Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Asperterpenes A and B, two unprecedented meroterpenoids from Aspergillus terreus with BACE1 inhibitory activities†
Changxing
Qi‡
a,
Jian
Bao‡
b,
Jianping
Wang‡
a,
Hucheng
Zhu
a,
Yongbo
Xue
a,
Xiaochuan
Wang
b,
Hua
Li
a,
Weiguang
Sun
a,
Weixi
Gao
a,
Yongji
Lai
a,
Jian-Guo
Chen
b and
Yonghui
Zhang
*a
aHubei Key Laboratory of Natural Medicinal Chemistry and Resource Evaluation, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. E-mail: zhangyh@mails.tjmu.edu.cn
bSchool of Basic Medicine, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
First published on 27th June 2016
Abstract
Asperterpenes A (1) and B (2), two 3,5-dimethylorsellinic acid-based meroterpenoids that contain a unique β-oriented Me-21 with an unprecedented 1,2,5-trimethyl-4,9-dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moiety, were obtained from Aspergillus terreus in very limited amounts of 3.6 mg and 1.8 mg, respectively. The absolute structure of 1 was determined using X-ray diffraction. Because of the low yield of 1, a comprehensive characterization of the BACE1 inhibitory activities of 1 was completed via molecular biological, cell and animal studies guided by in silico target confirmation (ISTC). ISTC assays suggested that compounds 1 and 2 might be BACE1 inhibitors. In cell-based tests, asperterpenes A and B, as natural products, exhibited promising inhibitory activities against BACE1, with IC50 values of 78 and 59 nM, respectively. LY2811376 (the positive control), one of the most potent clinical BACE1 inhibitors, has shown an IC50 value of 260 nM. In vivo, compound 1 exhibited activity similar to that of LY2811376 against Alzheimer's disease (AD) in 3xTg AD mice. Taken together, these findings demonstrate that asperterpene A, which contains a novel carbon skeleton, is the first terpenoid to exhibit effective BACE1 inhibitory activity. Moreover, 1 represents a potential lead compound and a versatile scaffold for the development of drugs for the treatment of AD.
Introduction
Alzheimer's disease (AD) is a major, chronic, neurodegenerative illness that is histopathologically characterized by brain β-amyloid (Aβ) plaques and neurofibrillary tangles, which result in a progressive decline in cognitive function and ultimately death.1,2 AD is a major public health problem in modern society. Over 30 million people worldwide were affected by this single major cause of dementia in middle-aged and elderly individuals in 2015. By 2050, the number of AD cases could exceed 100 million all over the world.3 Drugs such as acetylcholinesterase inhibitors and noncompetitive antagonists of NMDA receptors have been used clinically to alleviate cognitive impairment, but do not target the underlying pathology. New approaches to AD treatment have focused on reducing the production of Aβ, which has been confirmed to be the key contributor to AD by genetic and pathological studies.4,5 β-site amyloid precursor protein cleaving enzyme 1 (BACE1, β-secretase) initiates the production of Aβ by cleaving the extracellular domain of the amyloid precursor protein (APP).6,7 Consequently, BACE1 is considered a prime therapeutic target, and the development of BACE1 inhibitors is being intensely pursued.8 Compared with clinically used drugs, BACE1 inhibitors have direct implications for AD pathology without greatly affecting viability.9 In conclusion, there is an urgent demand for the development of BACE1 inhibitors.As part of our continued investigation of bioactive metabolites from fungi,10,11 asperterpenes A (1) and B (2), two meroterpenoids with unprecedented carbon skeletons, were isolated from the culture broth of Aspergillus terreus, along with a new biosynthetically related compound, asperterpene C (3) (Fig. 1). Asperterpenes A (1) and B (2) represent a new class of meroterpenoids, because the ring D is rotated by 180 degrees around the C-12/C-15 axis, which results in an unprecedented 8,16-cyclo-8,14-seco-6/6/6/6 skeleton that is distinct from those of meroterpenoids such as berkeleytrione and preaustinoid A.12 These structural differences lead to a novel carbon skeleton that possesses an unprecedented 1,2,5-trimethyl-4,9-dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moiety. In addition, 1 and 2 contain a unique β-oriented Me-21 that differs from previously reported meroterpenoids of 3,5-dimethylorsellinic acid origin, such as berkeleytrione and berkeleyones A–C.13 Both 1 and 2 were subjected to in vitro experiments guided by in silico target confirmation (ISTC) studies and in vivo studies were performed for 1. Compounds 1 and 2 exhibited promising BACE1 inhibitory activities in cell-based assays, with IC50 values of 78 and 59 nM, respectively, and were more active than LY2811376 (IC50 = 260 nM), a potent clinical BACE1 inhibitor produced by Eli Lilly (Fig. S6†).§14 Moreover, 1 significantly decreased BACE1 activity and Aβ42 levels in 3xTg AD mice, similar to LY2811376. These results demonstrate that 1, with a novel carbon skeleton, is the first terpenoid that acts as an effective BACE1 inhibitor and may be a potential lead compound for the development of AD drugs. Here, we report the isolation, structure elucidation, and plausible biosynthetic pathway of 1 and 2, and their remarkable ability to inhibit BACE1.
