Gd2O3 and GH combined with red blood cells to improve the sensitivity of contrast agents for cancer targeting MR imaging†
Kunchi
Zhang
,
Yi
Cao
,
Ye
Kuang
,
Min
Liu
*,
Yang
Chen
,
Zhili
Wang
,
Shanni
Hong
,
Jine
Wang
and
Renjun
Pei
*
Key Laboratory of Nano-Bio Interface, Division of Nanobiomedicine, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, China. E-mail: rjpei2011@sinano.ac.cn; Tel: +86-512-62872776
First published on 14th November 2016
Abstract
Herein, we fabricated efficient MR imaging probes by incorporating gadolinium oxide nanoparticles (Gd2O3) and gadolinium hybrid nanoparticles (GH) within RBCs. The Gd2O3 and GH encapsulated in the RBCs exhibited high relaxation rates and revealed high sensitivity for T1 MR imaging.
In recent years, gadolinium chelates have been encapsulated in targeting nanocarriers to resist rapid removal and decrease toxicity in vivo. These nanocarriers could satisfy several requirements, including biocompatibility, low toxicity, long circulation time and specificity etc.1 Researchers have previously developed contrast agents based on synthetic macromolecules, inorganic materials and liposomes. However, they all have drawbacks with regards to in vivo stability. Therefore, in order to avoid the inherent problems of nanocarriers, researchers have focused on the study of vectors that originate from cells, which have recently emerged as biological drug carriers, an interesting alternative to the regular carriers. Different cells, such as carrier erythrocytes (also called red blood cells, RBCs), bacterial ghosts, genetically engineered stem cells and dendritic cells have been used.2
As the most abundant and available cells in the human body, RBCs have gained the highest degree of interest owing to a series of advantages.3 The properties of availability, flexibility, inherent biocompatibility, high in vivo stability and long systemic circulation time make RBCs the most extensively studied therapeutic carrier among the vectors originally from cells.4–9 The structural characteristics of having no nuclei and good flexibility make it easy to load RBCs with various diagnosable and therapeutic molecules with high efficiency.10,11 Combined with other advantages, RBCs have been successfully applied in drug delivery and imaging.12–14 In the field of contrast agents (CAs), super paramagnetic iron oxide nanoparticles (SPIONs), gadolinium (Gd) chelates, and Au nanoparticles (AuNPs) have been encapsulated into RBCs to obtain CAs for MRI and X-ray imaging with long circulation time and high stability in the blood circulatory system.15–20 Also the Gd-loaded RBCs have been used as CAs to provide quantitative information on tumor vascularization. In this study, at first, we encapsulated Gd-DTPA into RBCs to manufacture CAs for T1 MRI. However, the relaxivity and sensitivity of Gd-DTPA@RBCs did not meet the requirements desired.
In recent studies, gadolinium-based nanoparticles have shown unique superiority compared to Gd chelates.21,28 In particular, gadolinium oxide nanoparticles (Gd2O3) as nanoclusters have been reported to possess proton relaxivity of 6–10 mM−1 s−1, which is superior to that of gadolinium chelates.22 We synthesized Gd2O3 nanoparticles according to the methods of Chang et al.23 The size of the as-prepared Gd2O3 nanoparticles was 1.0 nm. Nevertheless, it was found that complex procedures in the oily phase may have caused additional aqueous modification and thus limited their relaxivity. Therefore, we also synthesized gadolinium-based hybrid nanoparticles (GH). The GH synthesized were gadolinium oxide nanocrystals with albumin scaffolds and a mean size of 25.5 nm. Albumin-based biomineralization exhibited multiple advantages such as a facile and reproducible methodology, biocompatibility and good stability.22 The method for preparing the GH was simple, reproducible and environmentally benign and the produced GH exhibited high relaxivity.24
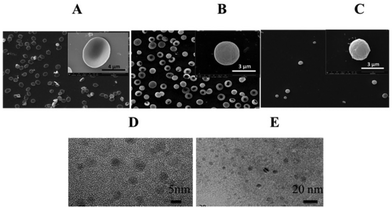
