SHG-active LnIII–[MoI(CN)5(NO)]3− (Ln = Gd, Eu) magnetic coordination chains: a new route towards non-centrosymmetric molecule-based magnets†
Masaya
Komine
a,
Szymon
Chorazy
ab,
Kenta
Imoto
a,
Koji
Nakabayashi
a and
Shin-ichi
Ohkoshi
*a
aDepartment of Chemistry, School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: ohkoshi@chem.s.u-tokyo.ac.jp
bFaculty of Chemistry, Jagiellonian Univ., Ingardena 3, 30-060 Kraków, Poland
First published on 10th November 2016
Abstract
The rare heteroligand pentacyanidonitrosylmolybdate(I) anion was employed in the construction of d–f bimetallic cyanido-bridged {[LnIII(dmf)6][MoI(CN)5(NO)]} (Ln = Gd, 1; Eu, 2) chains crystallizing in the non-centrosymmetric Pna21 space group. 1 exhibits Gd–Mo antiferromagnetic coupling giving rise to the ferrimagnetic spin chain behaviour with the onset of magnetic ordering below 2 K, and shows high second harmonic generation (SHG) activity with SH susceptibility of 1.2 × 10−10 esu, opening a novel family of non-centrosymmetric molecule-based magnets.
Molecule-based magnets which are based on paramagnetic metal complexes and/or organic radicals arouse considerable interest in chemistry, physics and materials science.1 They are especially attractive as efficient molecular platforms for multiple functionalities, that is the combination of intrinsic magnetic phenomena (magnetic coupling, magnetic ordering, slow magnetic relaxation or spin bistability) with additional physical properties such as luminescence,2 microporosity,3 and photo-induced4 or dehydration-driven phase transitions.5
The implementation of chirality or non-centrosymmetricity into molecule-based magnets is particularly fruitful due to the numerous physical cross-effects appearing as the product of coupling between the magnetic and optical properties of the material.6–11 For instance, the non-linear optical (NLO) effect of second harmonic generation (SHG) that occurs in the solid crystallizing in a non-centrosymmetric space group6 can be significantly enhanced in the spin ordered state due to the magnetic contribution to the intensity of SH light. The resulting cross-effect called magnetization induced second harmonic generation (MSHG) was presented in very special examples of molecule-based magnets based on cyanido- or oxalato-bridged networks.7 Lately, our group has showed an even more attractive effect, a 90 degree photoswitching of the SHG polarization plane in the FeII–[NbIV(CN)8]4− chiral photomagnet.8 The chirality introduced to the spin ordered systems also enabled the observation of magnetization enhanced magnetic circular dichroism (MCD)9 and magneto-chiral dichroism (MChD),10 or multiferroicity.11
In this context, there is great interest in searching for efficient synthetic strategies towards non-centrosymmetric molecule-based magnets.12 They are usually prepared by a straightforward method using chiral ligands or counterions to induce non-centrosymmetricity by coordination to metal ions,9,13 or through non-covalent interactions.10,11,14 Using this route, a number of non-centrosymmetric magnets, usually built of cyanido- or oxalato-bridged networks, were achieved.10,11,13,14 The alternative approach is based on the spontaneous resolution during the crystallization process.7,15 Such a synthetic route is difficult to control due to its usually unexplained mechanism. However, during our studies on chiral [M(CN)8]-based networks, we found that the spontaneous resolution can be induced by the selected non-chiral but sterically expanded ligands, such as 4-bromopyridine, inducing chirality in MnII–[NbIV(CN)8]4− ferrimagnets.15d Following these findings, we considered the application of metal complexes that can be good precursors for the non-centrosymmetric magnets. While broadly studied [M(CN)6]n− and [M(CN)8]n− ions are isotropic and cannot be used for symmetry breaking, we found that heteroligand pentacyanidonitrosyl metallates, [M(CN)5(NO)]n−, due to their decreased symmetry and structural anisotropy, can be of potential interest. We selected the rare [MoI(CN)5(NO)]3− anion which is paramagnetic,16 in contrast to diamagnetic nitroprusside [Fe/RuII(CN)5(NO)]2− ions, and its photochromic and photoswitchable properties were explored.17 [MoI(CN)5(NO)]3− was used only once in the synthesis of bimetallic coordination polymers, which is a K0.9Mn1.05[Mo(CN)5(NO)]·n(solv) Prussian blue analogue with ferrimagnetic ordering below 39 K but only prepared in the powder form which hampers the discussion on its structure.18 Here, we report the structure and properties of bimetallic CN-bridged {[LnIII(dmf)6][MoI(CN)5(NO)]} (Ln = Gd, 1; Eu, 2) chains crystallizing in a non-centrosymmetric space group, which can combine ferrimagnetic spin chain behaviour with SHG activity.
