Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel benzo-bis(1,2,5-thiadiazole) fluorophores for in vivo NIR-II imaging of cancer†
Yao
Sun‡
ab,
Chunrong
Qu‡
a,
Hao
Chen
b,
Maomao
He
a,
Chu
Tang
b,
Kangquan
Shou
b,
Suhyun
Hong
b,
Meng
Yang
c,
Yuxin
Jiang
c,
Bingbing
Ding
a,
Yuling
Xiao
a,
Lei
Xing
b,
Xuechuan
Hong
*a and
Zhen
Cheng
*b
aState Key Laboratory of Virology, Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (MOE) and Hubei Provincial Key Laboratory of Developmentally Originated Disease, Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China. E-mail: xhy78@whu.edu.cn
bMolecular Imaging Program at Stanford (MIPS), Bio-X Program, and Department of Radiology, Canary Center at Stanford for Cancer Early Detection, Stanford University, California 94305-5344, USA. E-mail: zcheng@stanford.edu
cChinese Academy of Medical Science, Peking Union Medical College Hospital, Department of Ultrasound, Beijing, 100730, China
First published on 16th June 2016
Abstract
Optical imaging of diseases represents a highly dynamic and multidisciplinary research area, and second near-infrared window (NIR-II, 1000–1700 nm) imaging is at the forefront of the research on optical imaging techniques. Small-molecule based NIR-II (1000–1700 nm) dyes are highly promising candidates for in vivo molecular imaging because of their high biocompatibility, fast excretion, and high clinical translation ability. However, research reports on small-molecule based NIR-II dyes and probes are rare. Herein, we designed a series of fluorescent compounds (Q1, Q2, Q3, and Q4) and investigated the relationships between their structures and absorption/fluorescence properties. Q4 (maximum emission at 1100 nm) stood out as the dye with the best physical properties and thus was selected as a scaffold for the facile construction of two types of water-soluble and biocompatible NIR-II probes (Q4NPs and SCH1100). Highly specific gastrin-releasing peptide receptor (GRPR) targeted NIR-II imaging of prostate cancer in living mice was achieved using the small-molecule probe SCH1100, which represents the first small peptide based NIR-II probe for targeted cancer imaging. The attractive imaging properties of Q4-based NIR-II probes open up many opportunities for molecular imaging and clinical translation in the unique NIR-II window.
Introduction
Optical imaging of diseases represents a highly dynamic and multidisciplinary research area. Over the last decade it has attracted extensive research attention from scientists working in a variety of fields such as chemistry, materials science, biotechnology, nanotechnology, biomedicine, etc.1–3 Optical imaging probes and techniques are expected to realize early cancer diagnosis and imaging guided therapy, and thus bring high impact to clinical cancer management.4,5In vivo fluorescence imaging of biological systems in the second near-infrared window (NIR-II, 1000–1700 nm) is at the forefront of the research on optical imaging techniques, and it holds great promise owing to minimal autofluorescence and tissue scattering in this region, leading to deep tissue imaging capability, high spatial resolution, and high contrast.6,7 Moreover, recent studies suggest that fluorophores with emission in the NIR-II region can dramatically improve the imaging quality and signal-to-noise ratio compared to those used in the traditional NIR window I (NIR-I) region (650–900 nm).8,9 Developing novel NIR-II fluorophores and molecular probes for in vivo imaging applications thus has high significance and direct impact on the field of biomedicine.To date, nanoparticle based systems, including single-walled carbon nanotubes (SWNTs),10,11 semiconducting quantum dots (QDs),12,13 rare-earth doped nanoparticles14 and conjugated polymers,15 have been actively explored for NIR-II fluorescence imaging. Considering the translation of NIR-II imaging agents into clinical applications, however, small-molecule based probes remain to be the most desirable and optimal candidates for this because of their high biocompatibility, fast excretion, quality control under the Current Good Manufacturing Practice (cGMP) conditions, and easy and robust preparation.16,17 Therefore, developing small-molecule based NIR-II fluorophores and probes with desirable chemical and physical properties, favorable excretion pharmacokinetics, minimal cellular toxicity, and clinical translation ability is crucial and highly demanded. It represents an emerging field in bioimaging and chemical research.
