnol., 2015, 10, 325–332 CrossRef CAS PubMed.
- M. X. You, Y. Chen, L. Peng, D. Han, B. C. Yin, B. C. Ye and W. H. Tan, Chem. Sci., 2011, 2, 1003–1010 RSC.
- J. F. Lovell, E. Huynh, T. D. MacDonald, Q. Lin and G. Zheng, Nanomedicine, 2011, 6, 1157–1158 CrossRef PubMed.
- D. Luque, et al. , Chem. Sci., 2014, 5, 575–581 RSC.
- F. Liu, J. Mu and B. G. Xing, Curr. Pharm. Des., 2015, 21, 1866–1888 CrossRef CAS PubMed.
- P. Meredith and T. Sarna, Pigm. Cell Res., 2006, 19, 572–594 CrossRef CAS PubMed.
- S. Takeuchi, W. Zhang, K. Wakamatsu, V. Hearing, K. Kraemer and D. Brash, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 15076–15081 CrossRef CAS PubMed.
- C. Qin, K. Cheng, K. Chen, X. Hu, Y. Liu, X. Lan, Y. Zhang, H. Liu, Y. Xu, L. Bu, X. Su, X. Zhu, S. Meng and Z. Cheng, Sci. Rep., 2013, 3, 1490 Search PubMed.
- R. Zhang, Q. Fan, M. Yang, K. Cheng, X. Lu, W. Huang and Z. Cheng, Adv. Mater., 2015, 27, 5063–5069 CrossRef CAS PubMed.
- Q. Fan, K. Cheng, X. Hu, X. Ma, R. Zhang, M. Yang, X. Lu, L. Xing, W. Huang, S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 15185–15194 CrossRef CAS PubMed.
- V. Hong, S. I. Presolski, C. Ma and M. G. Finn, Angew. Chem., Int. Ed., 2009, 48, 9879–9883 CrossRef CAS PubMed.
- M. Y. Yang, J. Li and P. R. Chen, Chem. Soc. Rev., 2014, 43, 6511–6526 RSC.
- J. A. Prescher and C. R. Bertozzi, Nat. Chem. Biol., 2005, 1, 13–21 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef CAS PubMed.
- V. Hong, N. F. Steinmetz, M. Manchester and M. G. Finn, Bioconjugate Chem., 2010, 21, 1912–1916 CrossRef CAS PubMed.
- L. M. Gaetke and C. K. Chow, Toxicology, 2003, 189, 147–163 CrossRef CAS PubMed.
- W. G. Lewis, F. G. Magallon and V. V. Fokin, J. Am. Chem. Soc., 2004, 126, 9152–9153 CrossRef CAS PubMed.
- C. Uttamapinant, P. Slade, K. R. Gee and A. Y. Ting, Angew. Chem., Int. Ed., 2012, 51, 5852–5856 CrossRef CAS PubMed.
- V. Bevilacqua, M. King, M. Chaumontet, S. Gabillet, D. Buisson, C. Puente, A. Wagner and F. Taran, Angew. Chem., Int. Ed., 2014, 53, 5872–5876 CrossRef CAS PubMed.
- R. H. Nagaraj, D. R. Shell, M. Prabhakaram, B. J. Ortwerth and V. M. Monnier, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 10257–10261 CrossRef CAS.
- J. T. Weiss, J. C. Dawson, K. G. Macleod, W. Rybski, C. Fraser, C. Torres-sanchez, E. E. Patton, M. Bradley, N. O. Carragher and A. Unciti-Broceta, Nat. Chem., 2014, 5, 3277 Search PubMed.
- J. T. Weiss, J. C. Dawson, C. Fraser, W. Rybski, C. Torres-sanchez, M. Bradley, E. E. Patton, N. O. Carragher and A. Unciti-Broceta, J. Med. Chem., 2014, 57, 5395–5404 CrossRef CAS PubMed.
- X. Qiu, M. Miyauchi, K. Sunada, M. Minoshima, M. Liu, Y. Lu, D. Li, Y. Shimodaira, Y. Hosoqi, Y. Kuroda and K. Hashimoto, ACS Nano, 2012, 6, 1609–1618 CrossRef CAS PubMed.
- G. Meloni, V. Sonois, T. Delaine, L. Guilloreau, A. Gillet, J. Teissie, P. Faller and M. Vasak, Nat. Chem. Biol., 2008, 4, 366–372 CrossRef CAS PubMed.
- Z. Zhou and C. J. Fahrni, J. Am. Chem. Soc., 2004, 126, 8862–8863 CrossRef CAS PubMed.
- L. Enrique, F. M. Eduardo and R. Ricardo, J. Am. Chem. Soc., 2009, 131, 5748–5750 CrossRef PubMed.
- P. Y. Liu, N. Jiang, J. Zhang, X. Wei, H. H. Lin and X. Q. Yu, Chem. Biodiversity, 2006, 9, 958–966 Search PubMed.
- A. Roxin, J. Chen, C. C. Scully, B. H. Rotstein, A. K. Yudin and G. Zheng, Bioconjugate Chem., 2012, 23, 1387–1395 CrossRef CAS PubMed.
- Y. Sun, X. W. Ma, K. Cheng, B. Y. Wu, J. L. Duan, H. Chen, L. H. Bu, R. P. Zhang, X. M. Hu, Z. X. Deng, L. Xing, X. C. Hong and Z. Cheng, Angew. Chem., Int. Ed., 2015, 20, 5981–5984 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc01536k |
| This journal is © The Royal Society of Chemistry 2016 |