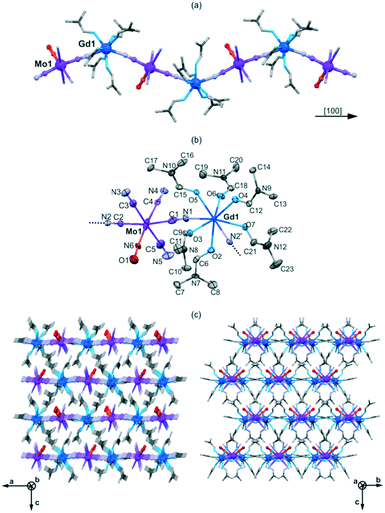
The yellow platelet single crystals of 1 and 2 were prepared under an argon atmosphere by crystallization from mixed solution of [MoI(CN)5(NO)]3− and the respective lanthanide(3+) ions in N,N′-dimethylformamide (dmf) solvent (Experimental section and Fig. S1, ESI†). Single crystal X-ray diffraction studies revealed that 1 and 2 are isostructural and composed of cyanido-bridged chains crystallizing in the non-centrosymmetric Pna21 space group (Fig. 1, S2 and S3, Tables 1, S1 and S2, ESI†). The coordination polymers of 1 and 2 are built of eight-coordinated [LnIII(μ-NC)2(dmf)6]+ (Ln = Gd, 1; Eu, 2) units of a nearly ideal square antiprism geometry, which are alternately arranged with octahedral [MoI(CN)5(NO)]3− moieties (Fig. 1, Table S2†). Two cyanides of Mo1 complexes are bridging the neighbouring Ln1 centers while three others are terminal. The NO ligands occupying the sixth position of the Mo1 complex are distinguishable by revealing Mo1–N bond lengths of ca. 1.93 Å, which is much shorter than the 2.13–2.18 Å Mo1–C bond of Mo1–CN linkages. Thus, the structural analysis revealed that the NO ligands of [MoI(CN)5(NO)]3− are also terminal. Each Ln center coordinates two bridging cyanides with a N–Ln1–N angle of ca. 138° resulting in the zig-zag topology of the chains.
| Compound | 1 | 2 | |
|---|---|---|---|
| Formula | Gd1Mo1C23H42N12O7 | Eu1Mo1C23H42N12O7 | |
| Formula weight [g mol−1] | 851.87 | 846.58 | |
| T [K] | 90(2) | ||
| λ [Å] | 0.71075 (Mo Kα) | ||
| Crystal system | Orthorhombic | ||
| Space group | Pna21 | ||
| Unit cell | a [Å] | 21.4968(12) | 21.5317(7) |
| b [Å] | 9.8959(5) | 9.9017(3) | |
| c [Å] | 16.3664(8) | 16.3749(5) | |
| V [Å3] | 3481.6(3) | 3491.14(19) | |
| Z | 4 | 4 | |
| Calculated density [g cm−3] | 1.625 | 1.611 | |
| Absorption coefficient [cm−1] | 2.304 | 2.195 | |
| F(000) | 1704 | 1700 | |
| Crystal size [mm × mm × mm] | 0.40 × 0.30 × 0.20 | 0.60 × 0.50 × 0.10 | |
| Crystal type | Yellow plate | Yellow plate | |
| Θ range [deg] | 3.063–27.464 | 3.060–27.442 | |
| Limiting indices | −27 < h < 27 | −27 < h < 27 | |
| −12 < k < 12 | −12 < k < 12 | ||
| −21 < l < 17 | −18 < l < 21 | ||
| Collected reflections | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 381 381 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 294 294 |
|
| Unique reflections | 7392 | 7521 | |
| R int | 0.0313 | 0.0313 | |
| Completeness [%] | 99.8 | 99.8 | |
| Max. and min. transmission | 0.630 and 0.418 | 0.803 and 0.485 | |
| Data/restraint/parameters | 7392/1/397 | 7521/2/397 | |
| GOF on F2 | 1.091 | 1.123 | |
| Flack x-parameter | 0.360(3) | 0.330(3) | |
| Final R indices | R 1 = 0.0405 [I > 2σ(I)] | R 1 = 0.0451 [I > 2σ(I)] | |
| wR2 = 0.0886 (all) | wR2 = 0.0932 (all) | ||
| Largest diff peak/hole | 3.646/−2.784 e A−3 | 3.689/−2.814 e A−3 | |
