A platform for the development of novel biosensors by configuring allosteric transcription factor recognition with amplified luminescent proximity homogeneous assays†
Shanshan
Li
a,
Li
Zhou
b,
Yongpeng
Yao
a,
Keqiang
Fan
 a,
Zilong
Li
a,
Lixin
Zhang
ab,
Weishan
Wang
*a and
Keqian
Yang
*a
a,
Zilong
Li
a,
Lixin
Zhang
ab,
Weishan
Wang
*a and
Keqian
Yang
*a
aState Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, 100101 Beijing, China. E-mail: yangkq@im.ac.cn; wangws@im.ac.cn
bInstitute of Health Sciences, Anhui University, 230601, Hefei, China
First published on 15th November 2016
Abstract
A wide range of chemicals can be sensed by allosteric transcription factors (aTFs) in bacteria. Herein, we report a biosensing platform by using isolated aTFs as recognition elements in vitro. Moreover, a general strategy to increase the sensitivity of the aTF-based biosensors is provided. As a proof-of-concept, we obtained by far the most sensitive uric acid and oxytetracycline biosensors by using aTF HucR and OtrR as recognition elements, respectively. As a large number of aTFs are present in bacteria, our work opens a novel route to develop sensitive aTF-based biosensors.
The development of novel biosensors for highly sensitive, selective, and rapid detection is of paramount importance for medical diagnostics, food safety screening, and environmental pollution monitoring.1 A biosensor is a compact analytical device or unit incorporating a biological or biologically derived sensitive recognition element integrated or associated with a physio-chemical transducer.2 Therefore, molecular recognition, which determines the specificity of a biosensor, is central to biosensing, and the availability of distinct recognition elements is a prerequisite of the development of novel biosensors.3
Bacteria contain large quantities of allosteric transcription factors (aTFs) that have the capacity to specifically sense different effectors.4 These aTFs usually have an effector sensory domain and a DNA binding domain.5 Effectors can enhance or attenuate the binding of aTFs on their target DNA sequences to regulate the expression of the corresponding gene(s).5 Hence, aTFs have the properties of recognition elements for specific effectors (target chemicals). Based on this knowledge, whole cell biosensors (WCBs) had been designed by coupling aTFs with different reporters (such as fluorescent proteins or luciferases) and exhibited great potential.6 However, poor robustness is an inevitable problem of WCBs, because the physiological state of cells is sensitive to variations of experimental conditions.7 As a new solution, we endeavored to establish a platform for the configuration of stable biosensors using isolated aTFs as recognition elements in vitro, which is similar to biosensors based on classical recognition elements such as antibodies, enzymes and aptamers.1,3b Meanwhile, this platform employed the amplified luminescent proximity homogeneous assay (Alpha) technology as the transducing element, which is a fast, sensitive and easy-to-use signal converter and amplifier.8 The robustness of biosensors developed based on this platform thereby could be ensured by the defined and homogeneous in vitro working conditions without cumbersome washing steps.
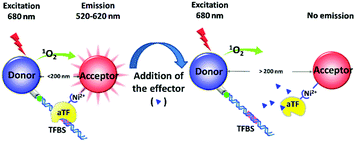
The principle of the platform is illustrated in Scheme 1. The biotinylated DNA sequence containing an aTF binding site (TFBS) and the his6-tagged aTF are anchored in the streptavidin-coated donor beads and nickel chelated acceptor beads, respectively. Upon laser excitation at 680 nm, the photosensitizer inside the donor beads converts ambient oxygen to an excited singlet state (1O2). Within its 4 μs half-life, 1O2 can diffuse approximately 200 nm in solution. The binding of aTF and TFBS shortens the distance between the donor and acceptor beads to less than 200 nm, enabling the energy to transfer from 1O2 to thioxene derivatives within the acceptor beads, ultimately producing extensive luminescence signals at 520–620 nm. While the effector (target chemicals) promotes the dissociation of aTF from TFBS, and thus separates the donor beads from the acceptor beads (>200 nm), causing 1O2 can not be received by the acceptor beads in time and finally resulting in the reduction of the luminescence signal. By this way, the concentration of the target chemicals can be determined by monitoring the change of luminescence signals.
