Open Access Article
Open Access ArticleMicrofluidic fabrication of polyethylene glycol microgel capsules with tailored properties for the delivery of biomolecules†
Luis P. B.
Guerzoni‡
 ,
Jan
Bohl‡
,
Jan
Bohl‡
 ,
Alexander
Jans
,
Alexander
Jans
 ,
Jonas C.
Rose
,
Jonas C.
Rose
 ,
Jens
Koehler
,
Jens
Koehler
 ,
Alexander J. C.
Kuehne
,
Alexander J. C.
Kuehne
 * and
Laura
De Laporte
* and
Laura
De Laporte
 *
*
DWI Leibniz Institute for Interactive Materials, Forckenbeckstrasse 50, Aachen, Germany. E-mail: kuehne@dwi.rwth-aachen.de; delaporte@dwi.rwth-aachen.de
First published on 23rd May 2017
Abstract
Microfluidic encapsulation platforms have great potential not only in pharmaceutical applications but also in the consumer products industry. Droplet-based microfluidics is increasingly used for the production of monodisperse polymer microcapsules for biomedical applications. In this work, a microfluidic technique is developed for the fabrication of monodisperse double emulsion droplets, where the shell is crosslinked into microgel capsules. A six-armed acrylated star-shaped poly(ethylene oxide-stat-propylene oxide) pre-polymer is used to form the microgel shell after a photo-initiated crosslinking reaction. The synthesized microgel capsules are hollow, enabling direct encapsulation of large amounts of multiple biomolecules with the inner aqueous phase completely engulfed inside the double emulsion droplets. The shell thickness and overall microgel sizes can be controlled via the flow rates. The morphology and size of the shells are characterized by cryo-SEM. The encapsulation and retention of 10 kDa FITC-dextran and its microgel degradation mediated release are monitored by fluorescence microscopy.
Introduction
Advanced drug delivery systems (DDS) show great potential to improve the efficacy of common drug therapies. As in systemic applications (e.g., orally in the form of pills), the pharmaceutically active compound is often metabolized directly after application,1 and DDS have been developed to alter and improve the pharmacokinetics and bio-distribution of the loaded drug.2 By incorporating specific binding ligands, targeted drug delivery becomes possible, reducing unwanted side effects, thus allowing the application of higher doses. At present, most of the FDA approved DDS are based on polymer–drug conjugates (Omontys®), liposomes or micelles (DepoDur®, AmBisome®, Doxyl/Caelix® and DepoCyt®), biodegradable carriers like poly(lactic-co-glycolic acid) (Ozudex®), or proteins, such as monoclonal antibodies (Brentuximab®).1,2 Polymers have also been applied to fabricate solid nano- and microspheres,3,4 microcapsules,5 or nano-6 and microgels7 to temporally and spatially control drug delivery. Microcapsules with a solid polymer shell are significantly more stable compared to micelles or liposomes. In addition, they have a large internal volume, which can be loaded directly during capsule formation. Due to these beneficial properties, they represent promising vehicles for targeted delivery and release of compounds, not only as DDS in medicine but also in cosmetics and food applications.8In contrast to solid particles and capsules, polymers can be crosslinked to form hydrogels. Hydrogels can be swollen with water at high volume fractions, depending on their crosslinking density.9–11 When such materials are produced with nano- to micrometer dimensions, they are termed microgels. These soft microgels represent ideal carrier entities for DDSs, as they exhibit unique properties in comparison with hard polymer carriers. The high surface to volume ratio of the polymer network enables them to entrap a large amount of therapeutic molecules, such as carbohydrates,12 proteins7 and DNA,13 which can be loaded via covalent14 or supramolecular binding.15 In addition, the incorporation of inorganic species, such as quantum dots and magnetic nanoparticles has also been reported.16–18 The microgels can also be modified with recognition ligands to target specific tissues19,20 and their chemistry can be fine-tuned to control the release mechanism and kinetics of the loaded drugs.21 Furthermore, the porous network structure enables diffusion-controlled release of small molecules, which can be further tailored when using degradable microgel networks. The in vitro release kinetics of bioactive compounds from hydrogel networks has been extensively investigated.22–24
Depending on the fabrication method, the size of the microgels can be modulated. Standard bulk emulsification or precipitation is mostly applied, resulting in nano- and microgels with diameters in the range of 100 nm to 10 μm.6,25,26 Here, drugs can be loaded during microgel preparation6 or bound to the network post production via diffusion or coupling.14,27 One of the main challenges here is efficient loading of the drug. Loading by diffusion results in a large fraction of the drug remaining in the dispersion medium, often leading to high losses and increased costs. In addition, emulsions produced by stirring result in polydisperse samples with limited control over the essential physical and mechanical particle properties, such as the mesh size and dimensions.28 These inhomogeneities impede precise temporal control over the release kinetics of the drug molecule.
