Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolic labelling of cholesteryl glucosides in Helicobacter pylori reveals how the uptake of human lipids enhances bacterial virulence†
Hau-Ming
Jan‡
ab,
Yi-Chi
Chen‡
ab,
Yu-Yin
Shih
a,
Yu-Chen
Huang
c,
Zhijay
Tu
a,
Arun B.
Ingle
f,
Sheng-Wen
Liu
a,
Ming-Shiang
Wu
 d,
Jacquelyn
Gervay-Hague
d,
Jacquelyn
Gervay-Hague
 e,
Kwok-Kong Tony
Mong
f,
Yet-Ran
Chen
e,
Kwok-Kong Tony
Mong
f,
Yet-Ran
Chen
 c and
Chun-Hung
Lin
c and
Chun-Hung
Lin
 *ab
*ab
aInstitute of Biological Chemistry, Academia Sinica, No. 128 Academia Road Section 2, Nan-Kang, Taipei, 11529, Taiwan. E-mail: chunhung@gate.sinica.edu.tw
bInstitute of Biochemical Sciences and Department of Chemistry, National Taiwan University, Taipei, 10617, Taiwan
cAgriculture Biotechnology Research Center, Academia Sinica, Taipei, 11529, Taiwan
dDivision of Gastroenterology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, 10002, Taiwan
eDepartment of Chemistry, University of California, Davis, CA 95618, USA
fDepartment of Applied Chemistry, National Chiao-Tung University, Hsin-Chu 300, Taiwan
First published on 3rd June 2016
Abstract
Helicobacter pylori infects approximately half of the human population and is the main cause of various gastric diseases. This pathogen is auxotrophic for cholesterol, which it converts upon uptake to various cholesteryl α-glucoside derivatives, including cholesteryl 6′-acyl and 6′-phosphatidyl α-glucosides (CAGs and CPGs). Owing to a lack of sensitive analytical methods, it is not known if CAGs and CPGs play distinct physiological roles or how the acyl chain component affects function. Herein we established a metabolite-labelling method for characterising these derivatives qualitatively and quantitatively with a femtomolar detection limit. The development generated an MS/MS database of CGds, allowing for profiling of all the cholesterol-derived metabolites. The subsequent analysis led to the unprecedented information that these bacteria acquire phospholipids from the membrane of epithelial cells for CAG biosynthesis. The resulting increase in longer or/and unsaturated CAG acyl chains helps to promote lipid raft formation and thus delivery of the virulence factor CagA into the host cell, supporting the idea that the host/pathogen interplay enhances bacterial virulence. These findings demonstrate an important connection between the chain length of CAGs and the bacterial pathogenicity.
Introduction
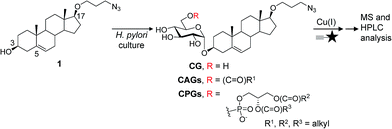
Infecting ∼50% of the world population, Helicobacter pylori causes chronic gastritis, which is asymptomatic in the majority of carriers but is considered a major risk factor for the development of gastric and duodenal ulcers and gastric malignancies, including mucosa-associated lymphoid tissue lymphoma and gastric adenocarcinoma.1 This bacterium has an exceptional ability to persist and establish chronic infection, unlike other Gram-negative bacterial pathogens, and represents a highly successful pathogen because it is capable of evading, subverting and manipulating the host's immune system.2,3 For example, cytotoxin-associated gene A (CagA)4 is an H. pylori virulence factor that enters host cells via type IV secretion system (T4SS)-mediated translocation.5 CagA then undergoes tyrosine phosphorylation by c-Src family kinases,6,7 and the phosphorylated CagA then activates several cellular signalling processes, including those leading to cancer development.8,9 Interestingly, the translocation process is not restricted to just the transfer from H. pylori to the host. The pathogen also receives molecules such as L-fucose10 and cholesterol3,11,12 from the host for various purposes.H. pylori assimilates cholesterol into its membrane by taking up cholesterol from the medium or from epithelial cells of the stomach.13 Upon uptake, the bacterial cells modify the cholesterol by α-glucosylation. Specifically, the glucosyltransferase encoded by hp0421 catalyses the transfer of glucose to the 3β-hydroxyl group of cholesterol, yielding cholesteryl α-D-glucopyranoside (CG). An acyl or phosphatidyl group is then attached to the O6′ of glucose in CG to produce cholesteryl-6′-O-acyl-α-D-glucopyranoside (CAG) or cholesteryl-6′-O-phosphatidyl-α-D-glucopyranoside (CPG), respectively.14,15 These cholesteryl glucoside derivatives (CGds) have been shown to be essential for several physiological activities, such as antibiotic resistance,12 CagA translocation,16 the prevention of phagocytosis by macrophages3 and the activation of invariant natural killer T cells.17,18 CAG and CPG are further diversified by the addition of different fatty acid chains to the acyl and phosphatidyl groups, respectively. Owing to limitations in the purification and analysis, typically these CGds are examined collectively instead of being separated and compared to determine whether the acyl or phosphatidyl chain plays a role in an activity of interest.
Herein, we developed a specific metabolite-tagging method to facilitate the purification and characterisation of H. pylori CGds. Based on ultrahigh-performance liquid chromatography mass spectrometry (UPLC-MS), this method not only allowed the structural determination and quantitative characterisation of all CGds, but also offered a great advantage in detection sensitivity and the isolation of various CGds that are difficult to obtain synthetically or by other means. Most importantly, the resulting analysis demonstrated the incorporation of the host phospholipids led to a significant increase in CAGs containing longer or/and unsaturated fatty acid chains. This change was further found to promote the translocation and subsequent phosphorylation of CagA through the enhanced formation of lipid rafts, demonstrating an interactive strategy to enhance bacterial virulence.
Results
Synthesis and evaluation of the tagged metabolites
Our metabolic labelling approach utilises azide-containing cholesterol analogues in bacterial cell culture (Fig. 1). After the cells were harvested, lipids were extracted and subjected to click chemistry (1,3-dipolar cycloaddition) with a fluorescently-tagged alkyne. We first examined the cholesterol analogues that are comparable with cholesterol in terms of biosynthetic incorporation in vivo. We synthesised three azide-containing cholesterol analogues, namely 17β-([3′′-azidopropoxy)-5-androsten-3β-ol (compound 1), 17β-([6′′-azidohexanoxy)-5-androsten-3β-ol (2) and 17β-([9′′-azidononanoxyl)-5-androsten-3β-ol (3), as shown in the ESI Scheme 1.† These analogues all have the same four fused rings as cholesterol, but differ in the azide-containing side chain introduced at O17β. To evaluate if the three analogues were suitable for incorporation into the biosynthetic pathway, the cholesteryl α-glucosyltransferase gene hp0421 was cloned and the protein was then overexpressed and purified as previously reported.15 Steady-state kinetics analysis (Table S1†)19,20 demonstrated that the catalytic efficiency (kcat/Km) values of compounds 1–3 are slightly smaller than that of cholesterol, being in the range of 84–59% that of cholesterol. 1 appeared to be the best among the three and was thus selected for the further investigations. Moreover, four other cholesterol analogues (the structures of which are shown in Table S1†) displayed much lower affinity for the enzyme, including ergosterol (13% that of cholesterol),15 stigmasterol (7%),15 22-NBD-cholesterol (not detectable) and 25-NBD-cholesterol (not detectable); with the last two compounds representing fluorescent analogues widely used for imaging studies.21–23 The side chain at C17 appeared to be critical to the enzymatic glucosylation.Compound 1 was evaluated by thin-layer chromatography and phospholipid composition analyses24 to confirm that it was equivalent to cholesterol in the biosynthesis of CGds (Fig. S1†). The latter examined the levels of several phospholipids in addition to CPG. The results indicated that 1 and cholesterol yielded similar percentages of the major phospholipids in H. pylori, including phosphatidylethanolamine (PE), cardiolipin and phosphatidylglycerol.
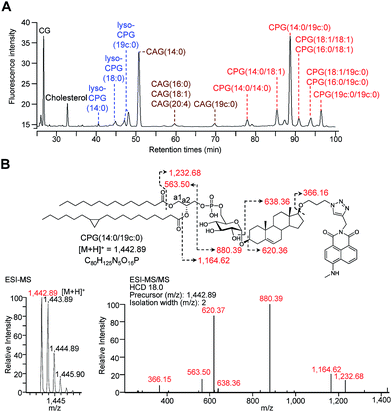
We considered two factors when preparing suitable alkyne-containing fluorescent compounds for the click chemistry, namely reactivity (potential yield) and stability. Two fluorophore-possessing alkynes were designed and synthesised, such as 4-N-methylamino-1,8-napthalimidopropyne (MAN) and 4-N,N-dimethylamino-1,8-naphthalimidopropyne (DAN)25 (ESI Scheme 2†). Both MAN and DAN, as well as several commercially available fluorescent dyes (Fig. S2B†), were examined by Cu[I]-catalysed click chemistry. The reaction yield and stability of MAN were higher than those of DAN and the other fluorescent dyes (Fig. S2†).
Structural determination and quantification of the CGds
For the metabolic labelling of CGds, H. pylori was treated with 1 and grown under microaerobic conditions at 37 °C. After 4 days, the bacterial cells were collected and subjected to Folch extraction.26 The extracts were reacted with MAN in the presence of tris(benzyltriazolylmethyl)amine, sodium ascorbate and CuSO4 for 1 h. The resulting compounds were separated by high-performance liquid chromatography and detected by fluorescence (HPLC-fluorescence; C18-reversed-phase column; λex = 428 nm, λem = 528 nm) (Fig. 2A). The CGds were also identified by UPLC-MS in the product ion scan mode (Fig. 2B, S3 and Table S2†).27 HPLC-fluorescence analysis revealed fluorophore-conjugated analogues of cholesterol and CG plus three major groups of compounds, namely CAGs, CPGs and lyso-CPGs (these fluorophore-conjugated analogues of CAGs, CPGs and lyso-CPGs derived from 1 are hereafter referred to as CAGs, CPGs and lyso-CPGs, respectively, unless otherwise specified). These compounds appeared in three groups in the HPLC chromatogram due to differences in polarity. The structure of each compound was identified by MS and confirmed by tandem MS (MS/MS). The errors for the precursor m/z and for relative isotope abundance obtained by UPLC-MS were <5 ppm and 5%, respectively (Table S2†). For instance, CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (see Fig. 2B for the molecular structure) consists of two esterified fatty acids
0) (see Fig. 2B for the molecular structure) consists of two esterified fatty acids![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) myristic acid (14
myristic acid (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and another (19c
0) and another (19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) that contains a cyclopropane at C9 of the acyl chain.28,29 In the MS/MS analysis of CPG(14
0) that contains a cyclopropane at C9 of the acyl chain.28,29 In the MS/MS analysis of CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), the predicted product ions for the functional groups of the fatty acids, phosphate, glucose, steroid, triazole and 4-N-methylamino-1,8-napthalimide (the fluorophore) were observed (Fig. 2B). The MS and corresponding MS/MS data for the other CGds are provided in Fig. S3.† We also performed 1H, 13C or/and 31P NMR analysis of CPG(14
0), the predicted product ions for the functional groups of the fatty acids, phosphate, glucose, steroid, triazole and 4-N-methylamino-1,8-napthalimide (the fluorophore) were observed (Fig. 2B). The MS and corresponding MS/MS data for the other CGds are provided in Fig. S3.† We also performed 1H, 13C or/and 31P NMR analysis of CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and CAG(14
0) and CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (ESI† spectra data 1, 2 and 3) to confirm that the phosphatidyl group is indeed attached to the O6 of glucose and that C1 of the glucose forms an α-linkage with O3 of the steroid.30 The phospholipases A1 and A2 (PLA1 and PLA2) were applied in the UPLC-MS analysis31,32 to assign where the two fatty acid chains of CPG(14
0) (ESI† spectra data 1, 2 and 3) to confirm that the phosphatidyl group is indeed attached to the O6 of glucose and that C1 of the glucose forms an α-linkage with O3 of the steroid.30 The phospholipases A1 and A2 (PLA1 and PLA2) were applied in the UPLC-MS analysis31,32 to assign where the two fatty acid chains of CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) are attached to the phosphatidyl glycerol. The fatty acids (14
0) are attached to the phosphatidyl glycerol. The fatty acids (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and (19c
0) and (19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) were found to be specifically linked to the a1- and a2-positions, respectively (Fig. S4A–C†). It was noted that acyl migration occurred after cleavage of CPG(14
0) were found to be specifically linked to the a1- and a2-positions, respectively (Fig. S4A–C†). It was noted that acyl migration occurred after cleavage of CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) at a1 using PLA1, i.e. the majority of the remaining ester at the a2 position moved to the a1 position via an intramolecular transfer (Fig. S4D–F†).33 Additionally, lyso-CPGs were found to exist as a mixture of regioisomers, with the acyl chain attached to either the a1 or a2 position (Fig. S4G†). These were designated as 2-lyso-CPG(19c
0) at a1 using PLA1, i.e. the majority of the remaining ester at the a2 position moved to the a1 position via an intramolecular transfer (Fig. S4D–F†).33 Additionally, lyso-CPGs were found to exist as a mixture of regioisomers, with the acyl chain attached to either the a1 or a2 position (Fig. S4G†). These were designated as 2-lyso-CPG(19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or 1-lyso-CPG(19c
0) or 1-lyso-CPG(19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), respectively, indicating that the ester at a2 or a1 is hydrolyzed.
0), respectively, indicating that the ester at a2 or a1 is hydrolyzed.
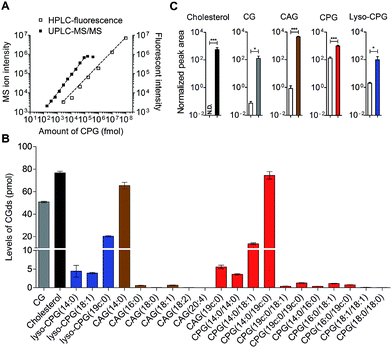
HPLC-fluorescence and UPLC-MS/MS were employed to quantify the levels of CGds in H. pylori. The former was based on the integration of signals in the LC chromatogram, whereas the latter relied on the peak area of selected specific fragments for the extracted ion chromatograms from using the Orbitrap UPLC-MS system operated in the product ion scan mode. Table S3† shows the m/z of the designated target and fragmented ions as well as the collision modes/energies. The chromatograms (Fig. S5†) show the selected ion chromatograms of all the CGds at m/z 366.16 or 620.36, which represent the m/z of the fragmented ions (daughter ions) shown in Table S3.† To quantify the CGds and determine the sensitivity of detection, CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) was purified to >99% purity (determined by HPLC-fluorescence and UPLC-MS) and used to establish calibration curves (Fig. 3A) in a linear range between 2.8 pmol and 100 nmol per 106 bacteria for HPLC-fluorescence analysis, and between 0.088 and 100 pmol for UPLC-MS/MS. The calibration curves of CG, CAG(14
0) was purified to >99% purity (determined by HPLC-fluorescence and UPLC-MS) and used to establish calibration curves (Fig. 3A) in a linear range between 2.8 pmol and 100 nmol per 106 bacteria for HPLC-fluorescence analysis, and between 0.088 and 100 pmol for UPLC-MS/MS. The calibration curves of CG, CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CPG(14
0), CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and lyso-CPG(19c
0) and lyso-CPG(19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) using UPLC-MS/MS and HPLC-fluorescence are also shown in Fig. S6.† In comparison with HPLC-fluorescence, UPLC-MS/MS was found to be more suitable for measuring the CGds in very low abundance (<5 pmol per 106 bacteria), such as CAG(18
0) using UPLC-MS/MS and HPLC-fluorescence are also shown in Fig. S6.† In comparison with HPLC-fluorescence, UPLC-MS/MS was found to be more suitable for measuring the CGds in very low abundance (<5 pmol per 106 bacteria), such as CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CAG(18
0), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), CPG(14
2), CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16
0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and CPG(18
0) and CPG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (Fig. 3B). The detection sensitivity in UPLC-MS/MS for the fluorophore-conjugated derivatives (0.088 pmol) was significantly enhanced relative to the unconjugated analogues, which is attributable to the dominant protonation of the incorporated fluorophore. In particular, whereas the native CG and CAGs have no charge, the fluorophore-conjugated analogues had 100- and 10
0) (Fig. 3B). The detection sensitivity in UPLC-MS/MS for the fluorophore-conjugated derivatives (0.088 pmol) was significantly enhanced relative to the unconjugated analogues, which is attributable to the dominant protonation of the incorporated fluorophore. In particular, whereas the native CG and CAGs have no charge, the fluorophore-conjugated analogues had 100- and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold enhanced intensities, respectively (Fig. 3C).
000-fold enhanced intensities, respectively (Fig. 3C).
Uptake of human phospholipids for CAG biosynthesis in H. pylori
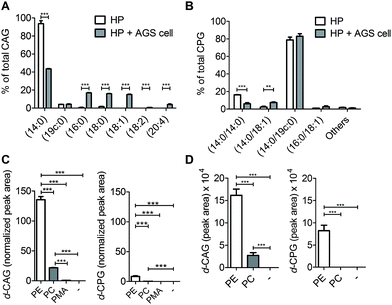
We next applied these labelling and analysis methods to compare the lipid composition of CAGs and CPGs between H. pylori cultured alone and co-cultures of H. pylori with AGS cells (a human gastric cancer cell line; i.e. H. pylori-infected AGS cells). Two major CAGs were observed in the bacterial cells cultured alone (Fig. 4A), namely CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (93.7%) and CAG(19c
0) (93.7%) and CAG(19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (4.0%), in agreement with the fact that these two fatty acids are dominant in H. pylori.14 In contrast, the co-cultured H. pylori generated significant amounts of CAGs containing longer or/and unsaturated acyl chains, such as CAG(16
0) (4.0%), in agreement with the fact that these two fatty acids are dominant in H. pylori.14 In contrast, the co-cultured H. pylori generated significant amounts of CAGs containing longer or/and unsaturated acyl chains, such as CAG(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CAG(18
0), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CAG(18
0), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CAG(18
1), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CAG(20
2) and CAG(20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (Fig. 4A and S7A†). These lipid chains rarely exist in H. pylori cultures, suggesting that they may have originated from the human cells. Contrary to prediction, there was no obvious difference in the lipid compositions or percentages of CPGs between the bacterial culture and the co-culture (Fig. 4B and S7B†).
4) (Fig. 4A and S7A†). These lipid chains rarely exist in H. pylori cultures, suggesting that they may have originated from the human cells. Contrary to prediction, there was no obvious difference in the lipid compositions or percentages of CPGs between the bacterial culture and the co-culture (Fig. 4B and S7B†).
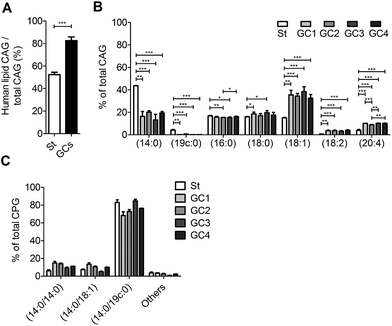
We further examined the CAG and CPG compositions when the AGS cells were co-cultured with different strains of H. pylori, including the standard type (26695) and four clinical strains isolated from patients with gastric cancer (GC1–GC4). UPLC-MS analysis indicated that on average 83% of the total CAGs obtained from the clinical isolates had incorporated human fatty acid chains, including (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), (18
0), (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), (18
0), (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (18
1), (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (20
2) and (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). In contrast, on average only 52% of CAGs from H. pylori 26
4). In contrast, on average only 52% of CAGs from H. pylori 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 showed such incorporation (Fig. 5A). In particular, the GC1–GC4 strains contained a higher content of CAG(18
695 showed such incorporation (Fig. 5A). In particular, the GC1–GC4 strains contained a higher content of CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CAG(18
1), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CAG(20
2) and CAG(20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) (Fig. 5B), which have one or more double bonds. There was no obvious difference in the lipid composition of CPGs among any of these H. pylori strains (Fig. 5C).
4) (Fig. 5B), which have one or more double bonds. There was no obvious difference in the lipid composition of CPGs among any of these H. pylori strains (Fig. 5C).
To search for a possible precursor in the biosynthesis, we first treated H. pylori cultures with deuterium-labelled PE, phosphatidylcholine (PC) or palmitic acid (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) for 7 h to examine the incorporation of deuterium-labelled acyl chains in CAGs and CPGs. PE was found to be the major precursor in the biosynthesis of CAGs and CPGs, although PC was also incorporated into CAGs to a small extent (Fig. 4C). Next, AGS cells were pretreated with 1 for 24 h, and incubated with deuterium-labelled PE or PC (the two major phospholipids in human cells) for 1 h. Because it is important to rule out the possibility of adsorbing any lipids from culture dishes during the H. pylori infection process, we extensively washed the phospholipid-pretreated AGS cells with phosphate-buffered saline (PBS) and then transferred the cells to new plates. The incorporation of each phospholipid into AGS cells was monitored by UPLC-MS. The cells were subsequently infected with H. pylori 26695 for 6 h and then analysed by UPLC-MS to determine the proportion of deuterium-labelled CAGs and CPGs. The analysis supported the previous observation and demonstrated PE to be the primary source for the acyl chains of CAGs and CPGs (Fig. 4D), and also showed that the bacteria can obtain phospholipids from the host cell for the synthesis of CAGs and CPGs. CPGs appeared to have a much lower level of incorporation of the host phospholipids than the CAGs (Fig. S7C and D†).
0) for 7 h to examine the incorporation of deuterium-labelled acyl chains in CAGs and CPGs. PE was found to be the major precursor in the biosynthesis of CAGs and CPGs, although PC was also incorporated into CAGs to a small extent (Fig. 4C). Next, AGS cells were pretreated with 1 for 24 h, and incubated with deuterium-labelled PE or PC (the two major phospholipids in human cells) for 1 h. Because it is important to rule out the possibility of adsorbing any lipids from culture dishes during the H. pylori infection process, we extensively washed the phospholipid-pretreated AGS cells with phosphate-buffered saline (PBS) and then transferred the cells to new plates. The incorporation of each phospholipid into AGS cells was monitored by UPLC-MS. The cells were subsequently infected with H. pylori 26695 for 6 h and then analysed by UPLC-MS to determine the proportion of deuterium-labelled CAGs and CPGs. The analysis supported the previous observation and demonstrated PE to be the primary source for the acyl chains of CAGs and CPGs (Fig. 4D), and also showed that the bacteria can obtain phospholipids from the host cell for the synthesis of CAGs and CPGs. CPGs appeared to have a much lower level of incorporation of the host phospholipids than the CAGs (Fig. S7C and D†).
Enhanced CagA translocation and lipid raft formation by CAGs containing longer acyl chains
Because we were able to isolate individual CAGs and CPGs, we further investigated the degree of influence of each derivative on the physiological activities. In the study of CagA translocation, we compared H. pylori 26695 to ΔCGT (a cholesterol glucosyltransferase-deficient strain (prepared as previously reported16)) and to ΔCGT pretreated for 30 min with either authentic CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (synthesised as previously reported34), fluorophore-labelled CAG(14
0) (synthesised as previously reported34), fluorophore-labelled CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or fluorophore-labelled CPG(14
0) or fluorophore-labelled CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (Fig. 6A). CAG(14
0) (Fig. 6A). CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and CPG(14
0) and CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
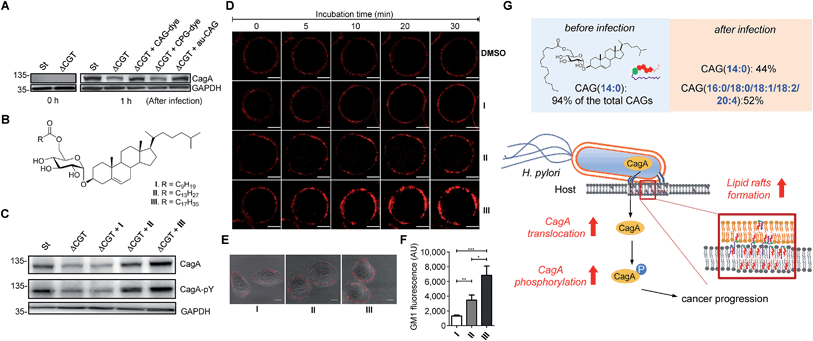
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) are the most abundant CAG and CPG in H. pylori, respectively. AGS cells were infected with the H. pylori strains for 1 h, and then the cell extracts were immunoblotted with a CagA-specific monoclonal antibody. There was no obvious difference between pretreatment with labelled and authentic CAG, indicating that fluorophore-conjugated CGd analogues are comparable to the corresponding native CGds and could be purified individually for further investigation. Additionally, CAG was found, for the first time, to promote a higher degree of CagA translocation than CPG (Fig. 6B). The result with authentic CAG was similar to that of the positive control (H. pylori 26695), indicating that CAGs are likely responsible for the observed activity among the mixture of CGds.
0) are the most abundant CAG and CPG in H. pylori, respectively. AGS cells were infected with the H. pylori strains for 1 h, and then the cell extracts were immunoblotted with a CagA-specific monoclonal antibody. There was no obvious difference between pretreatment with labelled and authentic CAG, indicating that fluorophore-conjugated CGd analogues are comparable to the corresponding native CGds and could be purified individually for further investigation. Additionally, CAG was found, for the first time, to promote a higher degree of CagA translocation than CPG (Fig. 6B). The result with authentic CAG was similar to that of the positive control (H. pylori 26695), indicating that CAGs are likely responsible for the observed activity among the mixture of CGds.
We further investigated the effect of chain length on the activity of CAGs. ΔCGT was pretreated for 30 min with three CAGs of increasing chain length (CAG(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CAG(14
0), CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and CAG(18
0) and CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0); Fig. 6B) and used to infect AGS cells. Analysis of CagA translocation and the subsequent tyrosine phosphorylation35 demonstrated that the acyl chain length of CAGs indeed plays a critical role in these activities (Fig. 6C). The degree of CagA translocation and the corresponding phosphorylation increased with the increasing chain length.
0); Fig. 6B) and used to infect AGS cells. Analysis of CagA translocation and the subsequent tyrosine phosphorylation35 demonstrated that the acyl chain length of CAGs indeed plays a critical role in these activities (Fig. 6C). The degree of CagA translocation and the corresponding phosphorylation increased with the increasing chain length.
In addition, we examined whether the chain length of CAGs correlates with lipid raft formation. Time-lapse fluorescence microscopy was performed to monitor the mobilisation of ganglioside GM1-associated rafts after each CAG was added to the AGS cells. GM1 in the AGS cells was labelled with Alexa-594-cholera toxin prior to treatment with CAG(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CAG(14
0), CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or CAG(18
0) or CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0). We found a time-dependent increase in fluorescence intensity for all three CAGs, and further found that CAG(18
0). We found a time-dependent increase in fluorescence intensity for all three CAGs, and further found that CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) induced the greatest accumulation of GM1 (Fig. 6D and ESI movies†). Furthermore, when AGS cells were treated with CAG(10
0) induced the greatest accumulation of GM1 (Fig. 6D and ESI movies†). Furthermore, when AGS cells were treated with CAG(10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CAG(14
0), CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or CAG(18
0) or CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) for 30 min and then labelled with GM1, the GM1 fluorescence intensity increased with increasing chain length (Fig. 6E and F). Taken together, these results suggest that CAGs with incorporated human fatty acids enhance the formation of lipid rafts in the membrane, which may lead to higher levels of CagA translocation and subsequent phosphorylation.
0) for 30 min and then labelled with GM1, the GM1 fluorescence intensity increased with increasing chain length (Fig. 6E and F). Taken together, these results suggest that CAGs with incorporated human fatty acids enhance the formation of lipid rafts in the membrane, which may lead to higher levels of CagA translocation and subsequent phosphorylation.
Discussion
Click chemistry-based tagging methods have been prevalently used in metabolite analysis. Our development appears to provide several advantages. First, the introduced fluorophore MAN is readily protonated in solution, significantly enhancing the ionisation level in mass spectrometry (102 to 105-fold, depending on the type of CGds). The combined mass spectrometric detection and enhanced ionisation of tagged metabolites made it possible to reach the detection limit at the femtomolar level. Second, MAN was found to display a much higher stability in comparison with several commercial fluorescent dyes (Fig. S2B†). The beneficial features of high sensitivity and stability support the idea that MAN should be useful for other profiling studies. Third, conventional analysis (e.g. 2D thin-layer chromatography) is usually entangled with contamination from phospholipids, explaining the reason why the relevant isolation or purification becomes troublesome. In contrast, our development makes the entire process more robust and it takes 2–3 weeks to obtain a single cholestyl glucosde derivative at the mg level, including 7–10 days of culturing the bacteria. After harvest, any of CGds can be made readily available with >95% purity after the bacterial cells are subjected to extraction with organic solvents, followed by two steps of column chromatography. Furthermore, the use of azide-containing compound 1 and the fluorophore MAN is compatible for the purpose of fluorescent imaging to demonstrate how these labelled CGds are translocated to and/or distributed in the host cell.Previous findings on the uptake of L-fucose from the host by H. pylori indicate that this is a beneficial strategy to acquire additional carbon and energy, as well as to evade host immune surveillance.10 Likewise, H. pylori has evolved to derive various CGds from exogenous cholesterol, for which it is auxotrophic (lacking biosynthetic machinery), but this is indispensable for its pathogenesis and virulence.3,11,14 In this study, by using a specific labelling method, we demonstrated that H. pylori also acquires phospholipids from the host for CAG biosynthesis. The acyl chain composition of H. pylori CAGs dramatically changed in the presence of human host cells. For instance, CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) constituted 94% of the total CAGs when bacteria were cultured alone, whereas when bacteria were cultured with human cells, CAG(14
0) constituted 94% of the total CAGs when bacteria were cultured alone, whereas when bacteria were cultured with human cells, CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) was reduced to 44% and was accompanied by CAGs containing human fatty acid chains (52%; (16
0) was reduced to 44% and was accompanied by CAGs containing human fatty acid chains (52%; (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), (18
0), (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), (18
0), (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (18
1), (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and (20
2) and (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); Fig. 4A and S7A†). This alteration led to a higher level of CagA translocation, indicating that accessing human phospholipids enhances bacterial virulence by altering the bacterial CAG compositions (Fig. 6G). Interestingly, Hatakeyama et al. found that host phosphatidylserine is aberrantly externalised from the inner to the outer leaflet of the host plasma membrane at the site of H. pylori attachment, which facilitates the delivery, intracellular localisation and pathophysiological activity of CagA.36 This finding suggests a mechanism for the release of host phospholipids during bacterial infection.36
4); Fig. 4A and S7A†). This alteration led to a higher level of CagA translocation, indicating that accessing human phospholipids enhances bacterial virulence by altering the bacterial CAG compositions (Fig. 6G). Interestingly, Hatakeyama et al. found that host phosphatidylserine is aberrantly externalised from the inner to the outer leaflet of the host plasma membrane at the site of H. pylori attachment, which facilitates the delivery, intracellular localisation and pathophysiological activity of CagA.36 This finding suggests a mechanism for the release of host phospholipids during bacterial infection.36
Cholesterol is an important component of cell membranes. Particularly in lipid rafts, this molecule serves as a glue to maintain the close packing of lipids and proteins. H. pylori CGds can be considered modified versions of cholesterol with the additional attachment of glucose, acyl and/or phosphatidyl groups. CAGs, CPGs and lyso-CPGs likely play different roles in membranes. Wang et al. reported that CagA translocation is mediated in a cholesterol-dependent manner, as the formation of CGds from host cholesterol promotes the translocation of CagA by affecting the membrane dynamics and enhancing lipid raft formation.11,16 Our study identified CAGs, but not CPGs, as being responsible for the enhanced translocation of CagA and for the formation of lipid rafts. An altered CAG composition therefore has multifaceted effects. Lipid rafts have been demonstrated to serve as the obligate structure for the entry of bacterial and viral pathogens.37,38
Our findings further demonstrate that only CAGs with longer acyl chains exert this effect to promote lipid raft formation and thus CagA translocation. This result is consistent with a number of previous reports. For instance, lipid rafts containing palmitoylated or stearoylated (C16/C18) GPI-anchored variant surface glycoprotein display a higher resistance to Triton X-100 extraction than those containing myristoylated (C14) GPI-anchored variant surface glycoprotein.39,40 Several raft-associated proteins are palmitoylated.41,42 When cytoplasmic proteins are subjected to lipidation, the presence of at least one palmitate chain, rather than the shorter myristoylate, is required for raft association.40,43 These studies support the idea that acquired host CAG(16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and CAG(18
0) and CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), rather than the plentiful bacterial CAG(14
0), rather than the plentiful bacterial CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), assist in the formation of lipid rafts to enhance the subsequent CagA translocation.
0), assist in the formation of lipid rafts to enhance the subsequent CagA translocation.
We also demonstrated that bacterial strains obtained from patients with gastric cancer displayed a significant increase in CAGs carrying unsaturated acyl chains, i.e. CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CAG(18
1), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CAG(20
2) and CAG(20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Brewster and Safran demonstrated that hybrid lipids (comprising one fully saturated and one partially unsaturated chain) serve as a surface-active component by occupying the interface between the lipid raft (facing the saturated anchor) and the less-ordered environment (facing the unsaturated chain).44 These reports suggest that the unsaturated chains of CAG(18
4). Brewster and Safran demonstrated that hybrid lipids (comprising one fully saturated and one partially unsaturated chain) serve as a surface-active component by occupying the interface between the lipid raft (facing the saturated anchor) and the less-ordered environment (facing the unsaturated chain).44 These reports suggest that the unsaturated chains of CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), CAG(18
1), CAG(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and CAG(20
2) and CAG(20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) may contribute to the raft structure by interacting with less-ordered lipids.
4) may contribute to the raft structure by interacting with less-ordered lipids.
Steryl glycosides (SGs) are known to prevalently exist in many organisms and some of SGs are also acylated at the C6′ position of the sugar moiety, like CAGs in H. pylori. In general, there are two possible routes regarding enzymatic acyl transfer: one requires acyl-CoA as the donor, while the other needs a different substrate for the same purpose. The acylation of several plant steryl glucosides were found to be independent of acyl-CoA. Instead, phosphoglycerolipids or/and galactolipids (fatty acids attached to C1 and C2) were employed for the enzymatic transfer of fatty acids.45 In AGS cells, PE and PC are the major phospholipids. PE is also the major phospholipid in H. pylori. To search for a possible precursor in the biosynthesis of CAG, PE, PC and fatty acid (PMA) were individually added to the bacterial culture to examine the incorporation of exogenous acyl chains (i.e. deuterium-labelled acyl chains in CAG). As shown in Fig. 4C, PE was found to be the major precursor in the biosynthesis of CAG, while little or no PMA was incorporated into the CAGs. This result supports there being a lipid-dependent acyltransferase involved in the biosynthesis of CAGs, and that PE is a better substrate for this enzyme.
To explain the observed uptake of human phospholipids, it appears to be essential to understand where the discussed acyltransferase is located in H. pylori, since the incorporation of deuterium-labelled acyl chain primarily occurred in CAGs but not in CPGs. We propose that the CAG-related enzyme resides at the bacterial outer membrane, making the direct access of human phospholipids possible. As a matter of fact, several studies have suggested that the biosynthetic location of CAGs is indispensable for their release to the surface of host cells and for the subsequent induction of the cytokines TNF-α and INF-γ.18 On the other hand, the unknown phosphatidyltransferase, to convert CG to CPG, might be located at either the inner membrane or at the cytoplasm, which would hamper the access of the host phospholipids.
Conclusions
We established a metabolite-tagging method for the robust detection of CGds at femtomolar sensitivity. The quantitative analysis of CGds indicated that these bacteria utilise phospholipids from human host cells to produce cholesteryl 6′-acyl α-glucosides (CAGs) containing longer/unsaturated fatty acid chains. Our findings demonstrate, for the first time to the best of our knowledge, that phospholipid hijack is the key to augment the pathogenicity. Because the incorporated fluorophore displayed great inherent stability and a high ionisation degree in mass spectrometry, the development should be applicable to other metabolic profiling.Experimental
Metabolic labelling of CGds
H. pylori cells were seeded on Brucella agar plates containing 1 (50 μM) for 2–4 days. Bacterial lipids were extracted by the Folch method,26 redissolved in chloroform/methanol/water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) and subjected to a click reaction with 0.25 mM MAN in the presence of 1.25 mM TBTA, 12.5 mM sodium ascorbate and 0.25 mM CuSO4 at room temperature for 1 h. The solvent was removed in vacuo, and the dried residue was redissolved in chloroform/methanol/water (5
1, v/v/v) and subjected to a click reaction with 0.25 mM MAN in the presence of 1.25 mM TBTA, 12.5 mM sodium ascorbate and 0.25 mM CuSO4 at room temperature for 1 h. The solvent was removed in vacuo, and the dried residue was redissolved in chloroform/methanol/water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) and analysed by HPLC and UPLC-MS.
1, v/v/v) and analysed by HPLC and UPLC-MS.
HPLC analysis
For the experiments described in Fig. 2A, an analytical Inspire C18 column (5 μm, 4.6 × 250 mm) was used at a flow rate of 0.4 mL min−1. To obtain fluorophore-labelled CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and CPG(14
0) and CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) for NMR characterisation, a preparative Inspire C18 column (5 μm, 21.5 × 250 mm) was used at a flow rate of 6.5 mL min−1. For quantification of the CGds using HPLC-fluorescence, an analytical Inspire C18 column (5 μm, 4.6 × 250 mm) was used at a flow rate of 0.4 mL min−1. In all the experiments, eluent A contained 30% acetonitrile and 70% aqueous 20 mM ammonium acetate, while eluent B contained 90% isopropyl alcohol and 10% aqueous 20 mM ammonium acetate.
0) for NMR characterisation, a preparative Inspire C18 column (5 μm, 21.5 × 250 mm) was used at a flow rate of 6.5 mL min−1. For quantification of the CGds using HPLC-fluorescence, an analytical Inspire C18 column (5 μm, 4.6 × 250 mm) was used at a flow rate of 0.4 mL min−1. In all the experiments, eluent A contained 30% acetonitrile and 70% aqueous 20 mM ammonium acetate, while eluent B contained 90% isopropyl alcohol and 10% aqueous 20 mM ammonium acetate.
UPLC-MS analysis
UPLC-MS analysis was performed on a linear ion trap-Orbitrap mass spectrometer (Orbitrap Elite; Thermo Fisher Scientific) coupled online with a UPLC system (ACQUITY UPLC; Waters). The mass spectrometer was operated in the positive electrospray ion mode set to one full FT-MS scan (m/z 200–1600; 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 resolution) and switched to different FT-MS product ion scans (15
000 resolution) and switched to different FT-MS product ion scans (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 resolution) for the different CGd precursors. The CGds were separated on a CSH C18 column (1.7 μm, 100 × 2.1 mm; Waters, USA) using gradients of LC buffer A (10 mM ammonium acetate in 40
000 resolution) for the different CGd precursors. The CGds were separated on a CSH C18 column (1.7 μm, 100 × 2.1 mm; Waters, USA) using gradients of LC buffer A (10 mM ammonium acetate in 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 [v/v] acetonitrile/water) and LC buffer B (10 mM ammonium acetate in 90
60 [v/v] acetonitrile/water) and LC buffer B (10 mM ammonium acetate in 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 [v/v] isopropyl alcohol/acetonitrile) at a flow rate of 0.4 mL min−1. To normalise the variations in sample preparation and UPLC-MS analysis, phosphatidylinositol containing deuterium-labelled palmitic acid (16
10 [v/v] isopropyl alcohol/acetonitrile) at a flow rate of 0.4 mL min−1. To normalise the variations in sample preparation and UPLC-MS analysis, phosphatidylinositol containing deuterium-labelled palmitic acid (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and unlabelled oleic acid (18
0) and unlabelled oleic acid (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used as the internal standard and added to the bacterial lysate (106 bacteria, determined by optical density at 600 nm). To determine the ionisation efficiency of the CGds with or without MAN labelling, the normalised peak area was defined as the peak area of CGds divided by the peak area of the internal standard. To quantify the MAN-labelled CGds from the bacterial lysates, the peak area of selected specific fragments for the extracted ion chromatograms was used. To establish the calibration curves of CGds, we added purified MAN-labelled cholesterol, CG, CAG(14
1) was used as the internal standard and added to the bacterial lysate (106 bacteria, determined by optical density at 600 nm). To determine the ionisation efficiency of the CGds with or without MAN labelling, the normalised peak area was defined as the peak area of CGds divided by the peak area of the internal standard. To quantify the MAN-labelled CGds from the bacterial lysates, the peak area of selected specific fragments for the extracted ion chromatograms was used. To establish the calibration curves of CGds, we added purified MAN-labelled cholesterol, CG, CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), CPG(14
0), CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and Lyso-CPG(19c
0) and Lyso-CPG(19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to the lysate from 106 bacteria along with the click chemistry reactants (TBTA, sodium ascorbate and CuSO4). The calibration curves for quantitation were obtained by analysing the peak areas of the serially diluted standard (0.088, 0.176, 0.35, 0.7, 1.4, 2.8, 5.6, 11.25, 22.5, 45 and 90 pmol per 106 bacteria) measured by UPLC-MS operated in the product ion scan mode. The amount of CGds in the bacterial lysate was determined by comparing the peak area in lysate to the standard curves. The relative isotopic abundance error was calculated as previously described.27
0) to the lysate from 106 bacteria along with the click chemistry reactants (TBTA, sodium ascorbate and CuSO4). The calibration curves for quantitation were obtained by analysing the peak areas of the serially diluted standard (0.088, 0.176, 0.35, 0.7, 1.4, 2.8, 5.6, 11.25, 22.5, 45 and 90 pmol per 106 bacteria) measured by UPLC-MS operated in the product ion scan mode. The amount of CGds in the bacterial lysate was determined by comparing the peak area in lysate to the standard curves. The relative isotopic abundance error was calculated as previously described.27
Co-culture experiments
Co-culture experiments were performed as previously described.16 In the experiments described in Fig. 4A, B and 5, AGS cells (8.0 × 106) were seeded in a 10 cm tissue culture dish and treated with 1 (6.2 μg mL−1) in Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Invitrogen) at 37 °C for 16 h. AGS cells were infected with H. pylori (26695 or clinically isolated strains) at an MOI of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 6 h and subsequently collected by centrifugation (6000 × g) for 5 min. CGds-containing lipids were isolated by Folch extraction and fluorescently labelled by click reaction for UPLC-MS analysis. With respect to the study shown in Fig. 4D, AGS cells (8.0 × 106) were seeded in a 10 cm tissue culture dish and treated with 1 (6.2 μg mL−1) in DMEM at 37 °C for 16 h. To label AGS cells with deuterium-labelled phospholipid, the AGS cells were incubated with serum-free medium (Ham's F12, Invitrogen) containing 0.1 mM 1-palmitoyl(d31)-2-oleoyl-sn-glycero-3-phosphoethanolamine (dPOPE; 16
1 for 6 h and subsequently collected by centrifugation (6000 × g) for 5 min. CGds-containing lipids were isolated by Folch extraction and fluorescently labelled by click reaction for UPLC-MS analysis. With respect to the study shown in Fig. 4D, AGS cells (8.0 × 106) were seeded in a 10 cm tissue culture dish and treated with 1 (6.2 μg mL−1) in DMEM at 37 °C for 16 h. To label AGS cells with deuterium-labelled phospholipid, the AGS cells were incubated with serum-free medium (Ham's F12, Invitrogen) containing 0.1 mM 1-palmitoyl(d31)-2-oleoyl-sn-glycero-3-phosphoethanolamine (dPOPE; 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 d31
0 d31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (18
(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) or 0.1 mM 1-palmitoyl(d31)-2-oleoyl-sn-glycero-3-phosphocholine (dPOPC; 16
1)) or 0.1 mM 1-palmitoyl(d31)-2-oleoyl-sn-glycero-3-phosphocholine (dPOPC; 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 d31
0 d31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (18
(18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) (Avanti Polar Lipids, USA) at 37 °C for 1 h. It is important to wash the cells with PBS five times to remove unbound 1 and dPOPE or dPOPC. Then the cells were transferred to a new plate for another 12 h. To demonstrate that the bacterial uptake of deuterium-labelled phospholipids was from the cells rather than the phospholipids remaining on the culture dish, empty culture dishes were first treated with 1 for 16 h, treated with POPE or dPOPC for another 1 h, washed with PBS five times, and then used to culture H. pylori without AGS cells for 6 h. No deuterium-labelled CGds were produced by H. pylori under these conditions. To perform the infection experiments, unloaded or dPOPE/dPOPC-loaded AGS cells were infected with H. pylori (26695) at an MOI of 200
1)) (Avanti Polar Lipids, USA) at 37 °C for 1 h. It is important to wash the cells with PBS five times to remove unbound 1 and dPOPE or dPOPC. Then the cells were transferred to a new plate for another 12 h. To demonstrate that the bacterial uptake of deuterium-labelled phospholipids was from the cells rather than the phospholipids remaining on the culture dish, empty culture dishes were first treated with 1 for 16 h, treated with POPE or dPOPC for another 1 h, washed with PBS five times, and then used to culture H. pylori without AGS cells for 6 h. No deuterium-labelled CGds were produced by H. pylori under these conditions. To perform the infection experiments, unloaded or dPOPE/dPOPC-loaded AGS cells were infected with H. pylori (26695) at an MOI of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 6 h and subsequently collected by centrifugation (6000 × g) for 5 min. CGds-containing lipids were isolated by Folch extraction and fluorescently labelled by click reaction for UPLC-MS analysis. For the CagA translocation experiments, AGS cells (8.0 × 105) were seeded in DMEM (Gibco, Invitrogen) in a 6-well culture plate at 37 °C for 16 h. To load H. pylori cells with CGds, H. pylori cells (26695 or ΔCGT) were incubated with serum-free medium (Ham's F12) containing 30 μM of MAN-labelled CAG(14
1 for 6 h and subsequently collected by centrifugation (6000 × g) for 5 min. CGds-containing lipids were isolated by Folch extraction and fluorescently labelled by click reaction for UPLC-MS analysis. For the CagA translocation experiments, AGS cells (8.0 × 105) were seeded in DMEM (Gibco, Invitrogen) in a 6-well culture plate at 37 °C for 16 h. To load H. pylori cells with CGds, H. pylori cells (26695 or ΔCGT) were incubated with serum-free medium (Ham's F12) containing 30 μM of MAN-labelled CAG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), MAN-labelled CPG(14
0), MAN-labelled CPG(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/19c
0/19c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) or unlabelled CAG standard at 37 °C for 30 min. The bacterial cells were then washed with PBS to remove unbound CPG or CAG and then used to infect AGS cells at an MOI of 200
0) or unlabelled CAG standard at 37 °C for 30 min. The bacterial cells were then washed with PBS to remove unbound CPG or CAG and then used to infect AGS cells at an MOI of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 1 h.
1 for 1 h.
Immunoprecipitation and blotting
After the infection of AGS cells with H. pylori (MOI of ∼200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the cells were washed three times with PBS and collected with a cell scraper. To detect the internalised CagA to AGS cells, saponin was used to lyse the AGS cells, but with care to keep the bacteria intact.5,35 The AGS cells were gently lysed with PBS containing 0.1% saponin, protease inhibitor cocktail (Calbiochem, USA) and phosphatase inhibitor cocktail (Calbiochem, USA) for 10 min at room temperature. The cell debris and intact bacteria were separated from the soluble fraction by centrifugation (6000 × g for 5 min), and the solute was filtered with a 0.22 μm-pore filter to obtain human cell proteins and the internalised CagA. The saponin lysis procedure was also performed on standard type H. pylori (26695) alone. Subsequent immunoprecipitation and blotting of CagA indicated that no CagA was detected, supporting the idea that this procedure did not release cytoplasmic proteins from H. pylori (data not shown). The filtrate (100 μg) was immunoprecipitated using 10 μg rabbit anti-CagA polyclonal antibody (Austral Biologicals, USA) and 30 μl Protein A Plus Agarose (Thermo scientific, USA) at 4 °C overnight. The obtained precipitates were washed three times with immunoprecipitation buffer (25 mM Tris–HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100) and once with TBS buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM CaCl2), then boiled in PAGE sample buffer (62.5 mM Tris–HCl, pH 6.8, 2% SDS, 10% (v/v) glycerol, 0.05% brilliant blue R) at 95 °C for 10 min, resolved by 6% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked with 5% (w/v) skimmed milk in TBS buffer containing 0.01% Tween 20 at room temperature for 1 h and incubated overnight with mouse monoclonal anti-CagA (Austral Biologicals; 1
1), the cells were washed three times with PBS and collected with a cell scraper. To detect the internalised CagA to AGS cells, saponin was used to lyse the AGS cells, but with care to keep the bacteria intact.5,35 The AGS cells were gently lysed with PBS containing 0.1% saponin, protease inhibitor cocktail (Calbiochem, USA) and phosphatase inhibitor cocktail (Calbiochem, USA) for 10 min at room temperature. The cell debris and intact bacteria were separated from the soluble fraction by centrifugation (6000 × g for 5 min), and the solute was filtered with a 0.22 μm-pore filter to obtain human cell proteins and the internalised CagA. The saponin lysis procedure was also performed on standard type H. pylori (26695) alone. Subsequent immunoprecipitation and blotting of CagA indicated that no CagA was detected, supporting the idea that this procedure did not release cytoplasmic proteins from H. pylori (data not shown). The filtrate (100 μg) was immunoprecipitated using 10 μg rabbit anti-CagA polyclonal antibody (Austral Biologicals, USA) and 30 μl Protein A Plus Agarose (Thermo scientific, USA) at 4 °C overnight. The obtained precipitates were washed three times with immunoprecipitation buffer (25 mM Tris–HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100) and once with TBS buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1 mM CaCl2), then boiled in PAGE sample buffer (62.5 mM Tris–HCl, pH 6.8, 2% SDS, 10% (v/v) glycerol, 0.05% brilliant blue R) at 95 °C for 10 min, resolved by 6% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked with 5% (w/v) skimmed milk in TBS buffer containing 0.01% Tween 20 at room temperature for 1 h and incubated overnight with mouse monoclonal anti-CagA (Austral Biologicals; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000), mouse monoclonal anti-phosphotyrosine (4G10, Millipore; 1
2000), mouse monoclonal anti-phosphotyrosine (4G10, Millipore; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) or mouse monoclonal anti-GAPDH (ab9482, Abcam, UK; 1
2000) or mouse monoclonal anti-GAPDH (ab9482, Abcam, UK; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) at 4 °C. The blots were washed and incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, USA) at a 1
5000) at 4 °C. The blots were washed and incubated with HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, USA) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilution, and the proteins of interest were visualised using an enhanced chemiluminescence assay (WBKLS0500, Millipore). See the ESI† for additional information.
5000 dilution, and the proteins of interest were visualised using an enhanced chemiluminescence assay (WBKLS0500, Millipore). See the ESI† for additional information.
Acknowledgements
We thank Dr Danny Yao for helpful discussion to revise the manuscript, Dr Chi-Fong Chang for assistance with the NMR experiments, and Dr Chi-Chi Chou for assistance with the mass spectrometry experiments. Confocal microscopy experiments were performed at the Imaging & Cell Biology Core Facility with Dr Chin-Chun Hung's assistance at the Institute of Biological Chemistry in Academia Sinica. The MS and NMR analyses were performed at the Metabolomics Facility of the Scientific Instrument Center and the High Field NMR Center of Academia Sinica, respectively. This work was financially supported by Academia Sinica and the Ministry of Science and Technology of Taiwan (99-213-M-001-006-MY3).Notes and references
- R. M. Peek Jr and M. J. Blaser, Nat. Rev. Cancer, 2002, 2, 28–37 CrossRef PubMed.
- W. Khamri, A. P. Moran, M. L. Worku, Q. N. Karim, M. M. Walker, H. Annuk, J. A. Ferris, B. J. Appelmelk, P. Eggleton, K. B. Reid and M. R. Thursz, Infect. Immun., 2005, 73, 7677–7686 CrossRef CAS PubMed.
- C. Wunder, Y. Churin, F. Winau, D. Warnecke, M. Vieth, B. Lindner, U. Zahringer, H. J. Mollenkopf, E. Heinz and T. F. Meyer, Nat. Med., 2006, 12, 1030–1038 CrossRef CAS PubMed.
- S. Censini, C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli and A. Covacci, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 14648–14653 CrossRef CAS.
- S. Odenbreit, J. Puls, B. Sedlmaier, E. Gerland, W. Fischer and R. Haas, Science, 2000, 287, 1497–1500 CrossRef CAS PubMed.
- H. Higashi, K. Yokoyama, Y. Fujii, S. Ren, H. Yuasa, I. Saadat, N. Murata-Kamiya, T. Azuma and M. Hatakeyama, J. Biol. Chem., 2005, 280, 23130–23137 CrossRef CAS PubMed.
- M. Selbach, S. Moese, C. R. Hauck, T. F. Meyer and S. Backert, J. Biol. Chem., 2002, 277, 6775–6778 CrossRef CAS PubMed.
- H. Mimuro, T. Suzuki, S. Nagai, G. Rieder, M. Suzuki, T. Nagai, Y. Fujita, K. Nagamatsu, N. Ishijima, S. Koyasu, R. Haas and C. Sasakawa, Cell Host Microbe, 2007, 2, 250–263 Search PubMed.
- L. Buti, E. Spooner, A. G. Van der Veen, R. Rappuoli, A. Covacci and H. L. Ploegh, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 9238–9243 CrossRef CAS PubMed.
- T. W. Liu, C. W. Ho, H. H. Huang, S. M. Chang, S. D. Popat, Y. T. Wang, M. S. Wu, Y. J. Chen and C. H. Lin, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 14581–14586 CrossRef CAS PubMed.
- C. H. Lai, Y. C. Chang, S. Y. Du, H. J. Wang, C. H. Kuo, S. H. Fang, H. W. Fu, H. H. Lin, A. S. Chiang and W. C. Wang, Infect. immun., 2008, 76, 3293–3303 CrossRef CAS PubMed.
- D. J. McGee, A. E. George, E. A. Trainor, K. E. Horton, E. Hildebrandt and T. L. Testerman, Antimicrob. Agents Chemother., 2011, 55, 2897–2904 CrossRef CAS PubMed.
- R. Ansorg, K. D. Muller, G. von Recklinghausen and H. P. Nalik, Zentralbl. Bakteriol., 1992, 276, 323–329 CrossRef CAS PubMed.
- Y. Hirai, M. Haque, T. Yoshida, K. Yokota, T. Yasuda and K. Oguma, J. bacteriol., 1995, 177, 5327–5333 CrossRef CAS PubMed.
- A. H. Lebrun, C. Wunder, J. Hildebrand, Y. Churin, U. Zahringer, B. Lindner, T. F. Meyer, E. Heinz and D. Warnecke, J. Biol. Chem., 2006, 281, 27765–27772 CrossRef CAS PubMed.
- H. J. Wang, W. C. Cheng, H. H. Cheng, C. H. Lai and W. C. Wang, Mol. Microbiol., 2012, 83, 67–84 CrossRef CAS PubMed.
- Y. Ito, J. L. Vela, F. Matsumura, H. Hoshino, A. Tyznik, H. Lee, E. Girardi, D. M. Zajonc, R. Liddington, M. Kobayashi, X. Bao, J. Bugaytsova, T. Boren, R. Jin, Y. Zong, P. H. Seeberger, J. Nakayama, M. Kronenberg and M. Fukuda, PLoS One, 2013, 8, e78191 CrossRef PubMed.
- M. Shimamura, Arch. Immunol. Ther. Exp., 2012, 60, 351–359 CrossRef CAS PubMed.
- P. Gonzalo, B. Sontag, D. Guillot and J. P. Reboud, Anal. Biochem., 1995, 225, 178–180 CrossRef CAS PubMed.
- H. Lee, P. Wang, H. Hoshino, Y. Ito, M. Kobayashi, J. Nakayama, P. H. Seeberger and M. Fukuda, Glycobiology, 2008, 18, 549–558 CrossRef CAS PubMed.
- S. Mukherjee, X. Zha, I. Tabas and F. R. Maxfield, Biophys. J., 1998, 75, 1915–1925 CrossRef CAS PubMed.
- J. P. Slotte, Biochim. Biophys. Acta, 1995, 1237, 127–134 CrossRef.
- W. P. Heal, B. Jovanovic, S. Bessin, M. H. Wright, A. I. Magee and E. W. Tate, Chem. Commun., 2011, 47, 4081–4083 RSC.
- X. Zhou and G. Arthur, J. Lipid Res., 1992, 33, 1233–1236 CAS.
- G. Loving and B. Imperiali, J. Am. Chem. Soc., 2008, 130, 13630–13638 CrossRef CAS PubMed.
- J. Folch, M. Lees and G. H. Sloane Stanley, J. Biol. Chem., 1957, 226, 497–509 CAS.
- Y. Xu, J. F. Heilier, G. Madalinski, E. Genin, E. Ezan, J. C. Tabet and C. Junot, Anal. Chem., 2010, 82, 5490–5501 CrossRef CAS PubMed.
- M. Haque, Y. Hirai, K. Yokota, N. Mori, I. Jahan, H. Ito, H. Hotta, I. Yano, Y. Kanemasa and K. Oguma, J. Bacteriol., 1996, 178, 2065–2070 CrossRef CAS PubMed.
- J. R. Vestal and D. C. White, Bioscience, 1989, 39, 535–541 CrossRef CAS PubMed.
- H. Q. Nguyen, R. A. Davis and J. Gervay-Hague, Angew. Chem., Int. Ed., 2014, 53, 13400–13403 CrossRef CAS PubMed.
- H. Kuge, K. Akahori, K. Yagyu and K. Honke, J. Biol. Chem., 2014, 289, 26783–26793 CrossRef CAS PubMed.
- M. K. Mishra, T. Kumaraguru, G. Sheelu and N. W. Fadnavis, Tetrahedron: Asymmetry, 2009, 20, 2854–2860 CrossRef CAS.
- J. Lessig, J. Schiller, J. Arnhold and B. Fuchs, J. Lipid Res., 2007, 48, 1316–1324 CrossRef CAS PubMed.
- R. A. Davis, C. H. Lin and J. Gervay-Hague, Chem. Commun., 2012, 48, 9083–9085 RSC.
- H. Tsugawa, H. Suzuki, H. Saya, M. Hatakeyama, T. Hirayama, K. Hirata, O. Nagano, J. Matsuzaki and T. Hibi, Cell Host Microbe, 2012, 12, 764–777 Search PubMed.
- N. Murata-Kamiya, K. Kikuchi, T. Hayashi, H. Higashi and M. Hatakeyama, Cell Host Microbe, 2010, 7, 399–411 Search PubMed.
- S. M. Campbell, S. M. Crowe and J. Mak, J. Clin. Virol., 2001, 22, 217–227 CrossRef CAS PubMed.
- J. S. Shin, Z. Gao and S. N. Abraham, Science, 2000, 289, 785–788 CrossRef CAS PubMed.
- R. J. Schroeder, S. N. Ahmed, Y. Zhu, E. London and D. A. Brown, J. Biol. Chem., 1998, 273, 1150–1157 CrossRef CAS PubMed.
- J. Benting, A. Rietveld, I. Ansorge and K. Simons, FEBS Lett., 1999, 462, 47–50 CrossRef CAS PubMed.
- S. Arni, S. A. Keilbaugh, A. G. Ostermeyer and D. A. Brown, J. Biol. Chem., 1998, 273, 28478–28485 CrossRef CAS PubMed.
- K. A. Melkonian, A. G. Ostermeyer, J. Z. Chen, M. G. Roth and D. A. Brown, J. Biol. Chem., 1999, 274, 3910–3917 CrossRef CAS PubMed.
- W. Rodgers, B. Crise and J. K. Rose, Mol. Cell. Biol., 1994, 14, 5384–5391 CrossRef CAS PubMed.
- R. Brewster and S. A. Safran, Biophys. J., 2010, 98, L21–L23 CrossRef CAS PubMed.
- S. Grille, A. Zaslawski, S. Thiele, J. Plat and D. Warnecke, Prog. Lipid Res., 2010, 49, 262–288 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General reagents and instruments of chemical synthesis, supplementary schemes, synthetic procedures, supplementary spectra data of selected compounds, supplementary methods, supplementary figures and supplementary tables. See DOI: 10.1039/c6sc00889e |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2016 |