As a proof-of-concept, we chose aTF HucR to configure novel uric acid (UA) biosensors. HucR can bind to the target TFBS hucO, while the HucR/hucO complex can be dissociated by UA.9 UA is a key biomarker for the diagnosis of several diseases, for instance, gout, hyperuricemia, renal disease and leukemia.10 Currently used UA biosensors employ uricase as the recognition element, integrating with various advanced signal amplification technologies,11 such as semiconductor nanoparticles12 and quantum dots.13 However, their limits of detection (LODs) are still relatively high and difficult to reduce below 0.1 μM. This might be primarily ascribed to the limit of the activity of the recognition element uricase (the Michaelis–Menten constant of the best reported one is 0.014 mM).14 From this point of view, aTF HucR might be an alternative recognition element to obtain more sensitive UA biosensors.
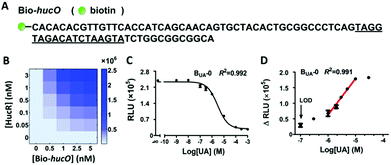
To prepare the isolated HucR protein, hucR gene was cloned from Deinococcus radiodurans and expressed in Escherichia coli. The recombinant HucR protein was purified to greater than 95% homogeneity (Fig. S1, ESI†). The gel shift assay showed that the isolated HucR had a high binding ability to hucO (Fig. S2, ESI†), and an equilibrium dissociation constant (KD) of 1.74 ± 0.03 nM was determined by the bio-layer interferometry (BLI) assay (Fig. S3, ESI†). These results indicate that the prepared HucR could be used to configure UA biosensors along with the purified biotinylated DNA sequence containing hucO (Bio-hucO, Fig. 1A).
To get stable biosensors, factors with potential influences on this biosensing platform were investigated, including buffer, pH, and foreign substances. Here, we obtained an optimized HBS-P buffer, which provided the lowest background signal and the highest ratio of signal to noise (S/N) in comparison to the commonly used buffers (Fig. S4, ESI†).8b,15 In addition, the HBS-P buffer could offer stable working conditions for biosensors with a broad range of pH from 5.0 to 8.0 (Fig. S5, ESI†) and a high concentration of various foreign substances up to 100 mM (Table S2, ESI†).
To accurately convert and amplify the binding of HucR with Bio-hucO to luminescence signals, it is crucial to avoid bead saturation and ensure sufficient S/N simultaneously. Therefore, lower concentrations of HucR and Bio-hucO are preferable, as long as the assay window is acceptable. To determine appropriate concentrations, cross-titration was carried out by using HucR and Bio-hucO with a series of concentrations below the saturation values of donor beads (30 nM) and acceptor beads (0.3–1 nM). It turned out that the relative luminescence unit (RLU) varied in a dose–response manner (Fig. 1B). The lowest concentrations of HucR and Bio-hucO that led to a credible signal (2.2 × 105 RLU; S/N = 24.4) were 0.1 and 1 nM, respectively, which are far below the capacities of the two beads. Thus, 0.1 nM of HucR and 1 nM of Bio-hucO were used to configure the original UA biosensor BUA-0 (Fig. 1C). The performance of BUA-0 was characterized by measuring luminescence signals emitted at a series of UA concentrations. The LOD and linear detection range of BUA-0 were 0.1 μM and 1–10 μM, respectively (Fig. 1D).
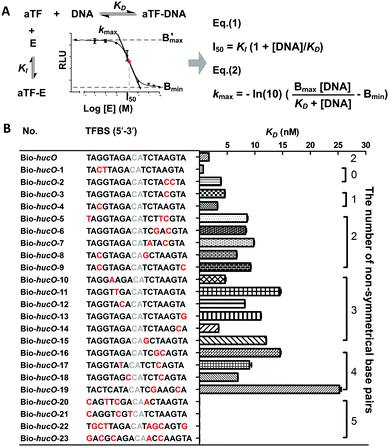
The biosensor community has long focused on achieving the highest possible sensitivity (lowest LOD) and an appropriate linear detection range.16 To achieve these goals, mathematical modeling was adopted to provide systematic theoretical guidance (Mathematic modeling and Fig. S6, ESI†). Here, two major equilibriums were considered in the biosensor (Fig. 2A), which was the premise of the modeling. As shown in Fig. 2A (inset), it is concluded that lowering I50 (concentration of the target chemical producing 50% signal reduction) and increasing kmax (the maximal slope of the dissociation curve) can improve the sensitivity and broaden the linear range of biosensors. From the resulting formulae, eqn (1) and (2) (Fig. 2A), we learnt that reducing the affinity between the aTF and TFBS (increasing KD) or increasing the affinity between the aTF and the target chemical (reducing the equilibrium inhibition constant KI) could lower I50 and increase kmax. An ideal mutant of aTF with both increased KD and reduced KI requires complex protein engineering and laborious screening. In contrast, the mutation of TFBS that just affects KD is much easier, so this strategy was preferred for the improvement of the UA biosensor BUA-0.
A series of point mutation was generated in the sequence of hucO (Fig. 2B). Interactions between HucR and 23 Bio-hucO mutants were all monitored by BLI (Fig. S7, ESI†), which determined their respective kinetic behavior (association and dissociation rate constants kon and koff, Table S3, ESI†). The results show that the number and position of point mutation in hucO could influence both kon and koff, and consequently affect KD (KD = koff/kon, Table S3, ESI†). The value of KD ranged from 0.68 to 25.10 nM, which generally increased with the number of non-symmetrical base pairs (Fig. 2B). It was also observed that the affinity became too weak to be detected when there were more than four non-symmetrical base pairs in the sequence of hucO (Fig. 2B).
The influence of KD on the performance of biosensors was then evaluated. Four Bio-hucO mutants, Bio-hucO-3, Bio-hucO-8, Bio-hucO-11 and Bio-hucO-19, showing gradually increased KD with HucR were selected to configure UA biosensors BUA-3, BUA-8, BUA-11 and BUA-19, respectively. These biosensors exhibited different responses to UA (Fig. S8, ESI†). Accompanying with the increased values of KD, I50 decreased while kmax increased (Table S4, ESI†), and accordingly, the LOD of the biosensor was lowered while the linear detection range was broadened (Fig. 3). These results agree with the conclusions drawn from mathematic modeling. Compared to BUA-0, the four improved biosensors were much more sensitive, and their linear detection ranges were changed. Among them, BUA-11 and BUA-19 could sense as low as 1 nM of UA (Fig. 3C, D and Table S4, ESI†), which were by far the most sensitive UA biosensors (Table S5, ESI†). In addition, BUA-11 exhibited a very wide linear range, spanning more than five orders of magnitude, from 1 to 3 × 104 nM (Fig. 3C). BUA-19 seems to be an exception. Despite it had the largest value of KD, its linear range was narrower than that of BUA-11 (Fig. 3 and Table S4, ESI†). This might be ascribed to the low affinity of HucR for Bio-hucO-19, which hampered the clear distinction of luminescence signals generated under different concentrations of UA. These results suggest that the point mutation of hucO is a feasible and flexible strategy to change the LOD and linear detection range of the UA biosensors. Further, BUA-11 with the best performance was selected to evaluate the specificity of the UA biosensor. In contrast to the effect of UA (1 nM), there was nearly a negligible luminescence response in the presence of different analogues even at a 10-fold concentration (Fig. S9, ESI†), demonstrating the high specificity of the HucR-based UA biosensor.
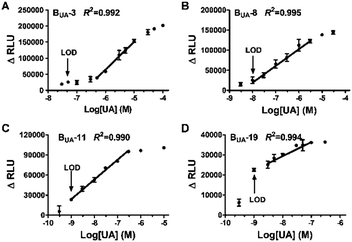 | ||
| Fig. 3 LOD and linear detection range of the improved UA biosensors: (A) BUA-3, (B) BUA-8, (C) BUA-11, and (D) BUA-19. | ||
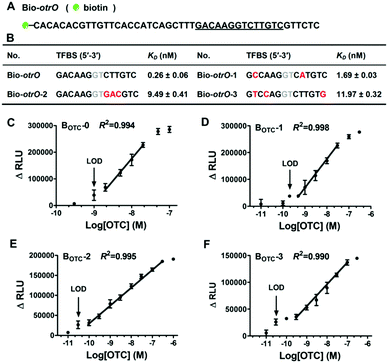
To prove the universality of this platform, another aTF OtrR from Streptomyces rimosus was chosen to develop oxytetracycline (OTC) biosensors. OTC has been commonly used as an additive in animal feed, and it is necessary to detect the residue level in dairy food, livestock, and soil to avoid the risks to human health.17 The aTF OtrR possesses strong affinity to the target sequence otrO, while OTC promotes the dissociation of the OtrR/otrO complex in a concentration-dependent manner.18 To configure OTC biosensors, purified OtrR (Fig. S10, ESI†) and a biotinylated DNA sequence containing otrO (Bio-otrO, Fig. 4A) were used. Then, appropriate concentrations of OtrR (0.03 nM) and Bio-otrO (0.3 nM) were determined via cross-titration (Fig. S11, ESI†). Further, the sequence of otrO was mutated to generate three variants (Bio-otrO-1, Bio-otrO-2, and Bio-otrO-3) with lower affinities to OtrR (Fig. 4B and Fig. S12 and Table S6, ESI†), and after that, four OTC biosensors (BOTC-0 to BOTC-3) were configured by using OtrR with Bio-otrO as well as its three variants simultaneously. As expected, the four biosensors showed different responses to OTC (Fig. S13, ESI†). Similar to UA biosensors, both the sensitivity and the linear detection range of the OTC biosensors were improved significantly with the increase of KD (Fig. 4C–F). Interestingly, the LOD of BOTC-2 and BOTC-3 was as low as 0.03 nM, which were more sensitive in comparison to the ever reported OTC biosensors (Table S7, ESI†). The specificity test of BOTC-2 revealed that it could respond to both OTC and chlortetracycline, but not to the structural analogues tetracycline and anhydrotetracycline (Fig. S14, ESI†). Nevertheless, our results imply that this biosensing platform can be extensively applied to develop other aTF-based biosensors, and the strategy of mutating TFBS is general to efficiently improve these biosensors with higher sensitivities and a desired linear detection range.
To assess the performance of the developed biosensors in practical applications, UA biosensors BUA-0, BUA-3, BUA-8, BUA-11 and BUA-19 were chosen to detect the concentration of UA in urine samples taken from healthy volunteers, as well as in commercial human serum. Meanwhile, HPLC was also adopted for UA detection as the control. The range of accuracy was 93.2–106.1% in urine samples (Tables S8 and S9, ESI†) and 91.33–108.90% in human serum (Table S10, ESI†), suggesting that UA concentrations determined by the five biosensors were in good agreement with the results obtained using HPLC. Moreover, precisions and recoveries of the five UA biosensors obtained from different samples (Tables S9 and S10, ESI†) were all within the acceptable range.19 These results imply the great potential of the HucR-based UA biosensors to be applied in clinical diagnosis.
In summary, a versatile and homogeneous platform was established for the development of novel biosensors by using isolated aTFs as recognition elements for the first time. Moreover, the systematic theoretical guidance and experimental strategy were presented for the efficient configuration of robust, sensitive and applicable aTF-based biosensors. Using this platform, we have obtained the most sensitive UA and OTC biosensors to date. As we know, numerous aTFs have been identified in bacteria, and many effectors of these aTFs are the target analytes in clinical diagnosis, food safety detection, environmental pollution, and so on (some are summarized in Table S11, ESI†). Hence we believe that this platform making full use of the natural tricks of aTFs in vitro presents a new avenue to configure various aTF-based biosensors for the detection of chemicals of interest.
This work was supported by the National Natural Science Foundation of China (No. 31300052, 31400034 and 31570031); the Ministry of Science and Technology of China (No. 2013CB734001), and Youth Innovation Promotion Association (No. 2016087), CAS. We thank Prof. Shuangyan Tang, Institute of Microbiology, CAS, for providing the genomic DNA of D. radiodurans. We also thank Prof. Xian-En Zhang for his valuable suggestions on this work.
Notes and references
- J. Kirsch, C. Siltanen, Q. Zhou, A. Revzin and A. Simonian, Chem. Soc. Rev., 2013, 42, 8733–8768 RSC.
- A. P. Turner, Science, 2000, 290, 1315–1317 CrossRef CAS PubMed.
- (a) I. Bazin, S. A. Tria, A. Hayat and J.-L. Marty, Biosens. Bioelectron., 2017, 87, 285–298 CrossRef CAS PubMed; (b) J. P. Chambers, B. P. Arulanandam, L. L. Matta, A. Weis and J. J. Valdes, Curr. Issues Mol. Biol., 2008, 10, 1–12 CAS.
- D. F. Browning and S. J. Busby, Nat. Rev. Microbiol., 2004, 2, 57–65 CrossRef CAS PubMed.
- V. Libis, B. Delepine and J. L. Faulon, Curr. Opin. Microbiol., 2016, 33, 105–112 CrossRef CAS PubMed.
- (a) M. Park, S. L. Tsai and W. Chen, Sensors, 2013, 13, 5777–5795 CrossRef CAS PubMed; (b) L. T. Bereza-Malcolm, G. Mann and A. E. Franks, ACS Synth. Biol., 2015, 4, 535–546 CrossRef CAS PubMed; (c) R. Fernandez-Lopez, R. Ruiz, F. de la Cruz and G. Moncalian, Front. Microbiol., 2015, 6, 648 CrossRef PubMed; (d) S. K. Checa, M. D. Zurbriggen and F. C. Soncini, Curr. Opin. Biotechnol., 2012, 23, 766–772 CrossRef CAS PubMed.
- (a) E. Michelini, L. Cevenini, M. M. Calabretta, S. Spinozzi, C. Camborata and A. Roda, Anal. Bioanal. Chem., 2013, 405, 6155–6163 CrossRef CAS PubMed; (b) A. Date, P. Pasini and S. Daunert, Adv. Biochem. Eng./Biotechnol., 2010, 117, 57–75 CAS.
- (a) E. F. Ullman, H. Kirakossian, S. Singh, Z. P. Wu, B. R. Irvin, J. S. Pease, A. C. Switchenko, J. D. Irvine, A. Dafforn and C. N. Skold, et al., Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 5426–5430 CrossRef CAS PubMed; (b) V. G. D’Agostino, V. Adami and A. Provenzani, PLoS One, 2013, 8, e72426 Search PubMed; (c) Y. Li, R. T. Cummings, B. R. Cunningham, Y. Chen and G. Zhou, Anal. Biochem., 2003, 321, 151–156 CrossRef CAS PubMed.
- S. P. Wilkinson and A. Grove, J. Biol. Chem., 2004, 279, 51442–51450 CrossRef CAS PubMed.
- K. L. Rock, H. Kataoka and J. J. Lai, Nat. Rev. Rheumatol., 2013, 9, 13–23 CrossRef CAS PubMed.
- P. E. Erden and E. Kilic, Talanta, 2013, 107, 312–323 CrossRef CAS PubMed.
- (a) D. Jin, M. H. Seo, B. T. Huy, Q. T. Pham, M. L. Conte, D. Thangadurai and Y. I. Lee, Biosens. Bioelectron., 2016, 77, 359–365 CrossRef CAS PubMed; (b) K. Jindal, M. Tomar and V. Gupta, Biosens. Bioelectron., 2012, 38, 11–18 CrossRef CAS PubMed.
- N. E. Azmi, N. I. Ramli, J. Abdullah, M. A. Abdul Hamid, H. Sidek, S. Abd Rahman, N. Ariffin and N. A. Yusof, Biosens. Bioelectron., 2015, 67, 129–133 CrossRef CAS PubMed.
- T. Ghosh, P. Sarkar and A. P. Turner, Bioelectrochemistry, 2015, 102, 1–9 CrossRef CAS PubMed.
- Y. Zhang, G. Pan, Z. Zou, K. Fan, K. Yang and H. Tan, Mol. Microbiol., 2013, 90, 884–897 CrossRef CAS PubMed.
- F. Ricci, A. Vallée-Bélisle, A. J. Simon, A. Porchetta and K. W. Plaxco, Acc. Chem. Res., 2016, 49, 1884–1892 CrossRef CAS PubMed.
- (a) D. Harcharik, Evaluation of certain veterinary drug residues in food: fiftieth report of the Joint FAO/WHO Expert Committee on Food Additives, Rome, Italy, February, 1998; (b) N. A. Mungroo and S. Neethirajan, Biosensors, 2014, 4, 472–493 CrossRef PubMed.
- W. Wang, T. Yang, Y. Li, S. Li, S. Yin, K. Styles, C. Corre and K. Yang, ACS Synth. Biol., 2016, 5, 765–773 CrossRef PubMed.
- D. Zhao, G. Yu, K. Tian and C. Xu, Biosens. Bioelectron., 2016, 82, 119–126 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc07244e |
| This journal is © The Royal Society of Chemistry 2017 |