Developing small-molecule NIR-II dyes is conceptually straightforward but highly challenging in reality. After several years' efforts, we have recently reported a NIR-II fluorophore based on a small organic molecule (CH1055) that is rapidly excreted renally (90% excreted within 24 h).18 This compound was further successfully conjugated with a small protein, an anti-epidermal growth factor receptor (EGFR) affibody, for molecular imaging of abnormalities in vivo. The resulting probe achieved superior tumor imaging quality and tumor-to-background signal ratios. However, the capability of using CH1055 as a generic NIR-II reporter for broad use remains unknown, especially using CH1055 for the modification of small peptides or small molecules has not been demonstrated. Moreover, despite this promising example, other small-molecule NIR-II agents for in vivo imaging have not been reported.
Hence, in this work, we design a new type of NIR-II dye with a different core structure from CH1055. The donor–acceptor–donor (D–A–D) type core is the key for obtaining NIR-II dyes.19 Based on the D–A–D scaffold, herein we incorporated a thiophene spacer to design and synthesize a small library of fluorescent compounds (Q1, Q2, Q3, and Q4) and investigated the relationships between their structures and absorption/fluorescence properties (Fig. 1a). Furthermore, the most promising dye, Q4, of these four compounds was identified and used to prepare two distinctive NIR-II probes. One probe includes Q4 encapsulated organic nanoparticles (Q4NPs) for NIR-II imaging of tumor blood vessels, the other is composed of Q4 successfully conjugated with a small bombesin peptide to prepare the probe SCH1100 for gastrin-releasing peptide receptors (GRPR) targeted NIR-II imaging of prostate cancer in living mice. These novel small-molecule based NIR-II dyes expand the library of NIR-II fluorophores to meet the demands of using the NIR-II imaging technique for a variety of applications.
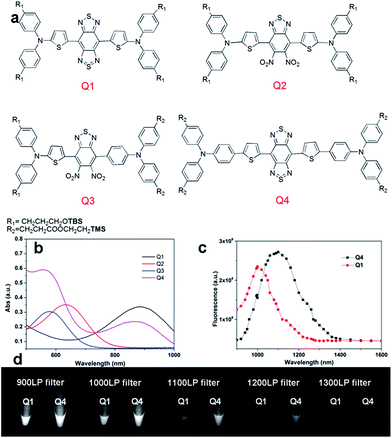 | ||
| Fig. 1 (a) Design of NIR-II dyes based on the D–A–D scaffold and the chemical structures of Q1–Q4, TBS = Si(Me)2tBu, TMS = SiMe3; (b) UV absorbance of Q1–Q4; (c) NIR-II fluorescence emission of Q1 and Q4 with peaks at ∼1000 nm and ∼1100 nm under 808 nm excitation (exposure time: 10 ms); the emission of Q2 and Q3 is in the NIR-I region (Fig. S2,† data not shown here). (d) NIR-II signals of Q1 and Q4 with various long-pass (LP) filters (900–1400 nm). | ||
Results and discussion
We first incorporated an electron-rich thiophene spacer relative to benzene in all four compounds (Q1–Q4), because a thiophene moiety facilitates an intramolecular charge transfer (ICT), resulting in a further bathochromic shift.20 The synthetic route of Q1–Q4 was more than 10 steps (see ESI†). Key steps utilized to assemble the core structure of the targeted compounds included Stille coupling, a Suzuki coupling reaction, iron reduction and N-thionylaniline induced ring closure. All compounds were verified by NMR and ESI-MS, and also exhibited good solubility in common organic solvents such as CH2Cl2 and