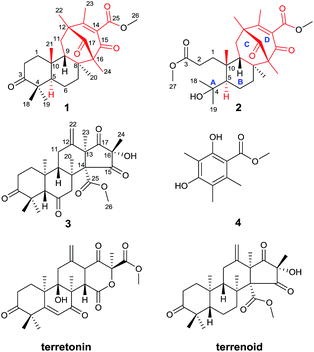 | ||
| Fig. 1 Structures of compounds 1–4 (red: new ring systems and fusion patterns of A/B rings that differ from normal analogues). | ||
Results and discussion
The strain of Aspergillus terreus was inoculated with solid medium containing 50 kg rice and 50 kg distilled water at 28 °C for 28 days. The growth of fungus was stopped by ethanol after incubation, followed by extraction with ethanol. The EtOH extract of the culture of A. terreus underwent repeated silica gel column chromatography, reversed-phase (RP) C18 medium pressure liquid chromatography, and Sephadex LH-20 chromatography, followed by semipreparative RP HPLC to afford asperterpenes A (3.6 mg) and B (1.8 mg).Asperterpene A (1) was isolated as colorless cubic crystals. Its molecular formula was determined as C26H36O5 by HRESIMS at m/z 451.2440 [M + Na]+ (calcd 451.2460). The 1H and 13C NMR data of 1 were partially similar to those of terrenoid, suggesting that 1 is likely to be a terrenoid or terretonin derivative with meroterpenoid features.15 The basic carbon skeleton was determined by detailed analyses of the 1H-1H COSY and HMBC spectra. The 1H-1H COSY cross-peaks of H-1/H-2 and H-5/H-6/H-7 and HMBC correlations from Me-18 and Me-19 to C-3, C-4 and C-5, from Me-20 to C-7, C-8 and C-9, and from Me-21 to C-1, C-9 and C-10 established the A/B ring system of 1, which is comparable to that of terrenoids.15 In addition to those signals assignable to rings A and B, two carbonyls (δC 198.2 and 210.2), a carboxyl (δC 166.8), an olefinic group (δC 131.3 and 163.9), two quaternary carbons (δC 67.4 and 49.5), a methylene (δC 39.8), three methyls (δC 14.2, 17.7, and 18.6), and a methoxyl (δC 52.5) remained, indicating considerable differences from terrenoids. An α,β-unsaturated carbonyl group (C-13–C-15) was proposed based on the characterized chemical shifts of a carbonyl (δC 198.2) and olefinic group (δC 131.3 and 163.9). This speculation, together with the HMBC correlations from Me-22 to C-12, C-13 and C-17, from Me-23 to C-12, C-13 and C-14, and from Me-24 to C-15, C-16 and C-17, established the presence of the six-membered ring D. Moreover, the 1H-1H COSY cross-peak of H-9/H-11 and HMBC interactions from Me-20 to C-16, from Me-24 to C-8, and from Me-22 to C-11 revealed that ring D is fused to ring B via C-8/C-16 and C-9/C-11/C-12 linkages to form a six-membered ring C. Finally, the HMBC correlation from Me-26 to C-25 suggested the presence of the methyl ester moiety, which was therefore located at C-14 to complete the structure of 1, considering the chemical shift and quaternary nature of C-14 as well as the molecular formula deduced by HRESIMS. Consequently, the planar structure of compound 1 was determined.
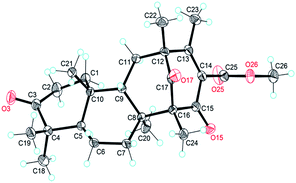
The NOESY interactions of Me-18/Me-21, H-5/Me-19, H-5/Me-20, and H-9/Me-21 indicated trans-fusion patterns for both the A/B and B/C rings. With respect to the conformation of the bi-cyclo[3.3.1]nonane system, the relative configurations of the remaining chiral centers at C-12 and C-16 of 1 should be 12R*,16R* or 12S*,16S*. A NOESY correlation of Me-24/H-7β was clearly observed, consistent with the molecular model of 12R*,16R* and thus establishing the relative configuration of 1. A single crystal of 1 suitable for X-ray diffraction was obtained by the evaporation of solvent (methanol and chloroform). The X-ray diffraction experiment (Fig. 2) confirmed the structure and the absolute configuration of 1 as 5R, 8S, 9S, 10R, 12R, 16R (for details, see the Experimental section).
Asperterpene B (2), obtained as an optically active white gum, possesses the molecular formula C27H40O7 as revealed by the analysis of its HRESIMS data. A comparison of the 1H and 13C NMR data of 2 with those of 1 suggested that their structures are identical in rings B, C, and D. The main difference between the two compounds was observed in ring A. Detailed analysis of the NMR data revealed that the quaternary carbon at δC 47.0 in 1 was oxygenated in 2 (δC 75.0), and the carbonyl at δC 218.8 in 1 was replaced by a carboxyl (δC 174.6). Moreover, an additional methoxyl was observed at δC 51.7 and δH 3.66. These findings, along with the eight degrees of unsaturation deduced from HRESIMS data, implied that ring A of 2 was open. In the HMBC spectrum, correlations from Me-27 to C-3 and from Me-18 and Me-19 to C-4 and C-5 confirmed the former speculation, and the planar structure of 2 was established.
In the same manner as for 1, the relative configuration of rings B, C, and D in 2 was confirmed to be identical to 1 using the NOESY spectrum. NOESY interactions of H-1/Me-20 and H-2/H-5 suggested that they are cofacial and α-oriented. In addition, correlations of H-9/Me-21 and H-9/H-7β revealed their β-orientations. Finally, the NOESY correlation Me-24/H-7β implied that the configuration of the bi-cyclo[3.3.1]nonane moiety of 2 was identical to that of 1. Thus, the relative configurations for all of the chiral centers in 2 were determined to be the same as those of 1. Considering the shared biosynthetic origin of 1 and 2, the absolute configuration of 2 was finally determined as 5R, 8S, 9S, 10R, 12R, 16R (Fig. 1).
