Near-infrared photoswitching of cyclodextrin–guest complexes using lanthanide-doped LiYF4 upconversion nanoparticles†
Nadja
Möller
a,
Tim
Hellwig
b,
Lucas
Stricker
a,
Sabrina
Engel
a,
Carsten
Fallnich
b and
Bart Jan
Ravoo
*a
aOrganic Chemistry Institute, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster, Germany. E-mail: b.j.ravoo@uni-muenster.de
bInstitute of Applied Physics, Westfälische Wilhelms-Universität Münster, Corrensstrasse 2, 48149 Münster, Germany. E-mail: fallnich@uni-muenster.de
First published on 29th November 2016
Abstract
This communication reports a new type of supramolecular cyclodextrin–guest complexes using cyclodextrin coated upconversion nanoparticles as hosts and monovalent and divalent azobenzenes and arylazopyrazoles as guests. A potentially biocompatible photocontrol of the interaction by isomerization of the azobenzene or arylazopyrazole was achieved by laser irradiation at 980 nm and a very low light intensity of 0.22 W cm−2.
The use of light responsive molecules in supramolecular chemistry is of particular interest, as their integration enables the photochemical, reversible or irreversible control over self-assembly processes. One leading representative is the class of azobenzenes, which show a reversible, photo-induced E/Z-isomerization.1 By light irradiation at 350 nm azobenzene can be isomerized from its thermodynamically stable trans- into its metastable cis-state. The reverse isomerization into its trans-state can be either obtained by irradiation at 440 nm or by thermal treatment. The photoresponse of azobenzene can additionally be exploited in host–guest interactions. Azobenzenes form inclusion complexes with α- and β-cyclodextrins (CDs). Whereas the trans-state of the azobenzenes is included in the cavity of CD, the interaction is inhibited in the cis-state due to the change in polarity and the sterical hindrance. This characteristic provides a photoswitchable control over host–guest-complexations and a broad range of applications for example light responsive hydrogels,2 molecular shuttles,3 micelles and vesicles,4 surfaces5 and drug delivery vehicles.6
However, in most cases the use of UV light is inevitable for the photoswitch, which becomes problematic in biological applications since UV light causes damage to those tissues. Therefore the use of low energy irradiation – such as near-infrared light (NIR) – would be desirable. Irradiation with NIR light can be – compared to UV – biocompatible and has the additional advantage of a deeper penetration depth into biological specimens.7 In principle, NIR excitation can be achieved by using upconversion nanoparticles (UCNPs). The advantage of using UCNPs is their property to show the anti-Stokes effect, i.e. UCNPs absorb several low energy photons and in return emit a high energy photon.8 Depending on the nanocrystal lattice and doping of the UCNPs even the emission of UV light can be achieved. Of special interest are lanthanide doped UCNPs, which exhibit, in contrast to conventional two-photon absorbing dyes or fluorophores, several long-lived excited states, enabling the irradiation at low light intensities, which significantly increases the biocompatibility of this approach.9 NIR laser intensities higher than several W cm−2 are categorized dangerous. The maximum permissible exposure of skin for a continuous irradiation at 980 nm is 0.726 W cm−2.10 Wu et al. recently showed a strategy to reduce the NIR light intensity in photochemical processes to an ultralow-intensity of 0.35 W cm−2.11
Various examples of photochemical processes in supramolecular systems involving UCNPs can be found in the literature ranging from photoisomerization of dithienylethenes,12 spiropyranes13 and azobenzenes14 to photolysis15 and photopolymerization.16 Furthermore CDs were occasionally applied as ligands for UCNPs to render them water-soluble or combine UCNPs with supramolecular chemistry.17
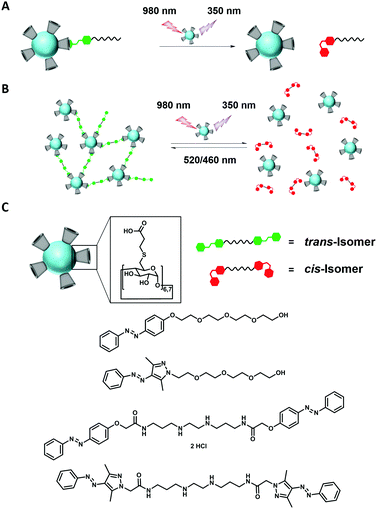
Herein, we describe NIR switchable supramolecular materials using LiYF4:Yb3+,Tm3+,Gd3+-UCNPs coated with α- and β-cyclodextrin acid (CDA@UCNPs) as host and azobenzene as guest (Scheme 1). We have previously shown that β-cyclodextrin persubstituted with carboxylic acid (per-6-deoxy(carboxylpropyl)thio-β-cyclodextrin, β-CDA) is an excellent ligand for magnetite nanoparticles.18 We now show that CDA@UCNPs can isomerize azobenzene by NIR irradiation at 980 nm and a very low light intensity of 0.22 W cm−2. Due to the host–guest interaction with the CD on the particle a close proximity between UCNPs and light-responsive guests was expected, leading to higher efficiencies for photoswitching. Furthermore we developed a reversible aggregation of the UCNPs by supramolecular cross-linking with a divalent azobenzene and redispersion of the resulting aggregates by NIR irradiation (Scheme 1). Finally, we show that also arylazopyrazole (AAP), which shows a strong binding to β-CD19 can be isomerized by NIR excitation of UCNPs.
Water-soluble UCNPs were synthesized in a modified ligand exchange reaction by adding per-6-deoxy(carboxylpropyl)thio-α- or β-cyclodextrin (α- or β-CDA) to a dispersion of methyl oleate (MO) coated LiYF4:Yb3+,Tm3+,Gd3+-UCNPs in ultra-pure water (MO@UCNPs) and sonication for 20 min.20 The CDA ligand, containing six or seven carboxylic acid functionalities, binds to the UCNPs in multivalent mode and easily replaces the weaker, monovalent MO ligand.
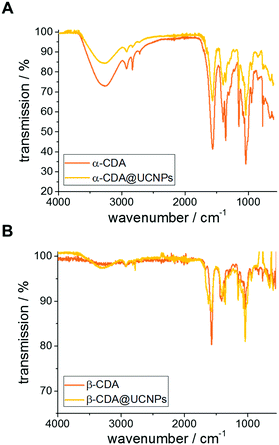
In order to verify that the ligands are on the surface of the UCNPs, Fourier transform infrared (FTIR) measurements were performed, comparing the spectrum of the free ligands α- and β-CDA with the spectrum of CDA@UCNPs (Fig. 1). In both cases, the free ligand spectra are in good agreement to the CDA@UCNPs spectra. Additionally the absence of the characteristic peaks of MO indicate that the ligand exchange was quantitative.
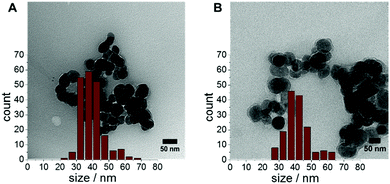
The water-soluble UCNPs were further characterized by transmission electron microscopy (TEM, Fig. 2). It can be seen from the TEM images that all particles have a diameter around 40 nm and did not significantly change in size or shape compared to the MO@UCNPs. For the α-CDA@UCNPs an average size of 39.6 ± 7.0 nm was obtained while the β-CDA@UCNPs have a size of 41.0 ± 7.5 nm. The TEM images further show some aggregation of the particles, which is most likely a consequence of the drying during sample preparation.
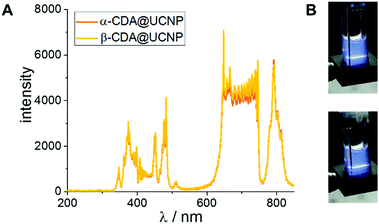
In addition the emission measurements of CDA@UCNPs upon irradiation with a NIR laser at 980 nm show identical spectra for both types of particles (Fig. 3A). The images of the corresponding samples are shown in Fig. 3B. We note that the emission around 350 nm should be suitable to photoisomerize azobenzene as well as AAP.
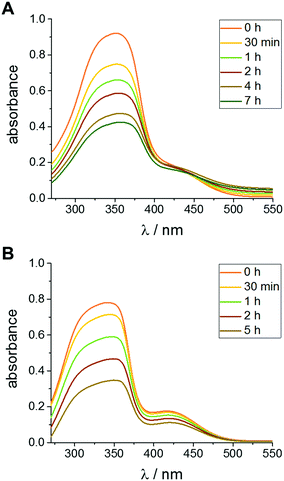
With the CDA functionalized UCNPs in hand we investigated if they are capable to isomerize azobenzene in an upconversion process by irradiation at 980 nm (see Scheme 1A). Indeed, a successful isomerization could be obtained for both α- and β-CDA@UCNPs. For α-CDA@UCNPs we prepared a water soluble, tetraethyleneglycol functionalized azobenzene derivative (Azo-TEG). For β-CDA@UCNPs we prepared tetraethyleneglycol functionalized arylazopyrazole (AAP-TEG). For the switching experiment a dispersion of CDA@UCNPs in ultra-pure water (2 mg mL−1) was mixed with a solution of either Azo-TEG or AAP-TEG and irradiated with a defocused laser beam at 980 nm and a light intensity of 0.22 W cm−2, which is under the maximum permissible exposure of skin, for a defined period. The reduced intensity is achieved by defocusing the laser beam. Control experiments showed that the UCNPs are still able to emit UV light at these intensities (see Fig. S12–S15, ESI†). The progress of the isomerization was monitored by consecutive UV/Vis measurements to determine the decreasing absorption maxima of the trans-isomers of azobenzene and AAP around 350 nm (Fig. 4). Although the upconversion-mediated NIR isomerization is slow (i.e. several hours) compared to a UV isomerization at 350 nm (i.e. several min), the switching is remarkably efficient considering the very low quantum yield of upconversion.8 These experiments confirm our hypothesis that host–guest interaction will bring the UCNPs and the azobenzene or AAP in close proximity and hence increase the efficiency of photoisomerization. The photostationary state of both systems after irradiation was determined by NMR to be 53% trans-isomer and 47% cis-isomer, which is less than for UV light induced photoisomerization (see Fig. S16–S20, ESI†).
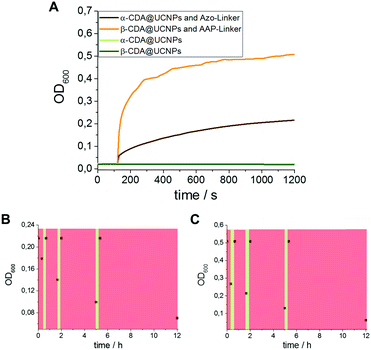
Furthermore, photoreversible aggregation experiments were performed using divalent cross-linkers (Azo-linker or AAP-linker, see Scheme 1B). The aggregation of the UCNPs upon addition of the trans-Azo-linker was monitored by optical density measurements at 600 nm (OD600). In all aggregation experiments the CDA@UCNPs concentration was constant at 0.085 μg mL−1.
During the initial equilibration time a constant OD600 value around 0.02 was measured derived from the scattering of the UCNPs dispersion. However, upon addition of the trans-Azo-linker a significant increase of the OD600 up to approximately 0.22 in 20 min was observed, indicating the formation of aggregates of UCNPs (Fig. 5A). Upon addition of the AAP-linker an even higher increase up to 0.51 in 20 min could be achieved (Fig. 5A). If no linker was added, OD600 remained constant for 20 min. In a control experiment using only the Azo-linker or AAP-linker without the α- or β-CDA@UCNPs no agglomeration was observed (see Fig. S10, ESI†). These observations indicate that aggregation of UCNPs is due to the host–guest-interaction of the CD-functionalized nanoparticles with the divalent cross-linker. The aggregation of UCNPs was verified by TEM measurements (see Fig. S5 and S6, ESI†) and showed large aggregates between 0.4 and 1.2 μm in diameter.
Finally, a successful, reversible aggregation and disaggregation cycle was obtained by alternating NIR irradiation at 980 nm (trans- to cis-isomer) and VIS irradiation at 450 nm (cis- to trans-isomer) (see Fig. 5B and C). However, it was found that the disaggregation of cross-linked UCNPs is slower compared to the switching experiments using the monovalent guest. Nevertheless, complete redispersion of the aggregates was achieved after 7 h, which enhances their potential application for host–guest interactions under biologically relevant conditions.
In this study we developed a versatile protocol for an efficient and broadly applicable combination of cyclodextrin coated upconversion nanoparticles and photoresponsive host–guest interactions. A near-infrared light stimulus was used to induce the photoisomerization of azobenzene and arylazopyrazole to control the formation of host–guest complex. Both the surface functionalization and aggregation of upconversion nanoparticles can be controlled by near-infrared irradiation. This attractive combination could become especially useful for the near-infrared induced local release of payload from cyclodextrin complexes under physiological conditions.4,6
We thank Christian Schwickert (MEET) for XRD measurements and Wilke de Vries and Andreas Rühling for helpful discussions. Martin Peterlechner is acknowledged for advice on TEM measurements.
Notes and references
- H. M. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809 RSC; S. Yagai, T. Karatsu and A. Kitamura, Chem. – Eur. J., 2005, 11, 4054 CrossRef CAS PubMed.
- D. Wang, M. Wagner, H.-J. Butt and S. Wu, Soft Matter, 2015, 11, 7656 RSC; I. Tomatsu, A. Hashidzume and A. Harada, Macromolecules, 2005, 38, 5223 CrossRef CAS; I. Tomatsu, A. Hashidzume and A. Harada, J. Am. Chem. Soc., 2006, 128, 2226 CrossRef PubMed; Y. L. Zhao and J. F. Stoddart, Langmuir, 2009, 25, 8442 CrossRef PubMed; X. Liao, G. Chen, X. Liu, W. Chen, F. Chen and M. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4409 CrossRef PubMed; Y. Takashima, S. Hatanaka, M. Otsubo, M. Nakahata, T. Kakuta, A. Hashidzume, H. Yamaguchi and A. Harada, Nat. Commun., 2012, 3, 1270 CrossRef PubMed; H. Yamaguchi, Y. Kobayashi, R. Kobayashi, Y. Takashima, A. Hashidzume and A. Harada, Nat. Commun., 2012, 3, 603 CrossRef PubMed.
- A. Ueno, H. Yoshimura, R. Saka and T. Osa, J. Am. Chem. Soc., 1979, 101, 2779 CrossRef CAS; H. Murakami, A. Kawabuchi, K. Kotoo, M. Kunitake and N. Nakashima, J. Am. Chem. Soc., 1997, 119, 7605 CrossRef; G. Wenz, B. H. Han and A. Müller, Chem. Rev., 2006, 106, 782 CrossRef PubMed.
- S. K. M. Nalluri and B. J. Ravoo, Angew. Chem., Int. Ed., 2010, 49, 5371 CrossRef CAS PubMed; S. K. M. Nalluri, J. B. Bultema, E. J. Boekema and B. J. Ravoo, Chem. – Eur. J., 2010, 17, 10297 CrossRef PubMed; J. H. Schenkel, A. Samanta and B. J. Ravoo, Adv. Mater., 2014, 26, 1076 CrossRef PubMed; S. K. M. Nalluri, J. Voskuhl, J. L. Bultema, E. J. Boekema and B. J. Ravoo, Angew. Chem., Int. Ed., 2011, 50, 9747 CrossRef PubMed.
- D. P. Ferris, Y. L. Zhao, N. M. Khashab, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 1686 CrossRef CAS PubMed; R. Klajn, Pure Appl. Chem., 2010, 82, 2247 CrossRef; J. Voskuhl, S. Sankaran and P. Jonkheijm, Chem. Commun., 2014, 50, 15144 RSC; P. B. Wan, Y. G. Jiang, Y. P. Wang, Z. Wang and X. Zhang, Chem. Commun., 2008, 5710 RSC.
- A. Samanta, M. C. A. Stuart and B. J. Ravoo, J. Am. Chem. Soc., 2012, 134, 19909 CrossRef CAS PubMed.
- P. Juzenas, A. Juzeniene, O. Kaalhus, V. Iani and J. Moan, Photochem. Photobiol. Sci., 2002, 1, 745 Search PubMed; D. E. Hudson, D. O. Hudson, J. M. Wininger and B. D. Richardson, Photomed. Laser Surg., 2013, 31, 163 CrossRef CAS PubMed; S. Wu and H.-J. Butt, Adv. Mater., 2016, 28, 1208 CrossRef PubMed.
- F. Azuel, Chem. Rev., 2004, 104, 139 CrossRef CAS PubMed; J. A. Capobianco, F. Vetrone, T. D'Alesio, G. Tessari, A. Speghini and M. Bettinelli, Phys. Chem. Chem. Phys., 2000, 2, 3203 RSC.
- R. Scheps, Prog. Quantum Electron., 1996, 20, 271 CrossRef CAS.
- American National Standard for safe use of lasers, Laser Institute of America, Orlando, FL, 2000.
- S. He, K. Krippes, S. Ritz, Z. Chen, A. Best, H.-J. Butt, V. Mailänder and S. Wu, Chem. Commun., 2015, 51, 431 RSC; Z. Chen, W. Sun, H.-J. Butt and S. Wu, Chem. – Eur. J., 2015, 21, 9165 CrossRef CAS PubMed.
- C.-J. Carling, J.-C. Boyer and N. Branda, J. Am. Chem. Soc., 2009, 131, 10838 CrossRef CAS PubMed; J.-C. Boyer, C.-J. Carling, B. D. Gates and N. Branda, J. Am. Chem. Soc., 2010, 132, 15766 CrossRef PubMed; X. Cui, J. Zhao, Y. Zhou, J. Ma and Y. Zhao, J. Am. Chem. Soc., 2014, 136, 9256 CrossRef PubMed; J.-C. Boyer, C.-J. Carling, S. Y. Chua, D. Wilson, B. Johnson, D. Baillie and N. Branda, Chem. – Eur. J., 2012, 18, 3122 CrossRef PubMed; T. Yang, Q. Liu, J. Li, S. Pu, P. Yang and F. Li, RSC Adv., 2014, 4, 15613 RSC.
- B. F. Zhang, M. Frigoli, F. Angiuli, F. Vetrone and J. A. Capobianco, Chem. Commun., 2012, 48, 7244 RSC; J. Lai, Y. Zhang, N. Pasquale and K.-B. Lee, Angew. Chem., Int. Ed., 2014, 53, 14419 CrossRef CAS PubMed; C. Zhang, C.-H. Xu, L. D. Sun and C.-H. Yan, Chem. – Asian J., 2012, 7, 2225 CrossRef PubMed; S. Chen, Y. Gao, Z. Cao, B. Wu, L. Wang, H. Wang, Z. Dang and G. Wang, Macromolecules, 2016, 49, 7490 CrossRef.
- J. Liu, W. Bu, L. Pan and J. Shi, Angew. Chem., Int. Ed., 2013, 52, 4375 CrossRef CAS PubMed.
- B. Yan, J.-C. Boyer, N. Branda and Y. Zhao, J. Am. Chem. Soc., 2011, 133, 19714 CrossRef CAS PubMed; Z. Chen, W. Sun, H.-J. Butt and S. Wu, Chem. – Eur. J., 2015, 21, 9165 CrossRef PubMed.
- S. Beyazit, S. Ambrosini, N. Marchyk, E. Palo, V. Kale, T. Soukka, B. T. S. Bui and K. Haupt, Angew. Chem., Int. Ed., 2014, 53, 8919 CrossRef CAS PubMed.
- A. Wang, W. Jin, E. Chen, J. Zhou, L. Zhou and S. Wei, Dalton Trans., 2016, 45, 3853 RSC; Q. Liu, C. Li, T. Yang, T. Yi and F. Li, Chem. Commun., 2010, 46, 5551 RSC; L. Grana-Suárez, W. Verboom, S. Sarkar, V. Mahalingam and J. Huskens, ChemistrySelect, 2016, 1, 4068 CrossRef CAS.
- A. Samanta and B. J. Ravoo, Angew. Chem., Int. Ed., 2014, 53, 12946 CrossRef CAS PubMed.
- L. Stricker, E.-C. Fritz, M. Peterlechner, N. L. Doltsinis and B. J. Ravoo, J. Am. Chem. Soc., 2016, 138, 4547 CrossRef CAS PubMed.
- B. Meesaragandla, V. N. K. B. Adusumalli and V. Mahalingam, Langmuir, 2015, 31, 5521 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Methods, synthesis, analytical data, additional experiments. See DOI: 10.1039/c6cc08321h |
| This journal is © The Royal Society of Chemistry 2017 |