In light of the advantages mentioned above, we encapsulated Gd-based nanoparticles into RBCs to build high relaxivity and sensitivity CAs for T1 MR imaging. The preparation process for Gd-DTPA-loaded erythrocytes included two steps (Scheme 1). The membrane pores of erythrocytes (RBCs) were enlarged by adding a hypotonic solution while the resealing procedure of the pores was the result of adding a hypertonic solution. The intracellular concentration of Gd3+ loaded into the RBCs was measured by ICP-AES. The concentration of Gd3+ in the Gd2O3@RBCs-cRGD (4.5 mM) was higher than that of Gd3+ in Gd-DTPA@RBCs-cRGD (1.05 mM) and GH@RBC-cRGD (0.2 mM) in the same number of RBCs (9.5 × 108 mL−1). The concentration of Gd3+ in GH@RBCs-cRGD was much lower than in Gd2O3@RBCs-cRGD, and even lower than in Gd-DTPA@RBCs-cRGD. This phenomenon should be related to the size of the GH. To further increase the sensitivity of the CAs, we designed a vector of RBCs with cross-linked cRGDyK as targeting molecules on the surface.25 To characterize the morphology of the cRGD modified RBCs, the RBCs were visualized using SEM. The images indicate that the change in the morphology of the RBCs is not observable through the preparation process (Fig. 1B and C), when compared with the native RBCs (Fig. 1A). Both the resealed RBCs and the RBCs reacting with cRGD are almost the same size as the native RBCs. The morphology of the RBCs loaded with GH/Gd2O3 is not obviously different to that of the native RBCs (data not shown). Since there were unreacted aldehyde groups on the membrane surface of RBCs-cRGD, the RBCs shown in Fig. 1C were diluted 100 fold to get the normal morphology. The results shown in Fig. 1A and B were diluted 30 fold. However, there were some wrinkles appearing on the membranes of the RBCs after modifying with cRGD (Fig. 1C). We deemed that these wrinkles were attributed to two behaviors. One was the shear force produced by magnetic stirring in the processes of loading and reaction. The other was immobilization of the proteins on the RBCs’ membrane during the process of glutaraldehyde treatment. The distribution of proteins and phospholipids on the cell membrane is inhomogeneous and results in directed flow. When the reaction between glutaraldehyde and the amino groups of the proteins occurred, the mobility was decreased and the wrinkles could have been produced. In spite of this, the wrinkles didn't have too much of an effect on the properties of the RBCs. Research has shown that a low concentration of glutaraldehyde is non-toxic and provides RBCs with increased stability.27 In this study, we used 0.8 mM glutaraldehyde to treat the RBCs, and found that when the concentration of glutaraldehyde was increased to 1 mM, aggregation appeared. The aggregation became serious at 10 mM and the number of RBCs severely decreased.
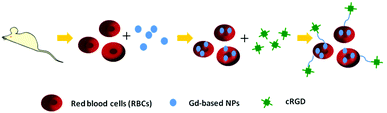 | ||
| Scheme 1 Illustration of the procedure for preparing tumor targeting Gd-based nanoparticle loaded RBC-cRGD. | ||
To study the in vivo stability properties of the RBCs, we measured the half-life of Gd2O3@RBCs-cRGD in blood circulation. The half-life of Gd2O3@RBCs-cRGD calculated by the first order kinetic equation was 21 h. Its circulation time in blood was much longer than that of Gd-DTPA and Gd-based nanoparticles. We have measured the interaction of Gd2O3@RBCs-cRGD with BSA. After Gd2O3@RBCs-cRGD was incubated with BSA for both 2 h and 6 h, the concentration of BSA exhibited almost no change, which indicated that there was almost no or very little nonspecific adsorption of BSA on Gd2O3@RBCs-cRGD (Fig. S1†).
Then we investigated the r1 of the RBCs (Fig. 2), and a good linear relation between the concentration of Gd3+ and 1/T1 was seen. As shown in Fig. 2, the r1 of Gd-DTPA@RBCs-cRGD (6.97 mM−1 s−1) was higher than that of Gd-DTPA (4.25 mM−1 s−1), as were those of GH@RBCs-cRGD (19.12 mM−1 s−1) and Gd2O3@RBCs-cRGD (6.4 mM−1 s−1). In the process of preparation, part of the GH might stay on the inner surface of the RBCs, since some proteins on the surface of the RBCs fell off under the hypotonic environment. The increase in r1 of GH@RBCs-cRGD mainly came from geometrical confinement and prolonged tumbling times caused by the GH on the inner surface of the RBC membrane. However, the Gd2O3 nanoparticles would not stay or adsorb on the inner surface due to their size and the hydroxyl groups on the surface, so there was no obvious change in r1. Our results confirmed that the relaxivity could be increased by the combination of Gd-based nanoparticles with RBCs by the means of encapsulating Gd-DTPA into RBCs.