THF. Functional groups such as carboxylic acid groups were introduced into these compounds to impart a certain aqueous solubility and allow facile conjugation to biomolecules, which expanded their in vivo imaging applications (see ESI†). The HOMO, LUMO, and band gap levels of Q1–Q4 analogs (to reduce the computational cost, R1 and R2 groups were replaced by a simple methyl group) were obtained from theoretical calculations (Tables 1 and S1†). The LUMOs of all compounds showed a strong contribution at the electron accepting aromatic moieties (benzo-bis(1,2,5-thiadiazole)). The HOMOs are well delocalized along the whole backbone for Q1 and Q4. Moreover, Q1 and Q4 presented higher-lying calculated HOMO and LUMO levels and showed lower band gaps compared to those of CH1055, as the electron-rich thiophene spacer significantly lowers the oxidation potential, accounting for the relatively stronger ICT effect.The UV-vis-NIR absorption spectrum of Q1–Q4 was measured in DCM. The absorption bands of Q1 and Q4 were at 800–1000 nm because of the formation of a strong charge-transfer structure between their D–A–D units (Fig. 1b). Meanwhile, NIR-II emission signals were only observed in Q1 and Q4 solutions under 808 nm excitation and with a 1000 long-pass (LP) filter (Fig. S1†), whereas Q2 and Q3 displayed NIR-I emission (Fig. S2†) and were not suitable for further NIR-II imaging applications. The fluorescence emission spectra of Q4 and Q1 were measured and they demonstrated a peak emission wavelength at ∼1100 nm and ∼1000 nm, respectively (Fig. 1c). Finally, we compared the NIR-II fluorescence signals of Q1 and Q4 under various LP filters (900–1400 nm, Fig. 1d). The results indicated that the fluorescence signals of Q4 were stronger than those of Q1 with each filter, and no signals were observed for either compound with a 1400 nm filter. All these data have demonstrated that Q4 is a new type of promising NIR-II fluorescence compound, which is suitable for further imaging applications.
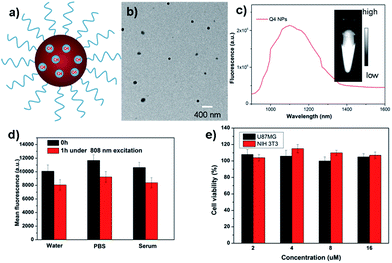
Q4 was then encapsulated into a PEGylated surfactant DSPE-mPEG5000 to prepare organic nanoparticle based water-soluble and biocompatible NIR-II nanoprobes, Q4NPs (Fig. 2a, see ESI†). The synthesized Q4NPs showed high monodispersity and homogeneity with an average particle size of ∼60.0 nm as determined by transmission electron microscopy (TEM, Fig. 2b) and a hydrodynamic diameter of ∼70.0 nm as determined by dynamic light scattering (DLS, Fig. S3†). The fluorescence emission spectrum of Q4NPs demonstrated a similar emission wavelength at ∼1100 nm as Q4 (Fig. 2c). The Q4NPs also exhibited high photostability in phosphate-buffered saline (PBS), water and mouse serum with negligible decay under continuous excitation for 1 h (Fig. 2d). The prepared Q4NPs were highly stable and can be stored in PBS without any precipitation in the refrigerator for one month. The result of the cytotoxicity study further indicated the high viability of U87MG and NIH-3T3 cells after 48 h of incubation with Q4NPs (2, 4, 8 and 16 μM), demonstrating the high biocompatibility of Q4NPs (Fig. S4†).