To better control the release kinetics, core–shell or hollow microgels have been established to entrap the drugs inside the lumen and control their release via the properties of the shell. The most common method to produce these microgel architectures is to apply a template, around which a shell is formed.29 For these geometries, we can envision only two approaches for loading of drugs into these soft containers: either the drug is already pre-loaded in the template, or the template is dissolved and the hollow lumen is filled with drugs via diffusion. The disadvantage of both approaches remains the low encapsulation efficiency and insufficient control over the release. To improve this, hollow PNIPAM nanogels have been produced by sequentially crosslinking PNIPAM and poly(N-isopropyl metharcylamide) (PNIPMAM) around silica particles, which can dissolve post fabrication.30 As both PNIPAM and PNIPMAM shells have different lower critical solution temperatures (LCSTs) of 32 and 42 °C, respectively, they have the ability to load drugs inside via diffusion when both shells are swollen, while the drugs can be entrapped in the cavity when the temperature is increased between both LCSTs, resulting in a collapse of the inner shell. Here, the drugs are still loaded post fabrication, and the microgels are produced in batch, which may lead to heterogeneous microgel properties.
To produce monodisperse microgels, enhance the encapsulation efficiency, and control their physical and mechanical properties, microfluidic techniques are applied where microgels are prepared in confined volumes.31–33 Standard (single emulsion) microfluidic dropmakers have been used for the production of monodisperse particles and microgels from the nano- to the microscale.34,35 Inside the microchannels, interfacial and viscous forces dominate over bulk forces and the droplet formation dynamics can be controlled by parameters, such as the flow rates of the fluids, their viscosities, interfacial tension, densities, surface chemistry, and channel geometry.36 After a microgel precursor-carrying phase is emulsified by an immiscible continuous phase, polymerization and/or crosslinking can be triggered inside the droplets to produce droplet-templated microgels.35,37 Using microfluidics for loading active compounds inside the microgels represents a formidable technique to circumvent the above described problems of diffusive loading or loading via a carrier. However, these microgel architectures remain limited in their capacity and temporal control of the release. For example, dextran-loaded core–shell microgels consisting of a polyacrylamide core and a PNIPAM shell have been produced via microfluidics.33 This approach is, however, still restricted, as it requires two polymer components, and limited, as the core of the particle is occupied by microgels.
On the other hand, droplet-based microfluidic techniques can be applied to form double emulsions with high precision and exquisite control over the number and size of the core droplets.28,38 This method enables direct loading of a large concentration of drugs inside the lumen and crosslinking of a second phase to form a protective shell, which can retain the drugs and control its release.21,39 Currently, double emulsion microfluidics has only been applied to make DDS with solid shells, which release the drug upon breaking. For example, solid microcapsules can be produced using acrylate based monomers, which are cured using a UV initiated crosslinking reaction.8 However, the degradation products are not biocompatible, obviating their application in biological environments.40,41 A biocompatible approach was realized by using polycaprolactone as the capsule shell material, in which proteins were encapsulated.42 While these approaches are promising in terms of shelf-life, it remains difficult to trigger and control the release of the active compound.
To circumvent the above described problems, a DDS system would ideally be loaded during microfluidic production and consist of a soft and responsive shell. The absence of such a system prevents the translation of high capacity drug carriers into clinical applications. While there are a variety of suitable hydrogel materials available11,43–48 with adjustable mechanical, chemical, and physical properties, there remains a lack of suitable microgel capsule approaches for DDS applications.