The cyanido-bridged chains of 1 and 2 are arranged in parallel within the supramolecular network which is stabilized only by the steric effects on the methyl groups of dmf ligands and the weak hydrogen bonds between the terminal cyanides and C–H groups of dmf (Fig. 1c and S2c, ESI†). The crystallization solvent molecules were not detected. Thus, the inter-chain space is controlled by the alignment of dmf ligands which results in the closest inter-metallic distances between neighbouring chains of ca. 8.28 Å between Mo1 and Ln1 along the c axis. Within the supramolecular networks of 1, the nitrosyl ligands of [MoI(CN)5(NO)]3− ions are aligned in the directions close to the c axis, following the 21 screw axis, while the coordination chains are aligned along the b axis. Moreover, the Mo1–N–O fragments of 1 are oriented towards the negative coordinates of the c direction which causes the overall lack of a centre of symmetry (Fig. 1c). In such a case, two enantiomorphic forms are expected but the crystals of 1 grow as the inversion twins with a Flack parameter of 0.360(3) indicating that the twin ratio is 0.360(3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.640(3) (Table 1).19 An identical behavior was found for 2 with a similar twin ratio of 0.330(3)
0.640(3) (Table 1).19 An identical behavior was found for 2 with a similar twin ratio of 0.330(3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.670(3). The structural model found from the single-crystal XRD experiment is valid for the bulk sample used in other physical measurements which was proved by the powder X-ray diffraction analysis (Fig. S3, ESI†).
0.670(3). The structural model found from the single-crystal XRD experiment is valid for the bulk sample used in other physical measurements which was proved by the powder X-ray diffraction analysis (Fig. S3, ESI†).
Due to their non-centrosymmetric Pna21 space group, 1 and 2 can be considered as promising functional materials for the occurrence of non-linear optical (NLO) properties. We checked for this attractive possibility by using 1 and executing the experiment of second harmonic generation (SHG), which is a well-known NLO effect of generating double frequency photons, 2ω, after interacting the photons of frequency ω with a solid medium showing at least a local lack of a centre of symmetry. After irradiating the polycrystalline sample of 1 with the fundamental light wavelength of 1064 nm, we repeatedly detected the second harmonic green light of 532 nm (see ESI,† Fig. S1 for the experimental details). The magnitude of SH susceptibility was determined to be 1.2 × 10−10 esu which is ca. 0.1 times the value of potassium dihydrogen phosphate (KDP), one of the most common NLO crystals. This value is, however, impressive among coordination polymers, especially those based on SHG-active cyanido-bridged materials (Table 2).15
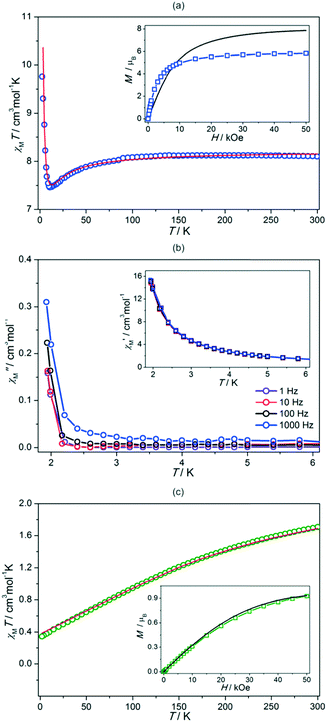
The direct-current (dc) magnetic properties of 1 and 2 are presented in Fig. 2. The room temperature χMT value for the {GdIIIMoI} unit of 1 is 8.1 cm3 mol−1 K which is close to the value of 8.2 cm3 mol−1 K expected for the free-ion contributions from GdIII (SGd = 7/2, gGd = 2.0) and MoI (SMo = 1/2, gMo = 2.0). Upon cooling, the χMT product decreases slowly down to 50 K, and then faster below this point. A minimum of 7.5 cm3 mol−1 K is observed at 10.8 K, and after that, a fast increase of χMT up to 10.0 cm3 mol−1 K at T = 2 K is detected (Fig. 2a). The minimum in the χMT vs. T plot suggests the presence of antiferromagnetic coupling between GdIII and MoI spin centers. This is also supported by the field dependence of magnetization at T = 2 K (Fig. 2a, the inset). With