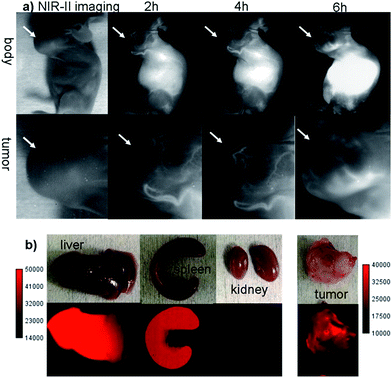
The U87MG tumor-bearing nude mice (n = 3) were injected with 100 μg of Q4NPs (0.0013 nmol, containing 24 μg of the Q4 molecule, calculated based on the UV-Vis measurement of Q4). Interestingly, the blood vessels of the tumor could be clearly visualized from the surrounding background tissue at 2 h p.i. using NIR-II imaging (Fig. 3a and S5†). After 6 h, the fluorescence signal and image quality of the tumor blood vessels reduced significantly, whereas a higher signal intensity was observed inside the tumor because of the non-specific diffusion and accumulation of Q4NPs in the tumor (Fig. S6†). This phenomenon could be explained by the passive targeting mechanism and enhanced permeability and retention (EPR) effect. The promising imaging result of the NIR-II Q4NP probe highlighted its possible use for monitoring tumor vasculatures and the EPR effect, which has not been achieved by previous NIR-I and NIR-II imaging in the literature (Fig. 3a, S5 and S6†). Ex vivo biodistribution studies were further performed at 96 h post-injection of the nanoprobe to evaluate the distribution of Q4NPs in major organs (Fig. 3b and S7†). It was found that Q4NPs mainly accumulated in the liver and spleen, suggesting the clearance routes of Q4NPs are predominantly through hepatobiliary systems, which is consistent with the clearance routes of many nanoparticle-based probes.21 In addition, a similar level of accumulation was observed in the tumor, indicating that Q4NPs can passively target tumors and be used for cancer theranostic applications (Fig. 3 and S7†).
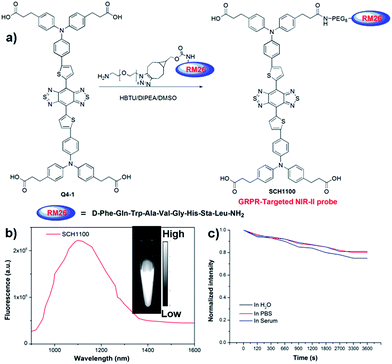
We next demonstrated the application of Q4 for receptor-targeted imaging of tumors such as prostate cancer (PCa). PCa is one of the leading causes of cancer-related death among men in the United States and Europe.22 If diagnosed early, chances for survival increase tremendously.23 In addition, imaging-guided fluorescence surgery would also be helpful to reduce the re-occurrence of PCa.24 GRPR has been reported to be overexpressed in PCa, and can serve as a promising target for PCa theranostics.25,26 Considering the advantages of NIR-II imaging, we explored the development of a novel small-molecule based GRPR-targeted NIR-II probe SCH1100, and investigated its imaging properties in vivo. SCH1100 was prepared through the direct conjugation of one of the carboxylic acid groups of Q4-1 (see ESI†) with a GRPR targeting ligand RM26 peptide. RM26 has been demonstrated as a promising targeting peptide for GRPR by our group and other groups.27,28 In order to increase the solubility of the SCH1100 probe, cyclooctyne functionalized RM26 was firstly conjugated with a small NH2-PEG8-azide through a Cu-free click reaction, and then amidation with one of the carboxylic acid groups of Q4-1 was performed to obtain SCH1100 (Fig. 4a and ESI†). SCH1100 was purified using HPLC and characterized using MALDI-TOF-MS [calcd. for C146H176N24O28S4: 2843.3630, found: m/z 2844.1526 (Fig. S8†)]. The fluorescence emission spectrum of SCH1100 demonstrated the maximum emission wavelength at ∼1100 nm (Fig. 4b). The fluorescence quantum yield of SCH1100 in water solution under an excitation of 808 nm was ∼0.2%, measured against a standard IR-26 dye as a reference (Fig. S9†). SCH1100 also exhibited high photostability in PBS, water and mouse serum with a slight decay under continuous excitation for 1 h (Fig. 4c). Furthermore, the high viability of human PCa cell lines (PC3) and NIH-3T3 cells after 24 h incubation with different concentrations of SCH1100 demonstrated the high biocompatibility of SCH1100in vitro (Fig. S10†). These results indicated that SCH1100 as an aqueous soluble, photo-stable and biocompatible NIR-II fluorescence probe is suitable for biological imaging.