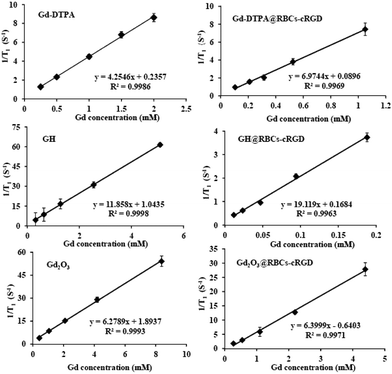 | ||
| Fig. 2 The relaxivity, r1, of Gd-DTPA, Gd-DTPA@RBCs-cRGD, GH, GH@RBCs-cRGD, Gd2O3 and Gd2O3@RBCs-cRGD. | ||
Following the studies on the relaxivity, the sensitivity of the RBCs was tested for cellular imaging. The peptide cRGDyK (cyclo Arg-Gly-Asp-Tyr-Lys) could bind with αvβ3-integrin. U87 cells, which overexpressed αvβ3-integrin, were selected as the cell line. Thus, the U87 cells were incubated with Gd-DTPA, GH, Gd2O3, Gd-DTPA@RBCs, Gd-DTPA@RBCs-cRGD, GH@RBCs, GH@RBCs-cRGD, Gd2O3@RBCs and Gd2O3@RBCs-cRGD for 1 h. Compared to the U87 cells incubated with Magnevist, significantly brighter images of the cells incubated with GH and Gd2O3 were observed (Fig. 3). The cellular images of the U87 cells incubated with the RBCs modified with cRGD on the surface had a significantly stronger contrast than those of the RBCs without cRGD (Fig. 3). Since the concentrations of Gd3+ used were all just 0.01 mM, the results further illustrated that the encapsulation of Gd-based nanoparticles into RBCs-cRGDs could enhance the sensitivity of CAs.
 | ||
| Fig. 3 MR images of U87 cells incubated with 0.01 mM Gd-DTPA, GH, Gd2O3, Gd-DTPA@RBCs, GH@RBCs, Gd2O3@RBCs, Gd-DTPA@RBCs-cRGD, GH@RBsC-cRGD and Gd2O3@RBCs-cRGD (from left to right). | ||
In order to verify the targeting function of cRGD, we undertook an investigation of the flow cytometry (Fig. 4). The fluorescence intensity of the U87 cells incubated with RBC-cRGD-FITC was much stronger than that of RBCs-FITC. However, there was some fluorescence intensity for the U87 cells incubated with RBC-FITC. This might have been due to the membrane fusion of cancer cells and RBCs by endocytosis. In this case, inverted fluorescence microscope images were taken for the further investigation of the targeting effect and the interaction of RBCs with U87 cells.
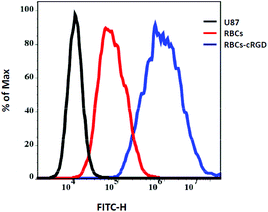 | ||
| Fig. 4 Flow cytometry data of U87 cells, U87 cells treated with RBCs-FITC and RBCs-cRGD-FITC, respectively. Experiments run at a RBC number of 7.5 × 107 per well. | ||
As shown in Fig. 5, obvious fluorescence was observed in the U87 cells incubated with RBCs-cRGD-FITC. The fluorescence intensity on the inside of the cells was weaker than on the surface, and this indicates that most of the RBCs didn't enter the cells. The images in Fig. 5 exhibited certain fluorescent signals for the U87 cells that were incubated with RBC-FITC without the modified cRGD, however, they were much weaker than the images with the modified cRGD. The results from the inverted fluorescence microscope images were consistent with the flow cytometry analyses. The data (Fig. 5) further confirmed that the presence of cRGD on the surface of the RBCs made the RBCs accumulate on the surface of the U87 cells.
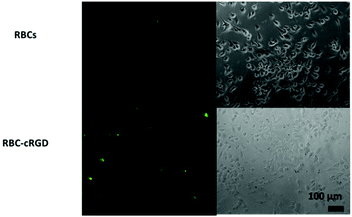 | ||
| Fig. 5 Inverted fluorescence microscope images of U87 cells incubated with RBCs-FITC and RBCs-cRGD-FITC. Experiments run at a RBC number of 7.5 × 107 per well. | ||
Hematoxylin-eosin (HE) staining was carried out to investigate the toxicity issues of Gd2O3@RBCs-cRGD. Fig. S2† shows the HE stained images of a heart, liver, spleen, lung and kidney. Compared to the control group (Fig. S2A†), the experimental group (Fig. S2B†) had normal and clear nucleus structures. In every organ, no sign of an inflammatory response was observed, nor any fibrosis tissue. The results demonstrated that Gd2O3@RBCs-cRGD was non-toxic and safe in vivo.
To investigate the biodistribution of Gd3+, the Gd3+ concentration in the organs was measured by ICP-AES 24 h after injection with Gd2O3@RBCs-cRGD. The Gd% enriched in the heart, liver, spleen, lung and kidney were 11.8%, 10.1%, 7.0%, 26.9% and 0.4%, respectively (Fig. S3†). For the organ with the highest maximum Gd3+ it could be considered that more RBCs were cleared through this organ. The results showed that 24 h after the intravenous injection of the RBCs, the maximal concentration of Gd3+ was in the lung. Meanwhile, it showed that the minimum concentration was in the kidney. It has been reported that intact erythrocytes tend to be enriched in the lungs because of their size and structure. The obtained results here were consistent with the literature.26
In conclusion, we demonstrated that cRGD modified RBCs loaded with Gd-based nanoparticles can act as CAs for tumor targeting MR imaging. The vector of RBCs encapsulating Gd-based nanoparticles has the potential to fabricate CAs with high relaxivity and sensitivity. The enhanced relaxivity was undoubtedly contributed to by the combined effect of RBCs and Gd-based nanoparticles. The specific binding of cRGD to the overexpressed αvβ3-integrin in the U87 cancer cells was verified by cellular MR imaging and flow cytometry studies. The results of cellular MR imaging proved that the modified cRGD could further increase the sensitivity of the RBCs. Since the size of the RBCs was on a micrometer scale, the RBCs would not have an enhanced permeability and retention effect in the tumor site. Therefore, targeting ligands were necessary. Limited by the low field strength of the MRI instrument, the results of in vivo MR imaging were not obtained. The in vivo sensitivity of the cRGD modified RBCs as MR imaging probes remains to be exploited in ongoing studies in our laboratory. Finally, owing to the safety, sensitivity and loading capacity of RBCs, they could be used as high potential and competitive MR imaging probes.
All animal experiments were performed in compliance with the relevant laws on experimental animal use and care in China and followed the institutional guidelines of the Institutional Animal Use and Care Committee of China. All animal experiments were approved by the Ethics Committee of Suzhou Institute of Nano-Tech and Nano-Bionics of Chinese Academy of Sciences.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21304106), Natural Science Foundation of Jiangsu Province (BK20161262) and the CAS/SAFEA International Innovation Teams program.Notes and references
- M. J. Ernsting, M. Murakami, A. Roy and S. D. Li, J. Controlled Release, 2013, 172(3), 782–794 CrossRef CAS PubMed.
- C. Gutiérrez Millán, C. I. Colino Gandarillas, M. L. Sayalero Marinero and J. M. Lanao, Ther. Delivery, 2012, 3(1), 25–41 CrossRef.
- S. Biagiotti, L. Rossi, M. Bianchi, E. Giacomini, F. Pierige, G. Serafini, P. G. Conaldi and M. Magnani, J. Controlled Release, 2011, 154(3), 306–313 CrossRef CAS PubMed.
- M. Chang, J. K. Hsiao, M. Yao, L. Y. Chien, S. C. Hsu, B. S. Ko, S. T. Chen, H. M. Liu, Y. C. Chen, C. S. Yang and D. M. Huang, Nanotechnology, 2010, 21(23), 235130–235133 CrossRef PubMed.
- N. Gupta, B. Patel and F. Ahsan, Pharm. Res., 2014, 31(6), 1553–1565 CrossRef CAS PubMed.
- M. Hamidi, P. Rafiei, A. Azadi and S. Mohammadi-Samani, J. Pharm. Sci., 2011, 100(5), 1702–1711 CrossRef CAS PubMed.
- T. D. Mai, F. d'Orlye, C. Menager, A. Varenne and J. M. Siaugue, Chem. Commun., 2013, 49(47), 5393–5395 RSC.