increasing field, M increases fast up to 4.9 μB at 10 kOe, and later much slower reaching 5.8 μB at H = 50 kOe. This value is close to the 6 μB expected for an antiparallel arrangement of GdIII and MoI spins and much lower than the 8 μB corresponding to ferromagnetic coupling. In addition, the experimental M–H curve deviates strongly from the sum of the Brillouin functions for GdIII and MoI (Fig. 2a, the inset). The rapid increase of magnetization at low magnetic field is due to magnetic ordering. The deviation at high magnetic field is explained by the saturation of magnetization in the ferrimagnetically ordered state. These all together indicate an antiferromagnetic nature of the Gd–Mo interaction leading to the ferrimagnetic spin chains of 1. Such magnetic interactions operate within the chains. In addition, at the lowest temperatures, the onset of long-range ferrimagnetic ordering is observed, as the frequency-independent peak of the out-of-phase magnetic susceptibility starts to appear. This, however, happens at the lowest available T, around 2 K, suggesting that the related critical temperature is below 2 K.
To confirm the type of GdIII–MoI magnetic coupling, the χMT–T plot was analysed based on Seiden's model assuming an alternate arrangement of classical spins of Gd (SGd = 7/2) and quantum spins of MoI (SMo = 1/2).20 In this model, the spin Hamiltonian with an exchange interaction (Jexc) is defined as 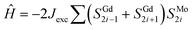 . Assuming gMo = 2.00, the values of J and gGd were fitted to the experimental data in the T range of 2–300 K giving Jexc = −1.02(5) cm−1 and gGd = 2.01(1) (Fig. 2a, see the ESI†). The negative J indicates an antiferromagnetic Gd–Mo interaction leading to the ferrimagnetic spin chain of 1.
. Assuming gMo = 2.00, the values of J and gGd were fitted to the experimental data in the T range of 2–300 K giving Jexc = −1.02(5) cm−1 and gGd = 2.01(1) (Fig. 2a, see the ESI†). The negative J indicates an antiferromagnetic Gd–Mo interaction leading to the ferrimagnetic spin chain of 1.
Fig. 2c shows the χMT vs. T plot for 2. The χMT at 300 K is 1.7 cm3 mol−1 K, which is slightly smaller than 1.875 cm3 mol−1 K calculated from isolated EuIII ion (1.5 cm3 mol−1 K) and MoI ion (0.375 cm3 mol−1 K).21 On cooling, the χMT decreases to reach 0.35 cm3 mol−1 K, which is close to the expected value for free-ion MoI. The magnetization curve in H = 50 kOe at T = 2 K reaches 0.93 μB, which is close to the 1 μB corresponding to the MoI spins. In fact, the whole M–H curve at 2 K follows the Brillouin function for S = 1/2 with g = 2.0. Such a magnetic behaviour of 2 is explained by the spin–orbit coupling effect of EuIII. This ion reveals the diamagnetic ground state of 7F0 but excited states are closely located, and can be thermally populated giving a non-zero contribution to the χMT. However, they are gradually depopulated on cooling, thus at 2 K only the 7F0 state is occupied, and MoI gives the sole contribution to magnetism of 2. Assuming these, we simulated the χMT–T plot with the spin–orbit coupling constant (λ) as a fitting parameter (see the ESI†). Good agreement with the experiment was obtained for λ = 365(3) cm−1. This indicates the negligible magnetic coupling between MoI and diamagnetic EuIII.