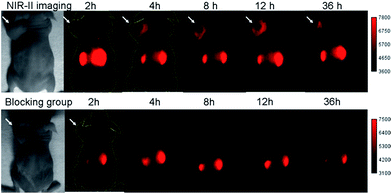
SCH1100 was then intravenously injected (100 μg) in PC3 tumor-bearing mice (n = 3 per group). From NIR-II imaging, the subcutaneous PC3 tumor could be clearly visualized from the surrounding background tissue from 4–36 h p.i. (Fig. 5, 1000 LP, 500 ms), and the tumor uptake reached maximum at 12 h. The targeting specificity of SCH1100 for GRPR was confirmed by the blocking experiment, and the tumor fluorescence signals were successfully reduced at all time points after co-injection of RM26 peptide (400 μg) with SCH1100 for NIR-II imaging (Fig. 5). An ex vivo biodistribution study was performed for SCH1100 to evaluate its distribution in major organs at 48 h (Fig. S11†). High accumulation was observed in the liver and kidney (ROI analysis indicated the fluorescence signal ratio in kidney/liver is ∼2), which suggested that the clearance routes of SCH1100 were through both hepatobiliary and renal systems, which was different from Q4NPs. In addition, the uptake of SCH1100 in the tumor was far higher than in most of normal organs except the kidneys, which further confirmed the excellent GRPR-targeting ability and specificity of SCH1100. Hence, SCH1100 represents a highly promising and clinically translatable NIR-II fluorescence probe for inexpensive and rapid detection and monitoring of PCa.
Conclusions
In conclusion, we have successfully developed a novel and versatile small-molecule based NIR-II fluorophore Q4, which shows to be highly promising for molecular imaging and clinical translation. Using this scaffold, organic nanoparticle based NIR-II imaging probes Q4NPs have been prepared, which allowed for in vivo and high resolution imaging of the blood vessels of the tumor, which has not been achieved in the NIR-I and NIR-II window before. Furthermore, a novel GRPR-targeted small NIR-II imaging probe SCH1100 has been successfully prepared and demonstrated specific GRPR-targeted imaging of PCa in vivo. The novel organic fluorescent compound Q4 provides unprecedented opportunities for constructing a variety of NIR-II probes for in vivo molecular imaging.Acknowledgements
This work was partially supported by the Office of Science (BER), U.S. Department of Energy (DE-SC0008397), NIH In vivo Cellular Molecular Imaging Center (ICMIC) grant P50 CA114747, NSFC (81573383, 81373254, 21390402 and 21204069), NSFHP (2014CFB704, 2012FFB04429), IS&TCPC (2015DFA30440, 2014DFB30020), the Applied Basic Research Program of WMB S&T (2015060101010031, 2014070404010200), the Fundamental Research Funds for the Central Universities and Innovation Seed Fund of Wuhan University School of Medicine, who are gratefully acknowledged. The authors thank Prof. Bao Zhang at Tianjin University for his help with theoretical calculation data of CH1055 and Q1–Q4.Notes and references
- R. Weissleder, C. H. Tung, U. Mahmood and A. Bogdanov, Nat. Biotechnol., 1999, 17, 375–378 CrossRef CAS PubMed.
- L. Bu, X. Ma, Y. Tu, B. Shen and Z. Cheng, Curr. Pharm. Biotechnol., 2014, 14, 723–732 Search PubMed.
- J. A. Thomas, Chem. Soc. Rev., 2015, 44, 4494–4500 RSC.
- V. Shanmugam, S. Selvakumar and C. S. Yeh, Chem. Soc. Rev., 2014, 43, 6254–6287 RSC.
- K. Licha and C. Olbrich, Adv. Drug Delivery Rev., 2005, 57, 1087–1108 CrossRef CAS PubMed.
- G. S. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. M. Xie, N. F. Huang, J. P. Cooke and H. J. Dai, Nat. Med., 2012, 18, 1841–1846 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. M. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- G. S. Hong, S. Diao, A. L. Antaris and H. J. Dai, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed.
- N. Won, S. Jeong, K. Kim, J. Kwaq, J. Park, S. G. Kim and S. Kim, Mol. Imaging, 2012, 11, 338–352 CAS.
- G. S. Hong, S. Diao, J. L. Chang, A. L. Antaris, C. X. Chen, B. Zhang, S. Zhao, D. N. Atochin, P. L. Huang, K. I. Andreasson, C. J. Kuo and H. J. Dai, Nat. Photonics, 2014, 8, 723–730 CrossRef CAS PubMed.