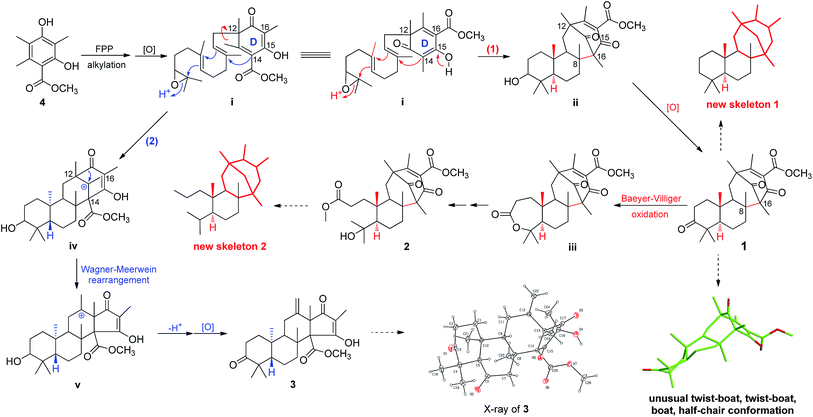
Asperterpenes A (1) and B (2) possess an intriguing tetracyclic ring system bearing an unprecedented 1,2,5-trimethyl-4,9-dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moiety that distinguishes them from known meroterpenoids. A biosynthetic pathway for 1 and 2 was proposed with the co-isolated compound 4 as a precursor (Scheme 1). The biosynthetic pathway of 1 and 2 appears similar to those of the known meroterpenoids with a 6/6/6/6 ring system (such as berkeleytrione, and preaustinoids A and B) and involves a series of cycloadditions and modifications. However, compared to the pathway for known meroterpenoids (from i to iv, with the key 6/6/6/6-fused tetracyclic intermediate 3), two main differences are apparent in the pathway for 1 and 2 in step 1. Firstly, although the fusion patterns of the A/B rings in both ii and iv are trans, ii bears an unusual β-oriented Me-21. Secondly, due to the rotation of the D ring by 180 degrees around the C-12/C-15 axis, the C-8/C-16 linkage leads to dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moieties.16 Chair and boat conformations are normally preferred in the cyclohexane of most terpenoids, and thus it is very interesting that 1 has an unusual twist-boat, twist-boat, boat, half-chair conformation, as revealed by its X-ray structure. The position selectivity of C-16 rather than C-14 and the stereoselectivity of Me-21 in the biosynthesis pathway of 1 and 2 are interesting and will attract much attention from the total and biosynthetic chemistry communities.
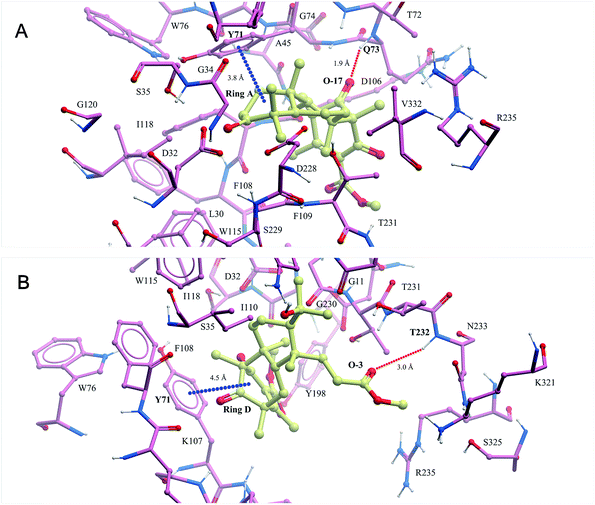
In recent years, our group has focused on the investigation of Alzheimer's disease.17–19 As a result, asperterpenes A and B were screened against eight significant enzymes in silico (PSH, GlpG, KMO, amyloid fibril, APOE4, acetylcholinesterase, NMDA receptor and BACE1) involved in AD processes using ISTR. Targets with lower calculated binding energies are considered to have higher binding affinities for the compounds. Our calculations had sufficient precision to predict that BACE1 exhibited significantly higher binding affinity for 1 and 2 than the other targets (Table S2†). As shown in Fig. 3, O-17 of 1 participates in hydrogen bonding with Q73, and ring A of this compound is involved in a stacking π–π interaction with Y71 in the BACE-1 catalytic pocket. O-3 of 2 participated in hydrogen bonding with T232, and ring D of compound 2 is involved in a stacking π–π interaction with Y71 in the pocket.
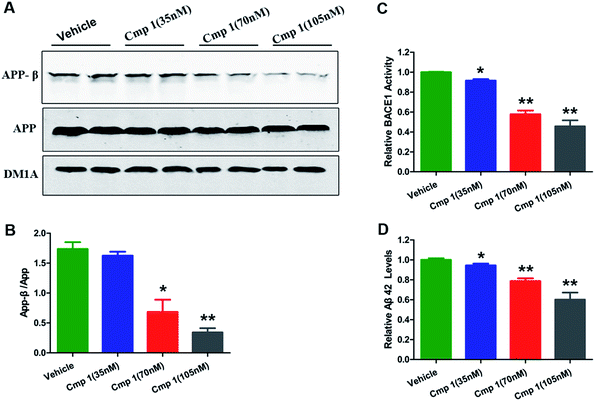
To confirm our hypothesis, the inhibitory activities of compounds 1 and 2 against BACE1 were further investigated in a cell-based Förster resonance energy transfer (FRET) peptide cleavage assay to validate this mode of action. FRET methods are widely used because they offer a homogeneous and sensitive assay in a multi-well format that is also easily adopted for high-throughput screening (HTS).201 and 2 exhibited significant inhibitory effects at the nanomolar scale with IC50 values of 78 and 59 nM with the HEK-293 cell line (positive control, LY2811376, IC50 = 260 nM), respectively (Table S3†). This investigation suggested that 1 and 2 exhibit greater BACE1 inhibitory activity than LY2811376 in cell-based assays. To further confirm this result, we investigated whether 1 has a similar effect on BACE1 in the N2a-APP cell line.21 BACE1 cleaves APP to generate a secreted APP-β fragment and a membrane bound carboxy-terminal fragment (C99). As shown in Fig. 4A and B, β cleavage of APP was significantly decreased in N2a-APP cells treated with 1 at concentrations of 70 and 105 nM, while the levels of full-length APP were unchanged. BACE1 activity and Aβ42 were also significantly reduced with these treatments (Fig. 4C and D). These data support the hypothesis that BACE1 activity is significantly inhibited by 1. In order to evaluate the general toxicity of compounds 1 and 2, MTT assays were performed, and the exposure of cells to 1 and 2 at concentrations up to 8 μM had no effect on cell viability (Fig. S3†).