In this present study, we develop a double emulsion microfluidic method to produce microgel capsules, where we directly load bioactive molecules inside the cavity during the formation of the microcapsule. The shell is formed by crosslinking 20% (w/w) six-armed acrylated star-shaped poly(ethylene oxide-stat-propylene oxide) using a biocompatible photoinitiator with absorption in the UV region. This results in capsules with a soft microgel shell, which can respond to their environment via hydrolysis to facilitate controlled drug release. Bulk hydrogels made with the same polymer composition have previously been reported to have an elastic modulus of approximately 3100 Pa, a swelling degree of about 280%, and a theoretically calculated mesh size of 2.5 nm.18,49 In addition, these hydrogels have been shown to be non-cytotoxic.18,50 As the shell degrades by breaking the ester bonds, no toxic free acrylates are present in the degradation products. The microfluidic technique applied here allows production of PEG-based microgel capsules with tunable dimensions, shell thickness and density, and degradation rate. We demonstrate the retention and release of fluorescently labelled dextran (MW = 10 kDa) as a proxy for an active component. As we apply a molecularly defined pre-polymer to produce the microgel shell, we have the opportunity to provide a platform of microgel capsules with tailored properties that can precisely control the release kinetics in a temporal manner, depending on the desired application. The microgel capsules reported here can be directly loaded with high concentrations of bioactive molecules during their microfluidic generation. The capsules have the potential for controlled release, while the kinetics can be tuned by varying the properties of the synthetic hydrogel shell. The capsules can be produced with tailored dimensions and shell thickness, and are synthesized in a monodisperse manner due to the microfluidic fabrication method.
Results and discussion
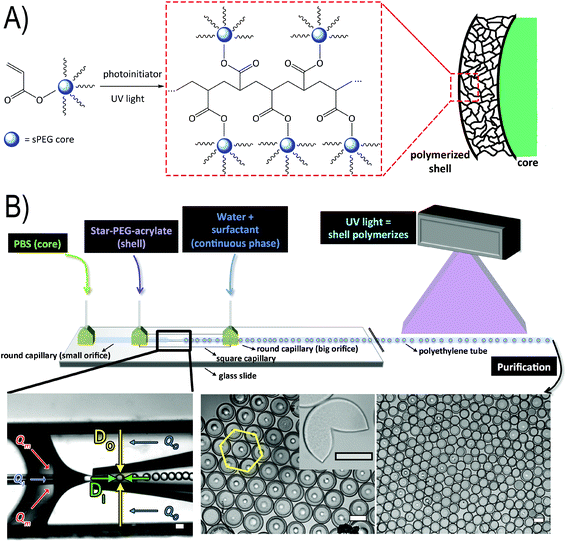
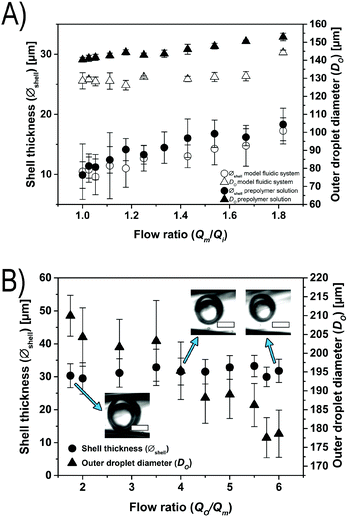
To form the microfluidic reactor, we use glass capillary technology, where tapered capillaries with a round cross-section are aligned inside a square capillary with an inner diameter slightly larger than the outer diameter of the round glass capillaries, using established protocols (see Fig. 1B).51 (The diameters of the tapered orifices of the small and big round capillaries are between 50 and 60 μm and between 200 and 225 μm, respectively, the inner diameter of the square capillary is 205 μm, and the distance between the two tapered orifices is between 50 and 110 μm.) We produce water-in-oil-in-water (W/O/W) emulsions by connecting the respective liquids to the dedicated inlets of the device and we drive the liquids using syringe pumps. The inner, middle, and outer phases are composed of PBS with 2% (w/w) Tween 80, toluene with 5% (w/w) Span 80, and water with 2% (w/w) Tween 80, respectively. To the middle toluene phase, we add an acrylate-functionalized six-armed star-shaped poly(ethylene oxide-stat-propylene oxide) (sPEG-A) pre-polymer with a molecular weight of 3 kDa. We also admix a radical photoinitiator (9% (w/w) Irgacure® 1173), which is activated by exposure to a UV emitting lamp (λem = 254/366 nm) (Fig. 1A and B). Movie 1 in the ESI† shows the microfluidic device producing W/O/W double emulsions that are crosslinked downstream.We manipulate the double emulsion formation by fixing the flow rates of the outer phase (Qo) at 10 mL h−1 and the middle phase (Qm) at 2 mL h−1, and varying the flow rate of the inner phase (Qi). With these settings, we can modify the oil shell thickness of the formed double emulsion droplets while maintaining the overall double emulsion size relatively constant. To investigate these conditions, we use two fluidic systems: a model fluidic system consisting of the aforementioned liquid phases in the absence of pre-polymer, and a