- J. T. Robinson, G. S. Hong, Y. Y. Liang, B. Zhang, O. M. Yaghi and H. J. Dai, J. Am. Chem. Soc., 2012, 134, 10664–10669 CrossRef CAS PubMed.
- Y. Zhang, G. S. Hong, Y. J. Zhang, G. C. Chen, F. Li, H. J. Dai and Q. B. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS PubMed.
- G. C. Chen, F. Tian, Y. Zhang, Y. Li and Q. B. Wang, Adv. Funct. Mater., 2014, 24, 2481–2488 CrossRef CAS.
- D. J. Naczynski, M. C. Tian, M. Zevon, B. Wall, J. Kohl, A. Kulesa, S. Chen, C. M. Roth, R. E. Riman and P. V. Moqhe, Nat. Commun., 2013, 4, 2199 CAS.
- G. S. Hong, Y. P. Zou, A. L. Antaris, S. Diao, D. Wu, K. Cheng, X. D. Zhang, C. X. Chen, B. Liu, Y. H. He, J. Z. Wu, J. Yuan, B. Zhang, Z. M. Tao, C. Fukunaga and H. J. Dai, Nat. Commun., 2014, 5, 4206 CAS.
- Y. Sun, X. Ma, K. Cheng, B. Wu, J. Duan, H. Chen, L. Bu, R. Zhang, X. Hu, Z. Deng, L. Xing, X. Hong and Z. Cheng, Angew. Chem., Int. Ed., 2015, 54, 5981–5984 CrossRef CAS PubMed.
- A. Louie, Chem. Rev., 2010, 110, 3146–3195 CrossRef CAS PubMed.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. S. Hong, C. R. Qu, S. Diao, Z. X. Deng, X. M. Hu, B. Zhang, X. D. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. C. Hong, Z. Cheng and H. J. Dai, Nat. Mater., 2015, 15, 235–242 CrossRef PubMed.
- G. Qian, Z. Zhong, M. Luo, D. B. Yu, Z. Q. Zhang, Z. Y. Wang and D. G. Ma, Adv. Mater., 2009, 21, 111–116 CrossRef CAS.
- Y. K. Suda, R. Nishiyabu and Y. J. Kubo, Tetrahedron, 2015, 71, 4174–4182 CrossRef CAS.
- Q. Fan, K. Cheng, X. Hu, X. Ma, R. Zhang, M. Yang, X. Lu, L. Xing, W. Huang, S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 15185–15194 CrossRef CAS PubMed.
- P. A. di Sant Agnese, Urology, 1998, 51, 121–124 CrossRef CAS.
- A. F. Prasanphanich, P. K. Nanda, T. L. Rold, L. X. Ma, M. R. Lewis, J. C. Garrison, T. J. Hoffman, G. L. Sieckman, S. D. Figueroa and C. J. Smith, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 12462–12467 CrossRef CAS PubMed.
- E. L. Kaijzel, G. Van der Pluijm and C. W. Lowik, Clin. Cancer Res., 2007, 13, 3490–3497 CrossRef PubMed.
- R. Mansi, A. Fleischmann, H. R. Macke and J. C. Reubi, Nat. Rev. Neurol., 2013, 10, 235–244 CAS.
- Q. Y. Cai, P. Yu, C. Besch-Williford, C. J. Smith, G. L. Sieckman, T. J. Hoffman and L. X. Ma, Prostate, 2013, 73, 842 CrossRef CAS PubMed.
- Y. Liu, X. Hu, H. Liu, L. Bu, X. Ma, K. Cheng, J. Li, M. Tian, H. Zhang and Z. Cheng, J. Nucl. Med., 2013, 54, 2132–2138 CrossRef CAS PubMed.
- S. R. Lane, P. Nanda, T. L. Rold, G. L. Sieckman, S. D. Figueroa, T. J. Hoffman, S. S. Jurisson and C. J. Smith, Nucl. Med. Biol., 2010, 37, 751–761 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc01561a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |