ORMOCHALCs: organically modified chalcogenide polymers for infrared optics†
D. A.
Boyd
*a,
C. C.
Baker
a,
J. D.
Myers
a,
V. Q.
Nguyen
a,
G. A.
Drake
b,
C. C.
McClain
 b,
F. H.
Kung
b,
S. R.
Bowman
a,
W.
Kim
a and
J. S.
Sanghera
a
b,
F. H.
Kung
b,
S. R.
Bowman
a,
W.
Kim
a and
J. S.
Sanghera
a
aOptical Sciences Division, Naval Research Laboratory, 4555 Overlook Ave SW, Washington DC 20375, USA. E-mail: darryl.boyd@nrl.navy.mil
bUniversity Research Foundation, 6411 Ivy Ln Ste 110, Greenbelt, MD 20770, USA
First published on 2nd December 2016
Abstract
A novel method combining elemental sulfur and selenium was developed, yielding crystalline sulfur–selenium compounds. The compounds were melted, and an organic comonomer added. Once the organic comonomer was consumed, the viscous compound was vitrified and allowed to cool yielding organic–inorganic hybrid polymers that are termed Organically Modified Chalcogenide (ORMOCHALC) polymers.
Materials with infrared (IR) optical capabilities are of great value in industrial, military and domestic applications such as heat sensing and night vision optics. IR optical materials are often composed of inorganic semiconductors (e.g. germanium and silicon) or glasses made of chalcogenides (i.e. sulfur, selenium, tellurium).1 Such materials exhibit low optical losses in the 1–10 μm light spectrum range. Yet, their broader use and implementation is limited because of their weight, difficult processing, and cost to manufacture.
Polymer-based optics overcome many of these disadvantages: they are typically inexpensive, lightweight, and can be readily fabricated with net-shape techniques, eliminating the costly need for grinding and polishing. Within the visible spectrum, polymers have been readily accepted as alternatives to glass-based optics, particularly in low-cost applications. Polymers that are commonly used in these applications are primarily composed of carbon and hydrogen-rich chains. As a result, they tend to suffer from fundamental absorptions in the mid-wave IR (MWIR) optical window (3–8 μm).2 One approach to broaden the transmission window of conventional polymers is through the addition of sulfur. Sulfur is an abundant element with several unique and beneficial properties, is known to provide transmission into the MWIR, and can be included in materials to allow for transmission in this region.3,4 However, because most sulfur-containing polymers are nevertheless rich in carbon and hydrogen, development of MWIR transmissive polymers without substantial fundamental carbon-related absorption peaks remains a major challenge.
A recent report by Pyun and co-workers detailed the synthesis of polymeric materials that used sulfur as the backbone instead of carbon.5 The method, termed inverse vulcanization, was used to fabricate polymers by the interaction between radical sulfur chains and a divinyllic aromatic monomer. Since that initial report, several other reports from researchers around the world have indicated inverse vulcanization as a potentially advantageous process in the development of novel materials.6–10 Sulfur, which exists primarily as S8 ring structures at room temperature, undergoes crystalline phase transitions between ∼96–120 °C, (a range that includes its melting temperature (TM)), and experiences ring-opening at temperatures greater than 159 °C.11 The ring-opening process reveals radicals at either end of each sulfur chain. As temperature increases from 120 °C, molten sulfur changes from yellow to orange to red in color while becoming increasingly more viscous.11 Heating to 200 °C causes the molten sulfur to polymerize into a viscous, solid-like red material. Upon cooling to room temperature, the metastable polymeric sulfur returns to its yellow crystalline (primarily) S8 ring structured state.11 Pyun and co-workers demonstrated that when in the high-temperature molten state, the radicals on the sulfur chains can be activated and a stable, translucent red thermoplastic polymer can be formed if the sulfur radicals and a comonomer are allowed to react at 200 °C for ∼20 min.5 Unlike the traditional vulcanization process, in which sulfur is utilized as a crosslinker between large carbon chains, in the inverse vulcanization process a carbon-rich aromatic comonomer serves as a branching agent to connect sulfur radicals.5
It is possible to further enhance the optical properties of polymers via the incorporation of other chalcogens, such as selenium. As members of Group 16 on the Periodic table, sulfur and selenium share similar characteristics.12,13 Because sulfur and selenium share characteristic similarities, they can be combined in significant proportions to form a crystalline material.12–14 The intrinsic character of sulfur and selenium allows superior optical characteristics to be accessed without the need to introduce components such as nanoparticles to bolster optical properties.15 With a nod in nomenclature to Organically Modified Silicon (ORMOSIL) sol–gel polymers, herein we present the first report of ORganically MOdified CHALCogenide (ORMOCHALC) polymers containing both sulfur and selenium, fabricated via inverse vulcanization. The specific advantage of incorporating selenium into predominately sulfur polymers is the impact that selenium incorporation will have on the ORMOCHALC optical properties, as a function of the atomic polarizabilities of sulfur (2.9 Å) and selenium (3.8 Å). The ORMOCHALC polymers were each composed of a S–Se precursor backbone, which was formed using a process for the combining of sulfur and selenium into a crystalline product. Upon heating, the molten precursor backbone then served as a comonomer, along with a divinyl aromatic compound, to form the ORMOCHALC polymers.
Elemental sulfur and elemental selenium were obtained, and each was further purified using a multi-step ‘bake out’ purification process to remove any remaining impurities (Fig. S1, ESI†). It has been shown that due to their elemental similarities, sulfur and selenium can be combined together in S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratios from 99
Se ratios from 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to ratios approaching 60
1 to ratios approaching 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, respectively.14 The S–Se compounds were made in various S
40, respectively.14 The S–Se compounds were made in various S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Se ratios (ESI†). The results were materials composed of atomically mixed sulfur and selenium. Energy dispersive X-ray spectroscopy (EDAX) data verified that the precursor ratios of sulfur and selenium agreed with the ratios seen in the final ORMOCHALC polymers (Fig. S4, ESI†). Differential scanning calorimetry (DSC) was used to experimentally determine the temperatures associated with the phase transitions of each S–Se compound, with this process depicted for S90Se10 in Fig. 1. A comparison of the 2nd heating ramp for multiple precursors is given in Fig. S5, ESI.†
Se ratios (ESI†). The results were materials composed of atomically mixed sulfur and selenium. Energy dispersive X-ray spectroscopy (EDAX) data verified that the precursor ratios of sulfur and selenium agreed with the ratios seen in the final ORMOCHALC polymers (Fig. S4, ESI†). Differential scanning calorimetry (DSC) was used to experimentally determine the temperatures associated with the phase transitions of each S–Se compound, with this process depicted for S90Se10 in Fig. 1. A comparison of the 2nd heating ramp for multiple precursors is given in Fig. S5, ESI.†
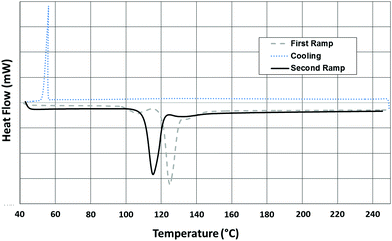 | ||
| Fig. 1 Differential scanning calorimetry data for crystalline S90Se10 ORMOCHALC precursor showing the 1st heating, cooling and 2nd heating ramps. | ||
Table 1 shows data obtained during the second heating for each precursor. The experimentally determined phase transitions for sulfur were consistent with the known sulfur transitions.14 Specifically, the peaks at ∼105 °C and ∼170 °C represent sulfur's melting (Tm) and ring opening transitions, respectively.14,16 In contrast to sulfur, each of the S–Se precursors were devoid of a definitive ring opening transition, exhibiting only the Tm between 110–115 °C, with much cooler and shallower peaks appearing at ∼145 °C for S95Se5 and ∼135 °C S90Se10 during the 1st heating ramp. This suggests that the atomically mixed S–Se precursors may not exist as ring structures despite the fact that the individual elemental components, sulfur and selenium, do exist as ring structures. However, like sulfur, the S–Se precursors form red, viscous material at ∼200 °C, and they return to a yellowish crystalline state at room temperature in the absence of a coupling comonomer.
| Precursor | 1st transition | 2nd transition |
|---|---|---|
| Sulfur | 105.3 | 169.8 |
| S95Se5 | 111.5 | 144.0 |
| S90Se10 | 115.1 | 131.2 |
Although the S–Se precursors were primarily composed of sulfur, it was clear to see that the addition of minimal selenium amounts had a notable affect, with the Tm of the S–Se compounds showing a steady increase in temperature. Specifically, the 2nd DSC heating ramp indicated that the Tm increased from 105.3 °C for sulfur to 111.5 °C for S95Se5, and even further to a value of 115.1 °C for S90Se10 (Table 1).
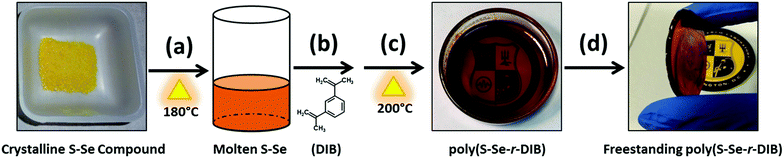
Once the S–Se crystalline compound was made, polymers were fabricated using a procedure similar to the one established by Pyun and co-workers (Scheme 1).5 Essentially, the S–Se crystalline compounds were melted (Scheme 1a), followed by the addition of 1,3-diisopropenyl benzene (DIB), which served as the comonomer (Scheme 1b). Once the DIB was added and the solution stirred for a few minutes, the viscous polymer was poured into a mold, vitrified at 200 °C and later retrieved upon cooling (Scheme 1c and d). ORMOCHALC fibers could also be drawn from the process (Fig. S3, ESI†). Although red in color to the naked eye, the red ORMOCHALC polymers were quite transparent and solid (Scheme 1c and d). The ORMOCHALC polymers were designated poly(SxSey-r-DIB), where S represents sulfur, Se represents selenium, r indicates the random nature of the comonomer branching in the polymer, and DIB is the comonomer. The subscripts x and y represent the atomic percentage of sulfur and selenium in the precursor compound, respectively. At the selenium percentages studied, the inverse vulcanization reaction appears to proceed the same as previously described.5 However, further studies are necessary to verify this preliminary observation.
DSC was also used to determine whether the crystalline character of the precursors was present or not upon the formation of poly(S–Se-r-DIB) following the introduction of DIB (Fig. 2). Indeed, there were no phase transitions observed in the second heating curve of the DSC for any of the ORMOCHALCS (Fig. S6, ESI†), indicating that the DIB was consumed within the molten precursor material, and uniform polymers were formed. Thus, the molten precursors were stabilized by the comonomer interactions, and did not revert to their yellowish crystalline state upon cool down to room temperature.
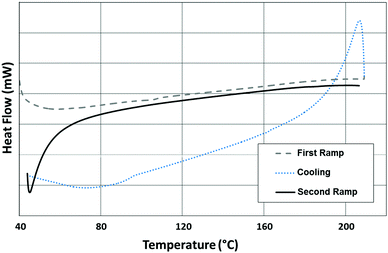 | ||
| Fig. 2 Differential scanning calorimetry data for poly(S90Se10-r-DIB) ORMOCHALC polymer showing the 1st heating, cooling and 2nd heating ramps. | ||
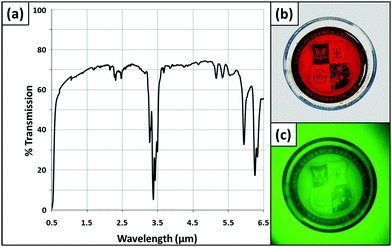
Analysis of these polymers indicates a wide area of high transmission spanning a portion of the visible, the near IR, short-wave IR (SWIR) and much of the MWIR regions of the spectrum from ∼0.7–6 μm (Fig. 3a). The transmission values were ∼70% within this range for S–Se polymers with a thickness of ∼150 μm. The lone portion of this region where transmission was low corresponds to absorption bands associated with the stretching vibrations of the DIB comonomer (i.e. C–H), and only spans a small range (between 3.2 and 3.5 μm). The high transmission from 3.5–6 μm represents an advancement in the MWIR transmission characteristics for polymers. It is also worth noting that although the desirable high IR transmission is not as broad and prevalent beyond 6 μm, there are some areas of the spectrum exhibiting between 40–60% transmission in the long-wave IR region including ∼8–9 μm and ∼10–11.5 μm, regions that are very important for various IR sensing and detection applications (Fig. S7, ESI†).
Thin films (<50 μm) of the polymers were yellow-orange in appearance and highly transparent. As film thickness increased to values greater than 100 μm, the polymers appeared more red in color (Fig. 3b). Bulk S–Se ORMOCHALC polymers were deep red in color, becoming increasingly darker in appearance as thickness increased, ultimately appearing black and opaque to the naked eye. However, even the polymers that appeared black to the naked eye appeared totally transparent when visualized using an IR imager. Fig. 3b depicts a backlit 5 mm thick bulk polymer that is red in appearance, and that is easily distinguishable from the clear glass Petri dish it is contained in. When visualized through a SWIR imager, the same polymer is indistinguishable from the glass Petri dish, appearing equally as transparent as the outer walls of the Petri dish (Fig. 3c). Although it is true that polymers such as poly(methyl methacrylate) (PMMA) and polycarbonate (PC) can transmit into the SWIR region of the spectrum, those polymers tend to be clear-transparent to the naked eye. Thus the fact that poly(S–Se-r-DIB) polymers are red-transparent to the naked eye but appear clear-transparent in the SWIR is notable. To further verify IR transmission capabilities, images of a human subject were captured through a poly(S95Se5-r-DIB) ORMOCHALC using a 3–5 μm MWIR camera lens (Fig. S8, ESI†). Finally, the FT-IR data clearly distinguishes the poly(S–Se-r-DIB) polymers from more common polymers like PMMA and PC, as those polymers do not substantially transmit further than 2 μm while the poly(S–Se-r-DIB) polymers displayed high transmittance well beyond 2 μm. The IR transmission and transparency of these polymers well into the MWIR region of the light spectrum highlight the potential for their use in IR optical applications.
With respect to stability, some of the ORMOCHALC polymers completely, or in part, changed in color from transparent red to opaque orange with time. There was also a range in hardness qualitatively observed for ORMOCHALCs made with the same quantities of comonomers. This suggests that in those instances there were regions of the polymers where not enough of the DIB comonomer was bound to the S–Se comonomer to prevent the S–Se from recrystallizing or softening. Thus efforts to both improve the stability of the ORMOCHALC polymers, as well as to further interrogate and better understand their properties, are ongoing. Nevertheless, many of the ORMOCHALC polymers have remained solid, transparent, and devoid of any apparent recrystallization for more than a year.
In summary, a novel method for the fabrication of sulfur and selenium into a crystalline compound was presented. Additionally, the synthesis and processing of novel ORMOCHALC polymers, which were composed of both sulfur and selenium in various proportions, was presented. The ORMOCHALC polymers exhibited significant transmission capabilities from ∼1–6 μm. Overall, the methods presented could be used to further develop other chalcogenide-based polymers to have enhanced optical properties, and the polymers presented could find use in various applications where IR transmission is necessary. Ultimately ORMOCHALC polymers represent a promising advancement and an enabling technology toward the development of lightweight, low cost IR optical systems.
References
- J. S. Sanghera, L. B. Shaw and I. D. Aggarwal, C. R. Chim., 2002, 5, 873–883 CrossRef CAS.
- C. Lu and B. Yang, J. Mater. Chem., 2009, 19, 2884–2901 RSC.
- J. J. Griebel, S. Namnabat, E. T. Kim, R. Himmelhuber, D. H. Moronta, W. J. Chung, A. G. Simmonds, K. J. Kim, J. van der Laan, N. A. Nguyen, E. L. Dereniak, M. E. Mackay, K. Char, R. S. Glass, R. A. Norwood and J. Pyun, Adv. Mater., 2014, 26, 3014–3018 CrossRef CAS PubMed.
- J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 CrossRef CAS PubMed.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- S. Zhuo, Y. Huang, C. Liu, H. Wang and B. Zhang, Chem. Commun., 2014, 50, 11208–11210 RSC.
- J. C. Bear, W. J. Peveler, P. D. McNaughter, I. P. Parkin, P. O'Brien and C. W. Dunnill, Chem. Commun., 2015, 51, 10467–10470 RSC.
- P. Liu, J. M. Gardner and L. Kloo, Chem. Commun., 2015, 51, 14660–14662 RSC.
- M. K. Salman, B. Karabay, L. C. Karabay and A. Cihaner, J. Appl. Polym. Sci., 2016, 133 DOI:10.1002/app.43655.
- M. Arslan, B. Kiskan and Y. Yagci, Macromolecules, 2016, 49, 767–773 CrossRef CAS.
- D. A. Boyd, Angew. Chem., Int. Ed., 2016, 55, 15486–15502 CAS.
- A. Eisenberg and A. V. Tobolsky, J. Polym. Sci., 1960, 46, 19–28 CrossRef CAS.
- A. V. Tobolsky and A. Eisenberg, J. Am. Chem. Soc., 1959, 81, 780–782 CrossRef CAS.
- V. Berbenni, A. Marini, V. Massarotti and D. Capsoni, Thermochim. Acta, 1994, 237, 253–260 CrossRef CAS.
- E. K. Macdonald and M. P. Shaver, Polym. Int., 2015, 64, 6–14 CrossRef CAS.
- B. Meyer, Chem. Rev., 1976, 76, 367–388 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and the relevant data are included. See DOI: 10.1039/c6cc08307b |
| This journal is © The Royal Society of Chemistry 2017 |