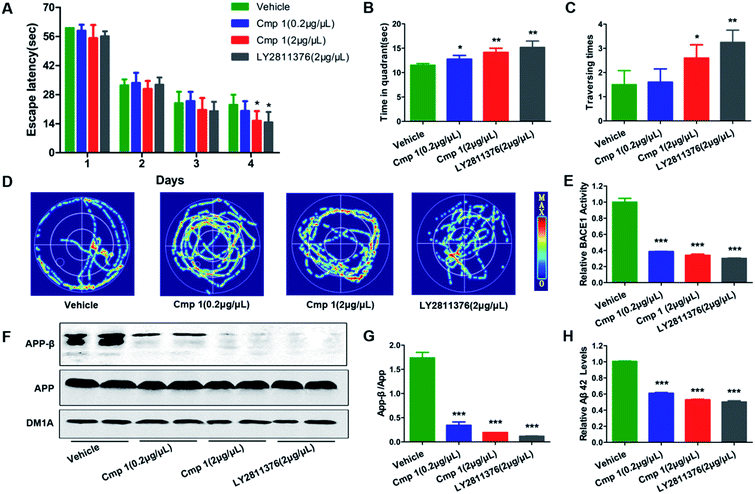
Many natural products exhibit excellent activity in vitro but are not effective in vivo. To comprehensively evaluate the BACE1 inhibitory activity of 1, we tested the bioactivity of 1 against AD-like pathological and behavioural alteration using 3xTg mice (the amount of compound 2 was too limited to complete the in vivo experiments). The Morris water maze was used to evaluate whether compound 1 could protect against learning and memory impairment in 3xTg mice.22 Because hippocampal-dependent memory impairments are the earliest cognitive symptoms detected in AD, we tested all 3xTg mice in a classic spatial reference memory task in the water maze, which relies heavily upon hippocampal integrity (Fig. 5A). We found that only treatment with 2 μg μL−1 of 1 or LY2811376 decreased the latency in finding a hidden platform at the 4th day, which partially indicated that compound 1 efficiently attenuated the hippocampal impairment of 3xTg mice. For the probe trial, the crossing numbers and time spent in the target quadrant were significantly increased in mice treated with 1 or LY2811376, which indicated better memory recall (Fig. 5B and C). The typical swimming tracks indicated that mice treated with 1 or LY2811376 searched for the platform in a more appropriate way than the vehicle group, resulting in the shorter latency to locate the platform and more excursions in the target quadrant (Fig. 5D). All of the mice exhibited comparable swimming speeds, indicating that 1 did not affect motor function (Fig. S2†). Thus, compound 1 can protect against learning and memory deficits in the AD mouse model. To test the effect of 1 on BACE1 activity, we employed β-Secretase Activity Assay Kit to detect BACE1 activity in the 3xTg mice.23 We found that treatment with 1 dramatically reduced BACE1 activity, similar to treatment with LY2811376 (Fig. 3E), and APP-β/APP was also dramatically decreased, which suggested that BACE1 activity was inhibited (Fig. 5F and G). As BACE1 contributes to the production of Aβ by cleaving APP, we also found a marked decrease in Aβ42 production in the hippocampus of 3xTg mice treated with compound 1 (Fig. 5H), while Nissl staining revealed no reduction in cell numbers in the hippocampus (Fig. S1†).
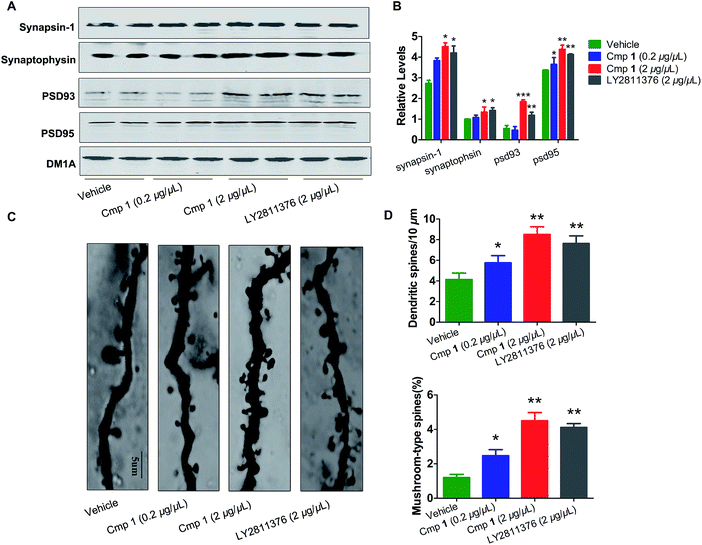
The morphological structures of neurons and the expression of synaptic proteins are critical components for learning and memory.24,25 Therefore, we explored the mechanism underlying the improvement in memory induced by compound 1. Golgi staining demonstrated that treatment with compound 1 remarkably increased dendritic branches and mushroom-type spines in the hippocampus (Fig. 6C and D). We also observed that the expression of several key learning- and memory-related proteins, synapsin-1, synaptophysin, PSD 93 and PSD 95, were increased in compound 1-treated 3xTg mice (Fig. 6A and B). Overall, 1 effectively improved Aβ-induced synapse impairments in vivo, producing effects similar to those elicited by treatment with LY2811376.
The yield of novel, natural isolates is typically too low to perform comprehensive biological assessments, particularly in animals, which severely restricts the discovery of new drugs and hampers the development of modern medicine. In this study, we only obtained 3.6 mg of compound 1 and 1.8 mg of 2 in total and with titres of 0.036 mg kg−1 and 0.018 mg kg−1, respectively. To compensate for the limited amounts of these compounds, a series of effective methods were used to evaluate BACE1 inhibitory activity guided by ISTC assays. Overall, these methods represent a novel starting point in the discovery of BACE1 inhibitors and may facilitate compound activity screening in medicinal chemistry.
Conclusions
In this study, asperterpenes A (1) and B (2), the first natural products with an unprecedented carbon skeleton featuring a 1,2,5-trimethyl-4,9-dioxobicyclo[3.3.1]non-2-ene-3-carboxylic acid moiety, were obtained from Aspergillus terreus. We subjected novel compounds 1 and 2 to anti-AD assays based on our previous study and determined that BACE1 was the most likely target guided by the method of ISTC. Asperterpenes A and B displayed significant inhibitory activity against BACE1 in vitro and were determined to be the most effective natural BACE1 inhibitors, with even higher BACE1 inhibitory activity than one of the most potent potential clinical BACE1 inhibitors, LY2811376, from Eli Lilly. Many BACE1 inhibitors have strong activity in vitro but no effect in vivo. However, compound 1 displayed excellent BACE1 inhibitory activity in animal studies, comparable to that of LY2811376. Overall, this is the first report of terpenoids as effective inhibitors of BACE1. Inhibitors of BACE1 are being intensely pursued, and these findings may provide a lead compound for promising drug candidates for the treatment of AD.Experimental section
General experimental procedures
HRESIMS data were obtained in the positive ion mode on a Thermo Fisher LC-LTQ-Orbitrap XL spectrometer. Extensive NMR spectra were obtained on a Bruker AM-600/400 spectrometer with tetramethylsilane as an internal standard, and the 1H and 13C NMR chemical shifts were normalized to the solvent or solvent impurity peaks for CDCl3 at δH 7.24 and δC 77.23. UV and FT-IR spectra were measured using Varian Cary 50 and Bruker Vertex 70 devices, respectively. Optical rotations were recorded using a Perkin-Elmer 341 polarimeter. Melting points were determined via a Beijing Tech X-5 micro-melting point instrument (uncorrected). Experimental ECD data were acquired through a JASCO J-810 spectrometer (Jasco, Easton, MD, USA). The X-ray diffraction experiments were carried out on a Bruker APEX DUO diffractometer using graphite monochromated CuKα radiation (λ = 1.54178 Å). HPLC procedures were implemented on an Agilent 1200 quaternary system with a UV detector using a reversed-phased C18 column (5 μm, 10 × 250 mm, Welch Materials, Inc.) at a flow rate of 2.0 mL min−1. CHIRALPAK®IC column (5 μm, 4.6 × 250 mm, Daicel Chiral Technologies Co., Ltd. China) was applied to the enantioseparation. Thin-layer chromatography (TLC) was performed with RP-C18 F254 plates (Merck, Germany) and silica gel 60 F254 (Yantai Chemical Industry Research Institute). ODS (50 μm, YMC Co. Ltd., Japan), Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Sweden), and silica gel (200–300 mesh; Qingdao Marine Chemical Inc., China) were used for column chromatography.Fungal material
The fungus Aspergillus terreus was isolated from the soil collected in the bottom of Yangzi River in May 2013 at Tongji Medical College, Hubei Province, China. The sequence data for this strain have been submitted to the DDBJ/EMBL/GenBank under accession no. KT360948. A voucher sample, QCX20130513, was preserved in the herbarium of Huazhong University of Science and Technology, P. R. China.Fermentation and isolation
To prepare the seed culture, the strain was cultured on potato dextrose agar (PDA) at 28 °C for 7 days. The agar plugs were cut into small pieces (approximately 0.6 × 0.6 × 0.6 cm3), then the strain was inoculated into 50 Erlenmeyer flasks (5 L) that were previously sterilized by autoclaving and each contained 1000 g rice and 1000 mL distilled water. The flasks were incubated at 28 °C for 28 days. After the incubation, the growth of fungus was stopped by adding 1000 mL ethanol to each flask, followed by extraction with ethanol. We used reduced pressure to remove the ethanol and yield a brown extract (880.0 g). The extracts were introduced to a silica gel chromatography column (CC) and eluted with CH2Cl2/MeOH (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain six fractions (Fr. 1–Fr. 6). We separated Fr. 4 with repeated silica gel CC to yield seven subfractions (Fr. 4.1–Fr. 4.7), and then subjected subfraction Fr. 4.4 to a Sephadex LH-20 CC (CHCl3–MeOH, 1
1) to obtain six fractions (Fr. 1–Fr. 6). We separated Fr. 4 with repeated silica gel CC to yield seven subfractions (Fr. 4.1–Fr. 4.7), and then subjected subfraction Fr. 4.4 to a Sephadex LH-20 CC (CHCl3–MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford four parts (Fr. 4.4a–Fr. 4.4d). The second part (Fr. 4.4b) was chromatographed on ODS (MeOH–H2O, 20–100%) to yield five mixtures (A–E). Mixture B was purified by repeated semi-preparative HPLC (MeOH–H2O, 60% and MeCN–H2O, 45%) to yield 2 (1.8 mg). Purifying mixture D by repeated semi-preparative HPLC (MeCN–H2O, 65% and MeOH–H2O, 55%) yielded 1 (3.6 mg). Finally, the mixture C was separated by semi-preparative HPLC (MeCN–H2O, 70%) to get 3 (3.1 mg) and 4 (3.2 mg).
1) to afford four parts (Fr. 4.4a–Fr. 4.4d). The second part (Fr. 4.4b) was chromatographed on ODS (MeOH–H2O, 20–100%) to yield five mixtures (A–E). Mixture B was purified by repeated semi-preparative HPLC (MeOH–H2O, 60% and MeCN–H2O, 45%) to yield 2 (1.8 mg). Purifying mixture D by repeated semi-preparative HPLC (MeCN–H2O, 65% and MeOH–H2O, 55%) yielded 1 (3.6 mg). Finally, the mixture C was separated by semi-preparative HPLC (MeCN–H2O, 70%) to get 3 (3.1 mg) and 4 (3.2 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) = 249 (3.99) and 202 (3.74) nm; IR νmax = 3392, 2973, 2951, 2867, 1740, 1736, 1660, 1620, 1460, 1433, 1378, 1337 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables S1;† HRESIMS [M + Na]+m/z 451.2410 (calcd for C26H36O5Na, 451.2460).
ε) = 249 (3.99) and 202 (3.74) nm; IR νmax = 3392, 2973, 2951, 2867, 1740, 1736, 1660, 1620, 1460, 1433, 1378, 1337 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables S1;† HRESIMS [M + Na]+m/z 451.2410 (calcd for C26H36O5Na, 451.2460).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) = 253 (3.12) and 209 (3.31) nm; IR νmax = 3396, 3263, 2957, 2881, 1741, 1725, 1711, 1453, 1433, 1388, 1368, 1345 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables S1;† HRESIMS [M + Na]+m/z 499.2637 (calcd for C27H40O7Na, 499.2672).
ε) = 253 (3.12) and 209 (3.31) nm; IR νmax = 3396, 3263, 2957, 2881, 1741, 1725, 1711, 1453, 1433, 1388, 1368, 1345 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables S1;† HRESIMS [M + Na]+m/z 499.2637 (calcd for C27H40O7Na, 499.2672).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) = 231 (3.28) and 202 (3.81) nm; IR νmax = 3528, 2990, 2944, 1776, 1754, 1728, 1618, 1446, 1387 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables S1;† HRESIMS [M + Na]+m/z 481.2199 (calcd for C26H34O7Na, 481.2202).
ε) = 231 (3.28) and 202 (3.81) nm; IR νmax = 3528, 2990, 2944, 1776, 1754, 1728, 1618, 1446, 1387 cm−1; for 1H NMR (400 MHz) and 13C NMR (100 MHz) data see Tables S1;† HRESIMS [M + Na]+m/z 481.2199 (calcd for C26H34O7Na, 481.2202).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Gaithersburg, MD, USA) and 200 μg mL−1 G418, and grown at 37 °C in a humid atmosphere with 5% CO2/95% air. Cells were seeded in 6-well culture plates (1 × 106 cells per well).21 After adherence and confluence were reached, the medium in each well was replaced with 1000 μL of the appropriate fresh medium, containing the test compound in DMSO or the vehicle alone in control wells. After 24 h of treatment, further analysis was continued.
1, v/v), supplemented with 10% fetal bovine serum (FBS, Gibco BRL, Gaithersburg, MD, USA) and 200 μg mL−1 G418, and grown at 37 °C in a humid atmosphere with 5% CO2/95% air. Cells were seeded in 6-well culture plates (1 × 106 cells per well).21 After adherence and confluence were reached, the medium in each well was replaced with 1000 μL of the appropriate fresh medium, containing the test compound in DMSO or the vehicle alone in control wells. After 24 h of treatment, further analysis was continued.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. For cell samples, cells were washed with PBS and lysed with 1× RIPA buffer. The protein concentrations of the supernatants were measured using the BCA method. The same amount of protein was separated using SDS-polyacrylamide gel electrophoresis (10%) and transferred to a nitrocellulose membrane. The membranes were blocked in 3% non-fat milk for 1 h and incubated with the following primary antibodies at 4 °C overnight: polyclonal anti-human sAPPβ antibodies from IBL (dilution 1
000g for 10 min. For cell samples, cells were washed with PBS and lysed with 1× RIPA buffer. The protein concentrations of the supernatants were measured using the BCA method. The same amount of protein was separated using SDS-polyacrylamide gel electrophoresis (10%) and transferred to a nitrocellulose membrane. The membranes were blocked in 3% non-fat milk for 1 h and incubated with the following primary antibodies at 4 °C overnight: polyclonal anti-human sAPPβ antibodies from IBL (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Aramachi, Gunma, JAPAN); polyclonal anti-APP antibodies from Cell Signaling Technology (dilution 1
1000, Aramachi, Gunma, JAPAN); polyclonal anti-APP antibodies from Cell Signaling Technology (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Beverly, MA, USA); polyclonal anti-PSD95 antibodies obtained from Abcam, (dilution 1
1000, Beverly, MA, USA); polyclonal anti-PSD95 antibodies obtained from Abcam, (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Cambridge, MA, USA); polyclonal anti-PSD93 antibodies obtained from Abcam (dilution 1
1000, Cambridge, MA, USA); polyclonal anti-PSD93 antibodies obtained from Abcam (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Cambridge, MA, USA); monoclonal anti-β-actin antibodies obtained from Abcam (dilution 1
1000, Cambridge, MA, USA); monoclonal anti-β-actin antibodies obtained from Abcam (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500 Cambridge, MA, USA); polyclonal synapsin-1 antibodies obtained from Millipore (dilution 1
1500 Cambridge, MA, USA); polyclonal synapsin-1 antibodies obtained from Millipore (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Temecula, CA, USA); and monoclonal anti-synaptophysin antibodies obtained from Sigma (dilution 1
1000, Temecula, CA, USA); and monoclonal anti-synaptophysin antibodies obtained from Sigma (dilution 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, MO, USA). The membranes were incubated with secondary antibody conjugated to IRDye (800 CW) for 2 h and visualized using the Odyssey Infrared Imaging System (LI-Cor Biosciences, Lincoln, NE, USA).
1000, MO, USA). The membranes were incubated with secondary antibody conjugated to IRDye (800 CW) for 2 h and visualized using the Odyssey Infrared Imaging System (LI-Cor Biosciences, Lincoln, NE, USA).
Acknowledgements
We thank Dr Xiao-nian Li from Analytical and Testing Center at Kunming Institute of Botany for X-ray analyses. This study was financially supported by the Program for New Century Excellent Talents in University, State Education Ministry of China (NCET-2008-0224) and by the National Natural Science Foundation of China (Nos 31370372, 31270395, 81573316, 31570361, 21502057, 81271405, and 31200258).Notes and references
- S. J. Vos, F. Verhey, L. Frölich, J. Kornhuber, J. Wiltfang, W. Maier, O. Peters, E. Rüther, F. Nobili and S. Morbelli, Brain, 2015, awv029 Search PubMed.
- H. Malkki, Nat. Rev. Neurol., 2015, 11, 548 CrossRef CAS PubMed.
- J. W. Wright and J. W. Hardinga, J. Alzheimer's Dis., 2015, 45, 985 CAS.
- A. B. Nigh, S. Vahedi, E. G. Davis, S. Weintraub, E. H. Bigio, W. L. Klein and C. Geula, Brain, 2015, 138, 1722 CrossRef PubMed.
- K. L. Viola and W. L. Klein, Acta Neuropathol., 2015, 129, 183 CrossRef CAS PubMed.
- D. Alcolea, P. Martínez-Lage, P. Sánchez-Juan, J. Olazarán, C. Antúnez, A. Izagirre, M. Ecay-Torres, A. Estanga, M. Clerigué and M. C. Guisasola, Neurology, 2015, 85, 626 CrossRef CAS PubMed.
- A. K. Ghosh and H. L. Osswald, Chem. Soc. Rev., 2014, 43, 6765 RSC.
- C. R. Ellis and J. Shen, J. Am. Chem. Soc., 2015, 137, 9543 CrossRef CAS PubMed.
- Y. Huang and L. Mucke, Cell, 2012, 148, 1204–1222 CrossRef CAS PubMed.
- H. Zhu, C. Chen, Y. Xue, Q. Tong, X. N. Li, X. Chen, J. Wang, G. Yao, Z. Luo and Y. Zhang, Angew. Chem., Int. Ed., 2015, 54, 13374 CrossRef CAS PubMed.
- C. Chen, H. Zhu, Q. Tong, X. N. Li, J. Yang, Y. Xue, Z. Luo, J. Wang, G. Yao and Y. Zhang, Angew. Chem., Int. Ed., 2016, 128, 3547 CrossRef.
- H. C. Lo, R. Entwistle, C. J. Guo, M. Ahuja, E. Szewczyk, J. H. Hung, Y. M. Chiang, B. R. Oakley and C. C. Wang, J. Am. Chem. Soc., 2012, 134, 4709 CrossRef CAS PubMed.
- D. B. Stierle, A. A. Stierle, B. Patacini, K. McIntyre, T. Girtsman and E. Bolstad, J. Nat. Prod., 2011, 74, 2273 CrossRef CAS PubMed.
- P. C. May, R. A. Dean, S. L. Lowe, F. Martenyi, S. M. Sheehan, L. N. Boggs, S. A. Monk, B. M. Mathes, D. J. Mergott and B. M. Watson, J. Neurosci., 2011, 31, 16507 CrossRef CAS PubMed.
- Y. Matsuda, T. Iwabuchi, T. Wakimoto, T. Awakawa and I. Abe, J. Am. Chem. Soc., 2015, 137, 3393 CrossRef CAS PubMed.
- Y. Matsuda and I. Abe, Nat. Prod. Rep., 2016, 33, 26 RSC.
- H. B. Luo, Y. Y. Xia, X. J. Shu, Z. C. Liu, Y. Feng, X. H. Liu, G. Yu, G. Yin, Y. S. Xiong, K. Zeng, J. Jiang, K. Q. Ye, X. C. Wang and J. Z. Wang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16586 CrossRef CAS PubMed.
- X. Wang, J. Blanchard, I. Grundke-Iqbal, J. Wegiel, H. X. Deng, T. Siddique and K. Iqbal, Acta Neuropathol., 2014, 127, 243 CrossRef CAS PubMed.
- X. Wang, J. Blanchard, E. Kohlbrenner, N. Clement, R. M. Linden, A. Radu, I. Grundke-Iqbal and K. Iqbal, FASEB J., 2010, 24, 4420 CrossRef CAS PubMed.
- J. Chlebek, A. D. Simone, A. Hošťálková, L. Opletal, C. Pérez, D. I. Pérez, L. Havlíková, L. Cahlíková and V. Andrisano, Fitoterapia, 2016, 109, 241 CrossRef CAS PubMed.
- X. Wang, T. Lu, Y. Lu, J. Peng, Y. Zhu, Y. Zhang and Z. Sun, FEBS Lett., 2015, 589, 726 CrossRef CAS PubMed.
- R. Morris, J. Neurosci. Methods, 1984, 11, 47 CrossRef CAS PubMed.
- Z. Najafi, M. Saeedi, M. Mahdavi, R. Sabourian, M. Khanavi, M. B. Tehrani, F. H. Moghadam, N. Edraki, E. Karimpor-Razkenari, M. Sharifzadeh, A. Foroumadi, A. Shafiee and T. Akbarzadeh, Bioorg. Chem., 2016, 67, 84 CrossRef CAS PubMed.
- B. Li, H. Yamamori, Y. Tatebayashi, B. Shafit-Zagardo, H. Tanimukai, S. Chen, K. Iqbal and I. Grundke-Iqbal, J. Neuropathol. Exp. Neurol., 2008, 67, 78 CrossRef CAS PubMed.
- E. Masliah, R. D. Terry, R. M. DeTeresa and L. A. Hansen, Neurosci. Lett., 1989, 103, 234 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- H. D. Flack, Acta Crystallogr., Sect. A: Found. Crystallogr., 1983, 39, 876 CrossRef.
- R. W. W. Hooft, L. H. Straver and A. L. Spek, J. Appl. Crystallogr., 2008, 41, 96 CrossRef CAS PubMed.
- S. Oddo, A. Caccamo, J. D Shepherd, M. P. Murphy, T. E. Golde, R. Kayed, R. Metherate, M. P. Mattson, Y. Akbari and F. M. LaFerla, Neuron, 2003, 39, 409 CrossRef CAS PubMed.
- R. Brandeis, Y. Brandys and S. Yehuda, Int. J. Neurosci., 1989, 48, 29 CrossRef CAS PubMed.
- L. J. Reed and H. Muench, Am. J. Hyg., 1938, 27, 493–497 Search PubMed.
- G. P. Demyanenko, A. Y. Tsai and P. F. Maness, J. Neurosci., 1999, 19, 4907 CAS.
- R. Emmers, Proc. Soc. Exp. Biol. Med., 1965, 119, 271 CrossRef CAS PubMed.
- A. Bensadoun and D. Weinstein, Anal. Biochem., 1976, 70, 241 CrossRef CAS PubMed.
- F. Mancini, A. De Simone and V. Andrisano, Anal. Bioanal. Chem., 2011, 400, 1979 CrossRef CAS PubMed.
- A. De Simone, G. F. Ruda, C. Albani, G. Tarozzo, T. Bandiera, D. Piomelli, A. Cavalli and G. Bottegoni, Chem. Commun., 2014, 50, 4904–4907 RSC.
- F. Prati, A. De Simone, P. Bisignano, A. Armirotti, M. Summa, D. Pizzirani, R. Scarpelli, D. I. Perez, V. Andrisano, A. Perez-Castillo, B. Monti, F. Massenzio, L. Polito, M. Racchi, A. D. Favia, G. Bottegoni, A. Martinez, M. L. Bolognesi and A. Cavalli, Angew. Chem., Int. Ed., 2014, 54, 1578 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 1D and 2D NMR, MS, UV, IR spectra for 1–3, 1D NMR for 4. CCDC 1416500 and 1416501. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc02464e |
| ‡ These authors contributed equally to this work. |
§ Crystal data for compound 1: C26H36O5, M = 428.55, orthorhombic, a = 7.9305(2) Å, b = 11.6311(3) Å, c = 25.2575(5) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 2329.76(10) Å3, T = 296(2) K, space group P212121, Z = 4, μ(CuKα) = 0.666 mm−1, 9987 reflections measured, 3701 independent reflections (Rint = 0.0304). The final R1 values were 0.0354 (I > 2σ(I)). The final wR(F2) values were 0.1015 (I > 2σ(I)). The final R1 values were 0.0358 (all data). The final wR(F2) values were 0.1021 (all data). The goodness of fit on F2 was 1.080. Flack parameter = 0.0(2). The Hooft parameter is −0.02(7) for 1531 Bijvoet pairs (CCDC 1416500). Crystal data for compound 3: C26H36O7, M = 474.55, orthorhombic, a = 15.9092(4) Å, b = 18.9397(5) Å, c = 8.0829(2) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 2435.50(11) Å3, T = 298(2) K, space group P21212, Z = 4, μ(CuKα) = 0.770 mm−1, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 reflections measured, 3931 independent reflections (Rint = 0.0479). The final R1 values were 0.0407 (I > 2σ(I)). The final wR(F2) values were 0.1136 (I > 2σ(I)). The final R1 values were 0.0411 (all data). The final wR(F2) values were 0.1140 (all data). The goodness of fit on F2 was 1.046. Flack parameter = 0.14(18). The Hooft parameter is 0.0119(8) for 1939 Bijvoet pairs (CCDC 1416501). 869 reflections measured, 3931 independent reflections (Rint = 0.0479). The final R1 values were 0.0407 (I > 2σ(I)). The final wR(F2) values were 0.1136 (I > 2σ(I)). The final R1 values were 0.0411 (all data). The final wR(F2) values were 0.1140 (all data). The goodness of fit on F2 was 1.046. Flack parameter = 0.14(18). The Hooft parameter is 0.0119(8) for 1939 Bijvoet pairs (CCDC 1416501). |
| This journal is © The Royal Society of Chemistry 2016 |