We report two bimetallic cyanido-bridged LnIII(dmf)–MoI (Ln = Gd, 1; Eu, 2) chains based on the rare [MoI(CN)5(NO)]3− anion. They are the second examples of coordination systems based on this exotic metalloligand, and the first obtained as single crystals. Due to the decreased symmetry and axial anisotropy of [MoI(CN)5(NO)]3− combined with the steric effects on the dmf ligands, 1 and 2 crystallize in the non-centrosymmetric Pna21 space group. This makes them attractive objects for the non-linear optical effects as shown by the SHG activity detected in 1. While 2 is a paramagnet without magnetic coupling, 1 reveals a ferrimagnetic chain behaviour due to the antiferromagnetic Gd–Mo interaction, and the onset of ferrimagnetic ordering below 2 K. Thus, 1 combines non-centrosymmetricity with the ferrimagnetic property showing that the insertion of heteroligand [MoI(CN)5(NO)]3− into coordination polymers is an efficient route towards non-centrosymmetric magnets with non-linear optical and magneto-optical properties.7 To observe such effects, a higher TC of magnetic ordering should be induced, and it can be realized by the increase of cyanide connectivity or replacement of 4f- with d-metal ions with strong exchange coupling. Moreover, [MoI(CN)5(NO)]3− like other nitroprusside ions can reveal photoinduced phase transitions due to the photosensitive NO ligand which together with non-centrosymmetricity and magnetism can lead to extra cross-effects.8 We are currently checking these exciting possibilities.
This work was financed by the Japan Society for the Promotion of Science within the Grant-in-Aid for Specially Promoted Research, 15H05697. We thank Cryogenic Research Center and Center for Nanolithography & Analysis, The Univ. of Tokyo, supported by MEXT. M. K. acknowledges the support of the Advanced Leading Graduate Course for Photon Science (ALPS).
Notes and references
- (a) J. A. Real, A. B. Gaspar and M. Carmen Munoz, Dalton Trans., 2005, 2062 RSC; (b) J. Miller, Chem. Soc. Rev., 2011, 40, 3266 RSC; (c) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed; (d) T. Grancha, J. Ferrando-Soria, M. Castellano, M. Julve, J. Pasan, D. Armentano and E. Pardo, Chem. Commun., 2014, 50, 7569 RSC.
- (a) J. Ferrando-Soria, H. Khajavi, P. Serra-Crespo, J. Gascon, F. Kapteijn, M. Julve, F. Lloret, J. Pasan, C. Ruiz-Perez, Y. Journaux and E. Pardo, Adv. Mater., 2012, 24, 5625 CrossRef CAS PubMed; (b) S. Chorazy, J. Wang and S. Ohkoshi, Chem. Commun., 2016, 52, 10795 RSC.
- P. Dechambenoit and J. R. Long, Chem. Soc. Rev., 2011, 40, 3249 RSC.
- (a) S. Ohkoshi, K. Imoto, Y. Tsunobuchi, S. Takano and H. Tokoro, Nat. Chem., 2011, 3, 564 CrossRef CAS PubMed; (b) C. Mathoniere, H.-J. Lin, D. Siretanu, R. Clerac and J. M. Smith, J. Am. Chem. Soc., 2013, 135, 19083 CrossRef CAS PubMed.
- (a) D. Maspoch, D. Ruiz-Molina, K. Wurst, N. Domingo, M. Cavallini, F. Biscarini, J. Tejada, C. Rovira and J. Veciana, Nat. Mater., 2003, 2, 190 CrossRef CAS PubMed; (b) B. Nowicka, M. Reczyński, M. Rams, W. Nitek, J. Żukrowski, C. Kapusta and B. Sieklucka, Chem. Commun., 2015, 51, 11485 RSC.
- L. R. Mingabudinova, V. V. Vinogradov, V. A. Milichko, E. Hey-Hawkins and A. Vinogradov, Chem. Soc. Rev., 2016, 45, 5408 RSC.
- (a) T. Nuida, T. Matsuda, H. Tokoro, S. Sakurai, K. Hashimoto and S. Ohkoshi, J. Am. Chem. Soc., 2005, 127, 11604 CrossRef CAS PubMed; (b) C. Train, T. Nuida, R. Gheorghe, M. Gruselle and S. Ohkoshi, J. Am. Chem. Soc., 2009, 131, 16838 CrossRef CAS PubMed; (c) Y. Tsunobuchi, W. Kosaka, T. Nuida and S. Ohkoshi, CrystEngComm, 2009, 11, 2051 RSC; (d) D. Pinkowicz, R. Podgajny, W. Nitek, M. Rams, A. M. Majcher, T. Nuida, S. Ohkoshi and B. Sieklucka, Chem. Mater., 2011, 23, 21 CrossRef CAS.
- S. Ohkoshi, S. Takano, K. Imoto, M. Yoshikiyo, A. Namai and H. Tokoro, Nat. Photonics, 2014, 8, 165 Search PubMed.