system containing a non-reactive sPEG-OH pre-polymer (MW = 3 kDa) to simulate the viscosity of the real sPEG-A loaded oil phase we apply further. Upon decreasing Qi from 2 mL h−1 to 1 mL h−1, the shell thickness of the model fluidic system double emulsions increases from 10.5 to 17.3 μm, while the overall droplet size remains relatively constant (see Fig. 2A). When using the non-reactive pre-polymer solution, the increased viscosity (2.224 mPa s versus the pre-polymer free solution at 0.664 mPa s) leads to a shell thickness window of 9.9 to 18.9 μm. The shell thickness (Øshell) of the double emulsion droplets is determined by measuring the outer diameter of the droplet (Do), subtracting the diameter of the inner droplet (Di), and dividing this value by half. Similar ranges of flow rates have been reported before.52
In a second experiment to control the droplet dimensions, a glass capillary device is used where the outer flow rate (Qo) is varied, while keeping the middle (Qm) and inner (Qi) flow rates constant at 4 and 1.5 mL h−1, respectively. With this configuration, we are able to tune the overall droplet size (from 178.6 to 210.0 μm) while maintaining the shell thickness constant (see Fig. 2B). Reducing the flow rates by half while keeping their ratio constant leads to an unstable system, which does not produce double emulsion droplets. In addition, higher flow rates result in jet formation and downstream turbulences, also forbidding the generation of monodisperse double emulsion droplets.
After successfully demonstrating the formation of double emulsion droplets, and the ability to control their size and shell thickness, the middle fluidic phase is exchanged to the reactive sPEG-A pre-polymer solution to generate microgel capsules. This pre-polymer solution contains 20% (w/w) sPEG-A and 9% (w/w) Irgacure® 1173 in toluene. A UV emitting lamp (λem = 254/366 nm) is applied for crosslinking to allow complete polymerization of the microgels within the short residence time in the microfluidic device. The double emulsion droplets are polymerized downstream in the tubing guiding the double emulsion away from the microfluidic chip. The obtained crosslinked monodisperse microgel capsules are shown in Fig. 1B. Small dots in the center of microgels appear due to their contact with the glass slide.
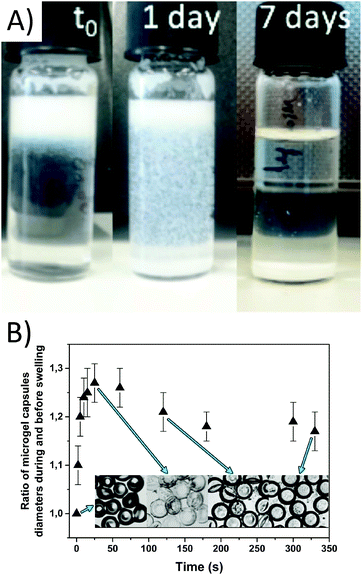
The microgel capsules are sequentially purified by evaporation of toluene and washing with water, isopropanol, and 1× PBS to remove traces of toluene and excess surfactant. After collection, the microgel capsules cream due to the low density of the remaining toluene in the middle phase. Upon removal of the toluene the microgels settle to the bottom of the collection vial over the course of 9 days (Fig. 3A). The purification is done in a much shorter time frame of approximately 1 hour via multiple centrifugation steps, after which the microgel capsules are still stable and remain monodisperse, as shown in Fig. 1B. The microgel capsules are stored at 5 °C and show no change in their macroscopic structure over the course of at least two weeks. To minimize microgel attachment to the inner surface of the tubing, low protein binding tubes are used. The robustness of the microgel capsules is verified by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes, after which the majority of the microgel capsules remain intact. Unlike solid capsules, the microgel capsules are also resistant towards sonication at 120 W. Only with the application of strong mechanical stress, for example by pressing two glass slides together, the microgel capsules break (Fig. 1B, inset).
000 rpm for 20 minutes, after which the majority of the microgel capsules remain intact. Unlike solid capsules, the microgel capsules are also resistant towards sonication at 120 W. Only with the application of strong mechanical stress, for example by pressing two glass slides together, the microgel capsules break (Fig. 1B, inset).
To determine the degree of swelling, the microgel capsules are dried and then again dissolved in water. The shell diameter is monitored over time upon swelling (see Fig. 3B). As the dried microgel capsules stick to the glass surface, they tend to flatten. The resulting oblate shape can be compared to the form of a red blood cell. During re-swelling, the microgel capsules show a fast increase in their diameters during the first thirty seconds. This steep increase is caused by the uptake of water molecules due to the hydrophilic character of the PEG shells53 and represents the fast uptake of total bound water. Water then infuses into the meshes of the hydrogel due to osmotic pressure, swelling the shell to its maximum, which is reached when the polymer chains attain their maximum extension lengths. This fast process is followed by water slowly migrating through the hydrogel network to fill up the inner hollow core of the microgels. In this case, no molecular interactions enhance the process and therefore the migration is slow compared to the first swelling. After 30 seconds, a slow decrease in the diameter of the microgel capsules is observed, as the microgels transition from a flat oblate shape to 3D spherical microgel capsules. This process can be observed in Fig. 3B, and in Movie 2 in the ESI.† Between 40 and 330 seconds, the outer diameter of the microgel capsules is increased by 12 μm, enhancing the total diameter by a factor of 1.2.
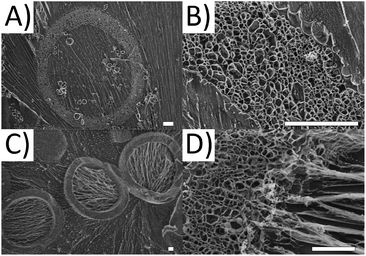
For more detailed insight into the structure of the microgel capsules, cryo-surface electron microscopy (cryo-SEM) is performed. The advantage of the cryo-SEM technique is that it allows investigating the structure of microgel capsules without altering the native shape and morphology. As a result, the three dimensional structure of the microgel capsules can be imaged as if they are swollen in water. During imaging, the vitrified water can be sublimated, revealing the naked polymer structure (see Fig. 4A and B). The shell thickness after 10 minutes of sublimation varies between 10 and 20 μm for the observed microgel capsules in Fig. 4A, which is consistent with the measured shell thicknesses for microgel capsules in Fig. 2A. The structure of the microgel shell is found to be highly porous. To also visualize the three-dimensional geometry of the microgel capsules, sublimation times are increased to 25 minutes to remove larger parts of the vitrified ice matrix (see Fig. 4C). All three microgels clearly show the empty microcapsule void. The pores in the shell of the microgel capsules appear larger than their real dimensions due to a typical artifact, which occurs during cooling and drying to prepare the sample for SEM imaging. During freezing, ice crystals form in the hydrogel structure, which push away the polymer chains and produce a porous structure. We hypothesize that the polymer strings, visible in the void, result from residual stabilizer or non-crosslinked sPEG-acrylate, as the strings are aligned with the direction of the ice crystals.
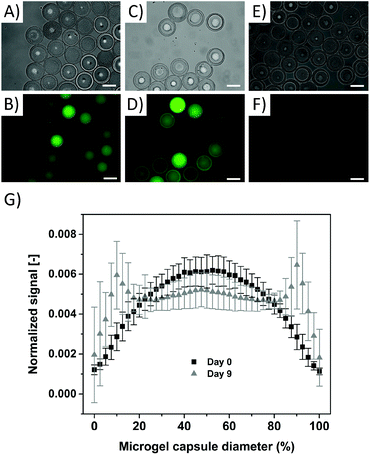
To demonstrate the encapsulation capability of the microgel capsules, a 10 kDa fluorescein functionalized (FITC) dextran (λabs = 492 nm, λem = 518 nm) is chosen to serve as a biological proxy. Fluorescently labelled dextran is available in a broad range of molecular weights and is often used to simulate the permeation and diffusion behaviour of biomolecules.42 10 kDa FITC-dextran remains inside our microgel capsules after all the previously described purification steps (Fig. 5A and B), proving that the mesh size of the microgel capsule, resulting from crosslinking of the 3 kDa sPEG precursor, is small enough to retain the dextran molecules. The differences in fluorescence intensity of the microgel capsules in Fig. 5B and D are due to the fact that not all microgels are in focus during fluorescence microscopy.
Monitoring the sample over nine days shows that dextran molecules are partly retained inside the microgel capsules, with an increase in fluorescence in the shell after 9 days. This is concomitant with a decrease in the fluorescence in the core, indicating that dextran diffuses through the microgel network, most likely supported by the hydrolysis of the ester bonds present in the hydrogel network50 (see Fig. 5C and D). In order to analyze the dextran distribution inside the microgel capsules, the distribution of the fluorescence signal intensity is analyzed across their diameter. Here, the fluorescence signal is related to the integral of all signals per microgel capsule, and normalized over the distance from the center of the microgel capsule. These results reveal a clear difference in dextran distribution, with a bell-shaped curve at day 0 and a maximal intensity in the center of the capsule, and two significant peaks at the location of the microgel shell at day 9 (Fig. 5G). This indicates that the generated microgel capsules can be applied to encapsulate biomolecules with size equal to or larger than the tested 10 kDa FITC-dextran with a hydrodynamic radius of 2.36 nm.54 The versatile fabrication method of the microgel capsules enables loading of differently sized biomolecules and, depending on the polymer composition of the shell, can control the release rate via the thickness and density of the shell and the network degradation rate.
Conclusions
We demonstrate a method for the production of microgel capsules and concomitant loading with biomolecules, actives, or drugs using microfluidics. Biomolecules can be retained by the small network mesh size and are released after hydrolysis of the network. While this study clearly indicates the high potential of microgel capsules for the loading and release of biomolecules, the effect of size dependent diffusion through the microgel network and the contribution of hydrolysis to degrading the molecular network will be further investigated. In the future, such porous microgel capsules with controlled mesh sizes and potential hydrolytic or light-induced degradation will enable precise delivery of actives for drug delivery, agriculture, and cosmetics.Experimental section
Materials
Round capillaries (borosilicate, outer diameter 1.0 mm, inner diameter 0.58 mm, Hilgenberg, Malsfeld, Germany); square capillaries (borosilicate, outer diameter 1.5 mm, inner diameter 1.22 mm, Hilgenberg, Malsfeld, Germany); epoxy resin two-component glue (Wiko, Gluetec, Greußenheim, Germany); a fine bore polyethylene (PE) tube (inner diameter: 0.86 mm, outer diameter: 1.52 mm, Portex, smiths-medical, Minnesota, U.S.A.); trimethoxy(octyl)silane (TMOS) (Sigma-Aldrich, Germany); Microlance 3 (20G × 1′′, 0.9 × 25 mm) needles.Microfluidic experiments
Six-armed star-shaped poly(ethylene oxide-stat-propylene oxide) (sPEG-OH) was synthesized from a sorbitol core with a molecular weight of 3 kDa (CHT R. Beitlich GmbH); sPEG-OH was modified with acrylate groups as described before;55 fluorescein isothiocyanate–dextran (FTIC-dextran, MW = 10 kDa, TCI); phosphate buffered saline, pH 7.2 (PBS, Lonza); 2-hydroxy-2-methylpropiophenone (Irgacure® 1173, BASF); isopropanol (ACS reagent, w ≥ 99.5, Sigma-Aldrich); Tween 80 (Sigma-Aldrich); Span 80 (Sigma-Aldrich), toluene (w = 98%, VWR).Methods
| Phase | Additive (% (w/w)) | Surfactant (% (w/w)) | Viscosity (mPa s) |
|---|---|---|---|
| Water | — | 2, Tween 80 | 0.94 |
| Toluene | — | 5, Span 80 | 0.66 |
| Toluene | 20, 3 kDa sPEG-OH | — | 2.21 |
Acknowledgements
We thank Prof. Dr Martin Möller for providing the acrylate-functionalized six-armed star-shaped poly(ethylene oxide-stat-propylene oxide) (sPEG-A) pre-polymer, and S. Moli, N. Jansen and S. Mallmann for experimental assistance. We acknowledge funding from the European Union's Horizon 2020 research and innovation programme under Marie Skłodowska-Curie grant agreement no. 642687 and the Deutsche Forschungsgemeinschaft (DFG) within the SFB 985 “Functional Microgels and Microgel Systems” (projects C3 and B5). This work was performed in part at the Center for Chemical Polymer Technology (CPT), which was supported by the EU and the federal state of North Rhine-Westphalia (grant EFRE 30 00 883 02).Notes and references
- T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS PubMed.
- Y. Zhang, H. F. Chan and K. W. Leong, Adv. Drug Delivery Rev., 2013, 65, 104–120 CrossRef CAS PubMed.
- D. S. Kohane, J. Y. Tse, Y. Yeo, R. Padera, M. Shubina and R. Langer, J. Biomed. Mater. Res., Part A, 2006, 77A, 351–361 CrossRef CAS PubMed.
- L. De Laporte, A. des Rieux, H. M. Tuinstra, M. L. Zelivyanskaya, N. M. De Clerck, A. A. Postnov, V. Préat and L. D. Shea, J. Biomed. Mater. Res., Part A, 2011, 98A, 372–382 CrossRef CAS PubMed.