- L. Rossi, F. Pierige, C. Carducci, C. Gabucci, T. Pascucci, B. Canonico, S. M. Bell, P. A. Fitzpatrick, V. Leuzzi and M. Magnani, J. Controlled Release, 2014, 194, 37–44 CrossRef CAS PubMed.
- L. Y. Wang, X. Y. Shi, C. S. Yang and D. M. Huang, Nanoscale, 2013, 5(1), 416–421 RSC.
- M. Delcea, N. Sternberg, A. M. Yashchenok, R. Georgieva, H. Bäumler, H. Möhwald and A. G. Skirtach, ACS Nano, 2012, 6(5), 4169–4180 CrossRef CAS PubMed.
- L. Picas, F. Rico, M. Deforet and S. Scheuring, ACS Nano, 2013, 7(2), 1054–1063 CrossRef CAS PubMed.
- A. A. Omran, J. Chem. Thermodyn., 2013, 66, 9–13 CrossRef CAS.
- A. A. Omran, Spectrochim. Acta, Part A, 2013, 104, 461–467 CrossRef CAS PubMed.
- M. Shahin, S. Ahmed, K. Kaur and A. Lavasanifar, Biomaterials, 2011, 32(22), 5123–5133 CrossRef CAS PubMed.
- M. Brähler, R. Georgieva, N. Buske, A. Müller, S. Müller, J. Pinkernelle, U. Teichgräber, A. Voigt and H. Bäumler, Nano Lett., 2006, 6(11), 2505–2509 CrossRef PubMed.
- S. Ahn, S. Y. Jung, E. Seo and S. J. Lee, Biomaterials, 2011, 32(29), 7191–7199 CrossRef CAS PubMed.
- M. Foroozesh, M. Hamidi, A. Zarrin, S. Mohammadi-Samani and H. Montaseri, J. Pharm. Pharmacol., 2011, 63(3), 322–332 CrossRef CAS PubMed.
- M. Shaillender, R. Luo, S. S. Venkatraman and B. Neu, Int. J. Pharm., 2011, 415(1–2), 211–217 CrossRef CAS PubMed.
- S. Tan, T. Wu, D. Zhang and Z. Zhang, Theranostics, 2015, 5(8), 863–881 CrossRef CAS PubMed.
- G. Ferrauto, D. Delli Castelli, E. Di Gregorio, S. Langereis, D. Burdinski, H. Grull, E. Terreno and S. Aime, J. Am. Chem. Soc., 2014, 136(2), 638–641 CrossRef CAS PubMed.
- W. J. Rieter, K. M. L. Taylor, H. An, W. Lin and W. Lin, J. Am. Chem. Soc., 2006, 128, 9024–9025 CrossRef CAS PubMed.
- Y. Wang, T. Yang, H. T. Ke, A. J. Zhu, Y. Y. Wang, J. X. Wang, J. K. Shen, S. Liu, C. Y. Chen, Y. L. Zhao and H. B. Chen, Adv. Mater., 2015, 27, 3874–3882 CrossRef CAS PubMed.
- C. R. Kim, J. S. Baeck, Y. M. Chang, J. E. Bae, K. S. Chae and G. H. Lee, Phys. Chem. Chem. Phys., 2014, 16, 19866–19873 RSC.
- B. B. Zhang, H. T. Jin, Y. Li, B. D. Chen, S. Y. Liu and D. L. Shi, J. Mater. Chem., 2012, 22, 14494–14501 RSC.
- R. Haubner, R. Gratias, B. Diefenbach, S. L. Goodman, A. Jonczyk and H. Kessler, J. Am. Chem. Soc., 1996, 118(32), 7461–7472 CrossRef CAS.
- C. A. Anselmo, V. Gupta, J. B. Zern, D. Pan, M. Zakrewsky, V. Muzykantov and S. Mitragotri, ACS Nano, 2013, 7(12), 11129–11137 CrossRef PubMed.
- E. Zocchi, M. Tonetti, C. Polvani, L. Guida, U. Benatti and D. A. Flora, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 2040–2044 CrossRef CAS.
- J. M. Moghaddam, D. L. Campo, M. Hirabayashi, A. P. Bean, J. L. Waddington, A. J. Scoble, G. Coia and J. C. Drummond, Biomater. Sci., 2014, 2, 924–935 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for RBCs and MR imaging experiments. See DOI: 10.1039/c6bm00627b |
| This journal is © The Royal Society of Chemistry 2017 |