- (a) K. Inoue, K. Kikuchi, M. Ohba and H. Okawa, Angew. Chem., Int. Ed., 2003, 42, 4810 CrossRef CAS PubMed; (b) S. Chorazy, R. Podgajny, W. Nitek, T. Fic, E. Gőrlich, M. Rams and B. Sieklucka, Chem. Commun., 2013, 49, 6731 RSC.
- C. Train, R. Gheorghe, V. Krstic, L. M. Chamoreau, N. S. Ovanesyan, G. Rikken, M. Gruselle and M. Verdaguer, Nat. Mater., 2008, 7, 729 CrossRef CAS PubMed.
- E. Pardo, C. Train, H. Liu, L. M. Chamoreau, B. Dkhil, K. Boubekeur, F. Lloret, K. Nakatani, H. Tokoro, S. Ohkoshi and M. Verdaguer, Angew. Chem., Int. Ed., 2012, 51, 8356 CrossRef CAS PubMed.
- C. Train, M. Gruselle and M. Verdaguer, Chem. Soc. Rev., 2011, 40, 3297 RSC.
- (a) H. Kumagai and K. Inoue, Angew. Chem., Int. Ed., 1999, 38, 1601 CrossRef CAS; (b) E. Coronado, J. Galan-Mascaros, C. J. Gomez-Garcia and A. Murcia-Martinez, Chem. – Eur. J., 2006, 12, 3484 CrossRef CAS PubMed; (c) A. Beghidja, P. Rabu, G. Rogez and R. Welter, Chem. – Eur. J., 2006, 12, 7627 CrossRef CAS PubMed; (d) K. Komori-Orisaku, K. Imoto, Y. Koide and S. Ohkoshi, Cryst. Growth Des., 2013, 13, 5267 CrossRef CAS.
- (a) N. S. Ovanesyan, V. Makhaev, S. M. Aldoshin, P. Gredin, K. Boubekeur, C. Train and M. Gruselle, Dalton Trans., 2005, 3101 RSC; (b) M. Clemente-Leon, E. Coronado, J. C. Dias, A. Soriano-Portillo and R. Willett, Inorg. Chem., 2008, 47, 6458 CrossRef CAS PubMed.
- (a) T. Hozumi, T. Nuida, K. Hashimoto and S. Ohkoshi, Cryst. Growth Des., 2006, 6, 1736 CrossRef CAS; (b) W. Kosaka, T. Nuida, K. Hashimoto and S. Ohkoshi, Bull. Chem. Soc. Jpn., 2007, 80, 960 CrossRef CAS; (c) S. Ohkoshi, S. Saito, T. Matsuda, T. Nuida and H. Tokoro, J. Phys. Chem. C, 2008, 112, 13095 CrossRef CAS; (d) T. Ohno, S. Chorazy, K. Imoto and S. Ohkoshi, Cryst. Growth Des., 2016, 16, 4119 CrossRef CAS.
- A. Müller, W. Eltzner, S. Sarkar, H. Bőgge, P. J. Aymonino, N. Mohan, U. Seyer and P. Subramanian, Z. Anorg. Allg. Chem., 1983, 503, 22 CrossRef.
- (a) M. D. Carducci, M. R. Pressprich and P. Coppens, J. Am. Chem. Soc., 1997, 119, 2669 CrossRef CAS; (b) Z.-Z. Gu, O. Sato, T. Iyoda, K. Hashimoto and A. Fujishima, Chem. Mater., 1997, 9, 1092 CrossRef CAS.
- N. Machida, S. Ohkoshi, Z. J. Zhong and K. Hashimoto, Chem. Lett., 1999, 907 CrossRef CAS.
- T. Weber, J. Dshemuchadse, M. Kobas, M. Conrad, B. Harbrecht and W. Steurer, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 308 CAS.
- J. Seiden, J. Phys., Lett., 1983, 44, 947 CrossRef CAS.
- Y. Wan, L. Zhang, L. Jin, S. Gao and S. Lu, Inorg. Chem., 2003, 42, 4985 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, setup for SHG measurement, detailed structure parameters, results of continuous shape measure analysis, additional structural views, powder X-ray diffraction patterns, and details of magnetic calculations. CCDC 1508558 and 1508559. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce02214f |
| This journal is © The Royal Society of Chemistry 2017 |