- L. Sun, X. Xiong, Q. Zou, P. Ouyang, C. Burkhardt and R. Krastev, J. Appl. Polym. Sci., 2017, 134, 44425 Search PubMed.
- H. Lee, H. Mok, S. Lee, Y.-K. Oh and T. G. Park, J. Controlled Release, 2007, 119, 245–252 CrossRef CAS PubMed.
- X.-Z. Zhang, P. Jo Lewis and C.-C. Chu, Biomaterials, 2005, 26, 3299–3309 CrossRef CAS PubMed.
- P. W. Chen, R. M. Erb and A. R. Studart, Langmuir, 2012, 28, 144–152 CrossRef CAS PubMed.
- J. Elisseeff, Nat. Mater., 2008, 7, 271–273 CrossRef CAS PubMed.
- A. C. Jen, M. C. Wake and A. G. Mikos, Biotechnol. Bioeng., 1996, 50, 357–364 CrossRef CAS PubMed.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345–1360 CrossRef CAS.
- G. Zhou, Y. Lu, H. Zhang, Y. Chen, Y. Yu, J. Gao, D. Sun, G. Zhang, H. Zou and Y. Zhong, Int. J. Nanomed., 2013, 8, 877–887 Search PubMed.
- A. Tamura, M. Oishi and Y. Nagasaki, Biomacromolecules, 2009, 10, 1818–1827 CrossRef CAS PubMed.
- S. V. Vinogradov, E. V. Batrakova and A. V. Kabanov, Bioconjugate Chem., 2004, 15, 50–60 CrossRef CAS PubMed.
- Y. Li, R. de Vries, T. Slaghek, J. Timmermans, M. A. Cohen Stuart and W. Norde, Biomacromolecules, 2009, 10, 1931–1938 CrossRef CAS PubMed.
- J. Chatterjee, Y. Haik and C. Jen Chen, Colloid Polym. Sci., 2003, 281, 892–896 CAS.
- U. Hasegawa, S.-I. M. Nomura, S. C. Kaul, T. Hirano and K. Akiyoshi, Biochem. Biophys. Res. Commun., 2005, 331, 917–921 CrossRef CAS PubMed.
- J. C. Rose, M. Cámara-Torres, K. Rahimi, J. Köhler, M. Möller and L. De Laporte, Nano Lett., 2017 DOI:10.1021/acs.nanolett.7b01123.
- Y. Hu, W. Liu and F. Wu, RSC Adv., 2017, 7, 10333–10344 RSC.
- S. Uthaman, S. Zheng, J. Han, Y. J. Choi, S. Cho, V. D. Nguyen, J.-O. Park, S.-H. Park, J.-J. Min, S. Park and I.-K. Park, Adv. Healthcare Mater., 2016, 5, 288–295 CrossRef CAS PubMed.
- T. Trongsatitkul and B. M. Budhlall, Polym. Chem., 2013, 4, 1502–1516 RSC.
- A. Arun and B. S. R. Reddy, Biomaterials, 2005, 26, 1185–1193 CrossRef CAS PubMed.
- B. J. Kong, A. Kim and S. N. Park, Carbohydr. Polym., 2016, 147, 473–481 CrossRef CAS PubMed.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS.
- H. Kawaguchi, Polym. Int., 2014, 63, 925–932 CrossRef CAS.
- B. R. Saunders, N. Laajam, E. Daly, S. Teow, X. Hu and R. Stepto, Adv. Colloid Interface Sci., 2009, 147–148, 251–262 CrossRef CAS PubMed.
- L. Bromberg, M. Temchenko and T. A. Hatton, Langmuir, 2002, 18, 4944–4952 CrossRef CAS.
- D. Chong, X. Liu, H. Ma, G. Huang, Y. L. Han, X. Cui, J. Yan and F. Xu, Microfluid. Nanofluid., 2015, 19, 1071–1090 CrossRef CAS.
- W. Richtering, I. I. Potemkin, A. A. Rudov, G. Sellge and C. Trautwein, Nanomedicine, 2016, 11, 2879–2883 CrossRef CAS PubMed.
- A. J. Schmid, J. Dubbert, A. A. Rudov, J. S. Pedersen, P. Lindner, M. Karg, I. I. Potemkin and W. Richtering, Sci. Rep., 2016, 6, 22736 CrossRef CAS PubMed.
- T. Femmer, A. Jans, R. Eswein, N. Anwar, M. Moeller, M. Wessling and A. J. C. Kuehne, ACS Appl. Mater. Interfaces, 2015, 7, 12635–12638 CAS.
- S. Seiffert, M. B. Romanowsky and D. A. Weitz, Langmuir, 2010, 26, 14842–14847 CrossRef CAS PubMed.
- S. Seiffert, J. Thiele, A. R. Abate and D. A. Weitz, J. Am. Chem. Soc., 2010, 132, 6606–6609 CrossRef CAS PubMed.
- T. Rossow, J. A. Heyman, A. J. Ehrlicher, A. Langhoff, D. A. Weitz, R. Haag and S. Seiffert, J. Am. Chem. Soc., 2012, 134, 4983–4989 CrossRef CAS PubMed.
- A. Jans, R. R. Rosencrantz, A. D. Mandic, N. Anwar, S. Boesveld, C. Trautwein, M. Moeller, G. Sellge, L. Elling and A. J. C. Kuehne, Biomacromolecules, 2017, 18(5), 1460–1465 CrossRef CAS PubMed.
- J. K. Nunes, S. S. H. Tsai, J. Wan and H. A. Stone, J. Phys. D: Appl. Phys., 2013, 46, 114002 CrossRef PubMed.
- S. Seiffert and D. A. Weitz, Soft Matter, 2010, 6, 3184–3190 RSC.
- A. S. Utada, L. Y. Chu, A. Fernandez-Nieves, D. R. Link, C. Holtze and D. A. Weitz, MRS Bull., 2007, 32, 702–708 CrossRef CAS.
- H. Bysell, R. Månsson, P. Hansson and M. Malmsten, Adv. Drug Delivery Rev., 2011, 63, 1172–1185 CrossRef CAS PubMed.
- C. D. A. L. Chaves, A. L. Machado, I. Z. Carlos, E. T. Giampaolo, A. C. Pavarina and C. E. Vergani, Dent. Mater., 2010, 26, 1017–1023 CrossRef PubMed.
- M. Armaka, E. Papanikolaou, A. Sivropoulou and M. Arsenakis, Antiviral Res., 1999, 43, 79–92 CrossRef CAS PubMed.
- J. Pessi, H. A. Santos, I. Miroshnyk, J. Yliruusi, D. A. Weitz and S. Mirza, Int. J. Pharm., 2014, 472, 82–87 CrossRef CAS PubMed.
- J. K. Tessmar and A. M. Göpferich, Adv. Drug Delivery Rev., 2007, 59, 274–291 CrossRef CAS PubMed.
- M. B. Browning, T. Wilems, M. Hahn and E. Cosgriff-Hernandez, J. Biomed. Mater. Res., Part A, 2011, 98A, 268–273 CrossRef CAS PubMed.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- M. Sheikhpour, L. Barani and A. Kasaeian, J. Controlled Release, 2017, 253, 97–109 CrossRef CAS PubMed.
- I. S. Kikuchi, R. S. Galante, K. Dua, V. R. Malipeddi, R. Awasthi, D. D. Ghisleni and T. de Jesus Andreoli Pinto, Curr. Drug Delivery, 2016, 13, 1–9 CrossRef.
- M. K. Nguyen and E. Alsberg, Prog. Polym. Sci., 2014, 39, 1235–1265 CrossRef CAS PubMed.
- S. P. Zustiak and J. B. Leach, Biomacromolecules, 2010, 11, 1348–1357 CrossRef CAS PubMed.
- M. B. Browning, S. N. Cereceres, P. T. Luong and E. M. Cosgriff-Hernandez, J. Biomed. Mater. Res., Part A, 2014, 102, 4244–4251 CAS.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537 CrossRef CAS PubMed.
- P. W. Chen, J. Brignoli and A. R. Studart, Polymer, 2014, 55, 6837–6843 CrossRef CAS.
- A. S. Hoffman, Adv. Drug Delivery Rev., 2002, 54, 3–12 CrossRef CAS PubMed.
- TdB Consultancy, FITC dextran product sheet, http://www.tdbcons.com/images/pdf/fitcdextran2.pdf (accessed December 2016).
- M. C. Lensen, P. Mela, A. Mourran, J. Groll, J. Heuts, H. Rong and M. Möller, Langmuir, 2007, 23, 7841–7846 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental data of the synthesis and analysis of star-shaped PEG-acrylate and movies of the W/O/W double emulsion formation in the microfluidic device and dried microgel capsules swelling in water. See DOI: 10.1039/c7bm00322f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |