Adsorption and diffusion of lithium in a graphene/blue-phosphorus heterostructure and the effect of an external electric field
Kaimin
Fan
 *a,
Jing
Tang
b,
Shiyun
Wu
*a,
Chengfu
Yang
a and
Jiabo
Hao
a
*a,
Jing
Tang
b,
Shiyun
Wu
*a,
Chengfu
Yang
a and
Jiabo
Hao
a
aSchool of Intelligent Manufacturing, Sichuan University of Arts and Science, Dazhou, 635000, China. E-mail: fankm128@163.com; wsywsy1968@163.com
bSchool of Chemistry and Chemical Engineering, Sichuan University of Arts and Science, Dazhou, 635000, China
First published on 30th November 2016
Abstract
The adsorption and diffusion behaviors of lithium (Li) in a graphene/blue-phosphorus (G/BP) heterostructure have been investigated using a first principles method based on density functional theory (DFT). The effect of an external electric field on the adsorption and diffusion behaviors has also been investigated. The results show that the adsorption energy of Li on the graphene side of the G/BP heterostructure is higher than that on monolayer graphene, and Li adsorption on the BP side of the G/BP/Li system is slightly stronger than that on monolayer BP (BP/Li). The adsorption energy of Li reaches 2.47 eV, however, the energy barriers of Li diffusion decrease in the interlayer of the G/BP heterostructure. The results mentioned above suggest that the rate performance of the G/BP heterostructure is better than that of monolayer graphene. Furthermore, the adsorption energies of Li atoms in the three different most stable sites, i.e., HG, TP and H1 sites, increase by about 0.49 eV, 0.26 eV, and 0.13 eV, respectively, as the electric field intensity reaches 0.6 V Å−1. The diffusion energy barrier is significantly decreased by an external electric field. It is demonstrated that the external electric field can not only enhance the adsorption but can also modulate the diffusion barriers of Li atoms in the G/BP heterostructure.
1. Introduction
Nowadays, the booming development of the intelligent electronics market and the ever-increasing demand for advanced battery systems have led to significant research efforts for high-performance electrode materials.1–4 As one of the secondary batteries, lithium ion batteries (LIBs) are regarded as the most promising energy storage and conversion battery system owing to their high energy density, flexible and lightweight design, longer lifespan,5,6 high reversible capacity,7 and environmental friendliness,8 and they are widely used in portable electronic devices, communication facilities, stationary energy storage systems and ever-enlarging markets for electric vehicles.9The electrochemical performance of LIBs strongly depends on the physical and chemical properties of anode and cathode materials.9,10 Typically, graphite has been used as the anode material in conventional LIBs due to its high abundance, outstanding kinetics, high energy stability, cycling stability and low cost.11,12 The graphite anode has a theoretical capacity of 372 mA h g−1,13,14 which limits its further demand for high power density or long term applications.
As a representative two-dimensional (2D) thin material, graphene has been widely investigated and utilized as an electrode material and obtained great success.15–17 Monolayer graphene has a theoretical Li ion storage capacity of 744 mA h g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 and a high specific surface area of 2965 m2 g−1.19 However, the volumetric energy density of graphene limits its implementation in commercial applications. The design of graphene-based composite materials is one of the feasible strategies to improve the rate capability, energy density, and power density of electrode materials.18 The design of novel composite materials is performed easily more and more with the progress of nanotechnology.
18 and a high specific surface area of 2965 m2 g−1.19 However, the volumetric energy density of graphene limits its implementation in commercial applications. The design of graphene-based composite materials is one of the feasible strategies to improve the rate capability, energy density, and power density of electrode materials.18 The design of novel composite materials is performed easily more and more with the progress of nanotechnology.
High-performance LIBs critically depend on excellent electrode materials with high rate capability, capacity, fast Li ion mobility, cheap price, good thermal stability, etc.20–23 In recent years, graphene-based composite materials, such as graphene/MoS2,24 graphene/MnO2,25 graphene/phosphorene26 and boron nitride/phosphorene,27 have attracted much attention due to their extraordinary mechanical, electronic and electrochemical properties.
Recently, blue phosphorus (P) nanosheets proposed by Zhu and Tománek have been reported to be equally stable as 2D black P, which should be exfoliated to form a quasi-2D material for potential electronic applications.28,29 In spite of blue P still not being fabricated by experiment, it has also attracted sufficient attention due to its interesting electrochemical properties with a graphene type structure.28–32
Both blue and black P nanosheets exhibit excellent adsorption capability to Li, Na, and Mg atoms, and the adsorption energies of the adatoms on blue and black P are stronger than that on the BN, SiC, and MoS2 sheets.30,33 Different from black P, blue P has a wider band gap.28 Li et al.32 have reported that Li diffusion on the surfaces of black and blue P has a relatively low energy, and the blue P nanosheet has high charge capacities. The nanocomposite structure is an effective way to integrate the properties of the isolated components.26 Reports from experimental research showed that phosphorus–carbon nanocomposites have high capacities (1700 mA h g−1),34 and the phosphorus/graphene nanosheet hybrid structure displays extraordinarily high cycling stability as an anode material in LIBs.35 Recently, a study on the silicon/carbon nanotube/BaTiO3 nanocomposite indicated, as an anode of the LIBs, that this composite can improve the cycle performance and discharge capacity.36
Detailed work on the graphene/blue-phosphorus (G/BP) heterostructure is lacking. In the present work, we have provided significant insights into the Li atom adsorption and diffusion behaviors and investigated the effect of an external electric field on adsorption and diffusion in the G/BP heterostructure.
2. Theoretical methods
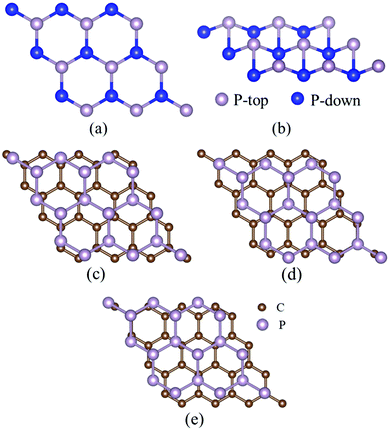
A first-principles method based on DFT has been used in the present work, and the Spanish Initiative for Electronic Simulations with Thousands of Atoms (SIESTA) code37 has been employed using the GGA (generalized gradient approximation)38 with the PBE (Perdew–Burke–Ernzerhof) functional39 in our calculations. 3 × 3 × 1 and 5 × 5 × 1 Brillouin zone k-point sampling and the Monkhorst–Pack (MP) mesh are used for testing. The results show that the adsorption energies of Li on graphene more or less depend on the k-point sampling, but the order of the adsorption energies is almost unacted on k-points. For computational efficiency, 3 × 3 × 1 Brillouin zone k-point sampling and the Monkhorst–Pack (MP) mesh are used in our calculations. Valence electron wave functions were expanded using the double-ζ basis set37 with a plane wave energy cutoff of 150 Ry. The convergence of the total energy with respect to the plane wave energy cutoff has been tested. The total energies of the graphene unit cell and blue P unit cell convergence at an energy cutoff of 150 Ry with the total energy change of 0.0005 and 0.007 eV, respectively, have been evaluated. A large vacuum of 30 Å has been added to avoid the interaction of layers due to periodic repeats.Firstly, the geometry optimizations of 3 × 3 × 1 blue P and 4 × 4 × 1 graphene supercells with a full relaxation of the atomic positions, the shape and the size of the supercell have been performed. The norm-conserving Troullier–Martins pseudopotentials40 from the SIESTA databases have been employed, which have been proved to well describe the properties of graphene and carbon nanotubes,41 the Si–P system,42 and Li adsorption in the MoS2 monolayer.43 The calculated bond-lengths of C–C in graphene and P–P in blue P are 1.42 Å and 2.30 Å agreeing well with the experimental value of 1.42 Å44 and the other theoretical value of 2.27 Å,28 respectively. The relaxed stable structure of blue P is shown in Fig. 1(a) and (b).
In order to test the effect of supercell size on the calculated results, 5 × 5 × 1 supercells with 50 atoms for graphene and blue P have been used to calculate the adsorption energies of Li, and the results show that adsorption energies depend slightly on the supercell size, which agrees with the reported calculations.32 Therefore, the G-(4 × 4 × 1)/BP-(3 × 3 × 1) supercell has been mainly employed in the present calculations.
The stacking patterns of the G/BP heterostructure have been tested in our calculations. Three different stacking patterns have been investigated as shown in Fig. 1(c)–(e). Fig. 1(c) shows that the bridge of blue P (BP) appears to be directly over the hexagons of graphene, called the S1 structure. One top-layer P atom (P-top) of BP overlaps the center of the hexagons of graphene, denoted as S2, see Fig. 1(d). Fig. 1(e) shows that one down-layer P atom (P-down) of BP overlaps the center of the hexagons of graphene denoted as S3. The results show that all the three structures are stable configurations, and the S1 structure is more stable with a slightly larger binding energy of about 11 meV than the S2 and S3 configurations. The bond lengths of C–C and P–P are 1.44 Å and 2.30 Å in the relaxed G/BP structure being preserved well compared to the pristine graphene (1.42 Å) and BP (2.30 Å), respectively, which demonstrates the structure stability of the present stacking structure pattern. To assess the role of van der Waals (vdW) interactions in the G/BP heterostructure, the calculations on the stacking structures of G/BP with the vdW correction (vdW-DF)45 have been performed. Our calculations show that S1, S2 and S3 structures also are all stable configurations with nearly equal binding energy, and the bond lengths of C–C and P–P are 1.43 Å and 2.31 Å in G/BP with vdW-DF, which indicates that the PBE exchange–correlation functional (PBE) can correctly describe the G/BP heterostructure. On the basis of the above calculations from PBE and vdW-DF, we mainly consider the S1 structure to investigate the properties of adsorption and diffusion for Li in the G/BP heterostructure.
3. Results and discussion
3.1. Adsorption and diffusion
The adsorption energies of Li in the monolayer graphene, monolayer BP and G/BP heterostructure have been determined. The adsorption energy is defined by| Ead. = EG/BP + εLi − EG/BP+Li, | (1) |
These Li adsorption sites in the Li/G/BP, G/BP/Li and G/Li/BP systems are illustrated in Fig. 2. HG, BG, and TG represent Li on the graphene side (Li/G/BP), and HP, BP, and TP represent Li on the blue P side (BP side, i.e., G/BP/Li), as shown in Fig. 2(a) and (b), respectively. Four Li adsorption sites (H1, H2, H3 and H4) in the interlayer of the G/BP heterostructure (G/Li/BP) have been considered. As shown in Fig. 2(b), H1, H2, H3 and H4 sites represent that Li atoms locate in the hollow site (H site) of the graphene in the G/BP heterostructure. Our calculations show that the H4 site is not a stable adsorption position for the Li atom; the Li atom markedly deviates from the hollow site (H site) of the graphene due to the P-top atom of BP.
As shown in Table 1, Li adsorption in hollow (H), bridge (B) and top (T) sites on the monolayer graphene and BP has been considered. The configurations of Li adsorption on the monolayer graphene and BP are the same as in ref. 46 and ref. 32, respectively, and can also be referred to those in Fig. 2. It is worth mentioning here that the T site is one Li atom on the top of the P-down atom in the monolayer BP. We have also investigated that the adsorption site is one Li atom on the top of the P-top atom, which shows that it is not a stable Li adsorption position.
| Li site | E ad. | Li site | E ad. | |||
|---|---|---|---|---|---|---|
| PBE | G/Li | H | 1.17 | BP/Li | H | 1.96 |
| PBE | B | 0.83 | B | 1.90 | ||
| PBE | T | 0.82 | T | 2.05 | ||
| PBE | Li/G/BP | HG | 1.37 | G/BP/Li | HP | 1.98 |
| vdW-DF | HG | 1.29 | HP | 1.86 | ||
| PBE | BG | 1.02 | BP | 1.92 | ||
| vdW-DF | BG | 1.03 | BP | 1.81 | ||
| PBE | TG | 1.01 | TP | 2.07 | ||
| vdW-DF | TG | 1.02 | TP | 1.92 | ||
| PBE | G/Li/BP | H1 | 2.47 | |||
| vdW-DF | H1 | 2.20 | ||||
| PBE | H2 | 2.41 | ||||
| vdW-DF | H2 | 2.16 | ||||
| PBE | H3 | 2.39 | ||||
| vdW-DF | H3 | 2.12 |
The vdW interactions may affect the results of binding energies of Li in the G/BP heterostructure. Therefore, the adsorption energies of Li in the Li/G/BP, G/BP/Li and G/Li/BP systems with the vdW correction (vdW-DF) have been investigated. As shown in Table 1, the adsorption energies with the vdW correction slightly decrease compared to those resulting from PBE without vdW-DF. Nevertheless, for the Li/G/BP, G/BP/Li and G/Li/BP systems, respectively, the order of the adsorption energies of Li is independent of the vdW corrections. Therefore, PBE without vdW corrections has been used in the following calculations.
Table 1 shows that the most stable adsorption site of Li is the H site for the monolayer graphene (G/Li). The adsorption energy of the B site is 0.01 eV higher than the value of the T site, which is in good agreement with the previous studies.46,47 The Li adsorption on the graphene side of the Li/G/BP system is stronger than that on monolayer graphene. For the monolayer BP, the Li atom prefers to occupy the T site with an adsorption energy of 2.05 eV, which agrees well with the previous calculations (2.02 eV).32 The adsorption energies of Li on the BP side of the G/BP/Li system is slightly higher than that on monolayer BP (BP/Li). It is evident that the adsorption energies of Li in the G/Li/BP system are 2.47, 2.41 and 2.39 eV, 1.18, 1.12 and 1.10 eV higher than the value of the H site on the monolayer graphene, respectively. The H1 site is almost the most stable site on both monolayer graphene and BP (see Fig. 2(b)). Table 1 shows that, in G/Li/BP, H1 is the most stable Li adsorption site, followed by H2 and H3, which indicates that the stability of the Li adsorption on G/Li/BP depends on both graphene and BP.
To further understand the interaction between Li and the G/BP heterostructure, we have investigated the densities of state (DOS) of Li, C and P atoms in G/BP, Li/G/BP and G/Li/BP systems. The present calculations indicate that the major electron contribution is mainly attributed to the s state of Li and the p state of C and P atoms, so, the DOS of Li-2s for Li and C-2p and P-3p for the nearest neighbor C and P atoms of the Li atom are plotted in Fig. 3.
Fig. 3(a) shows the DOS of C and P atoms in G/BP without Li adsorption. It can be seen that there is no hybridization between the C-2p and P-3p states due to the physical adsorption nature of graphene on BP with a binding energy of 0.06 eV per C atom between graphene and BP. As seen from Fig. 3(b) and (c), the DOS of C-2p and P-3p shift to lower energies, i.e., Li adsorption shifts the Fermi level into the conduction band, which indicates that charge transfers to graphene and BP as well as the titanium-graphene system.46,48,49 The prominent peaks of the Li-2s and C-2p states at about 1.0 eV appear as shown in Fig. 3(b), which demonstrates the strong hybridization interaction between the two states. Fig. 3(c) shows that the DOS of P-3p is strongly altered due to the presence of the Li atom compared to that in G/BP and in Li/G/BP systems. One can see from Fig. 3(c) that, as Li intercalates into the interlayer of G/Li/BP, the peaks of Li-2s, C-2p and P-3p almost appear at the same position, which indicates the hybridization interaction between Li-2s and C-2p (P-3p) states, which is similar to that of Li intercalation in graphene/black P.26 Strong hybridization results in the strong bonding between the Li and C (P) atoms, which may account for the higher adsorption energy of H1 in G/Li/BP compared to that of HG in Li/G/BP.
For graphene, the disadvantage of zero-gap restricts the applications in electronic and optoelectronic devices.24,50 The band structures of the G/BP and G/Li/BP systems have been investigated, as shown in Fig. 4. Fig. 4(a) shows the band structure of G/BP without Li adsorption. The band structure of G/Li/BP with a Li intercalating interlayer is plotted in Fig. 4(b). It is found that the Dirac point shifts to the valence band due to the presence of Li atoms, which may be attributed to the electron interaction between Li and the G/BP heterostructure as shown in Fig. 3(c), and a similar phenomenon has also been observed in potassium–graphene46 and graphene/Li/MoS224 systems. With Li atom intercalating into the G/BP heterostructure, as well as the case of Li in graphene/MoS2,24 the band gap has been found to be 0.05 eV as shown in Fig. 4(b). The relatively small gap opening is advantageous for the electrode conductivity.51
 | ||
| Fig. 4 Band structures of (a) the G/BP heterostructure without Li and (b) G/Li/BP with a Li intercalating interlayer. | ||
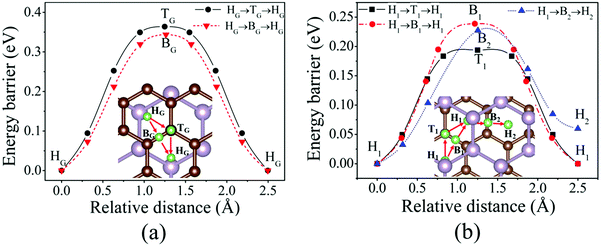
The diffusion properties have been investigated due to the importance of the Li-ion mobility in an electrode material. As the NEB method has not been implemented in the newest siesta 4.0 code, the energy barriers of Li diffusion are calculated by using a constrained method in this work, in which Li ions are constrained in the direction along the diffusion path, whereas the relaxation in the directions perpendicular to the diffusion path is allowed.52 The calculations on Li diffusion are carried out at a fixed volume,52 but a full relaxation of the atomic positions (Li atoms are constrained to relax in the directions perpendicular to the diffusion path) has been performed. We assume that Li moves between two adjacent favored sites via a high-symmetry site. We mainly focus on the change mechanism of the diffusion barrier. Therefore, the energy difference between one favored site and the high-symmetry site is referred to as the diffusion barrier.46Fig. 5(a) shows two different possible paths and the corresponding energy barriers of Li diffusion on the graphene side (Li/G/BP). The first path on the graphene side is that one Li atom moves from the HG site to the adjacent HG site across the BG site and the second path is that one Li atom moves from the HG site to the adjacent HG site passing over the TG site. Fig. 5(b) illustrates three possible paths and the corresponding energy barriers of Li diffusion in G/Li/BP. Similar to that on the graphene side, the H1 → B1 → H1 path is the Li diffusion between two H1 sites across the B1 site, and the H1 → T1 → H1 path is the diffusion between two H1 sites across the T1 site. We have also investigated the third possible path that one Li atom moves from the H1 site to the adjacent metastable adsorption site (H2 site) across B2 (H1 → B2 → H2) as shown in Fig. 5(b).
The diffusion barrier calculated in the present work is 0.34 eV for Li diffusion on the monolayer graphene, which agrees with the values of 0.30 eV26 and 0.32 eV53 reported previously. As shown in Fig. 5(a), the Li atom prefers to move along HG → BG → HG with an energy barrier of 0.34 eV on the graphene side. However, Fig. 5(b) shows that the energy barriers decrease as Li atoms diffuse in the interlayer of the G/BP heterostructure. One Li atom moves between two H1 sites via H1 → B1 → H1 and H1 → B2 → H2 paths by overcoming 0.23 and 0.22 eV energy barriers, respectively, which clearly decrease compared to that on the monolayer graphene and on the graphene side of the Li/G/BP system. The energy barrier of Li diffusion along the H1 → T1 → H1 path decreases about 0.16 eV. At the same time, the mobility of Li on the BP side of the G/BP heterostructure is preserved with an almost equal energy barrier of 0.09 eV to the monolayer BP, which is similar to that of Li on graphene/black P.26 The above results suggest that the rate performance of the G/BP heterostructure is better than that of monolayer graphene.
3.2. Effect of an external electric field
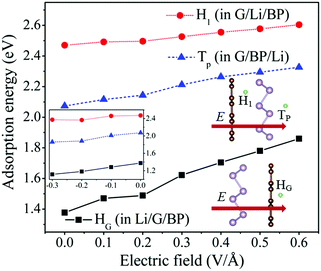
Recently, Shi et al.43 have proposed an effective method to improve the charging rate by applying an external electric field. In this work, the effect of the external electric field on the adsorption behavior and diffusion properties of Li atoms has also been investigated. The effect of the electric field direction on the results has been tested, which shows that the electric field perpendicular to the G/BP surface can bring about a more significant effect on the adsorption energies and diffusion barriers of Li than the other directions. As proposed by Ortiz-Medina et al.,54 the electric field applied for all the systems is a static and homogeneous external potential. The electric field is perpendicular to the G/BP surface, and its positive direction is shown in the inset of Fig. 6.In Fig. 6, we have plotted the adsorption energies of HG, TP and H1 sites in Li/G/BP, G/BP/Li and G/Li/BP, respectively. The adsorption energies of the HG, TP and H1 adsorption sites increase from 1.37 to 1.86, from 2.07 to 2.33 and from 2.47 to 2.60 eV with the electric field intensities (E), ranging from 0 to 0.6 V Å−1, respectively. The adsorption energies almost show a linear increase with increasing E. We have also investigated the case of the applied reverse electric field. As shown in the inset of Fig. 6, the adsorption energies decrease with E, ranging from 0 to −0.3 V Å−1, at HG, TP and H1 sites, respectively. In other words, the adsorption energies of Li in the G/BP heterostructure decrease when the reverse E increases. That is to say, as well as the case of the gas molecule on the hexagonal boron nitride sheet,55 the external electric field can significantly enhance the adsorption of Li on the G/BP heterostructure, but this enhancement of Li adsorption depends on the direction of E.
To investigate the effect of the external electric field on the diffusion properties, the diffusion of Li atoms on the graphene side of the Li/G/BP system is chosen as the representative case. Fig. 7 illustrates the schematic of Li diffusion along the HG → BG → HG path for 6 values of E ranging from 0.1 to 0.6 V Å−1, and the positive direction of E is shown in the inset of Fig. 6. It is found clearly that the energy barrier for the HG → BG → HG path decreases with increasing E. The energy barrier decreases about 0.13 eV as E reaches 0.6 V Å−1. The diffusion behavior is sensitive to the external electric field.
 | ||
| Fig. 7 The diffusion of Li on the graphene side of the Li/G/BP system with E ranging from 0.0 to 0.6 V Å−1. | ||
To investigate the effect of the external electric field on the interaction between Li and G/BP, the Bader charge analysis has been performed to explore the charge transfer from Li to G/BP in HG and BG sites of the Li/G/BP system. As shown in Fig. 8, Ne represents the amount of charge transferred from Li to G/BP in HG and BG sites with E ranging from 0.0 to 0.6 V Å−1.
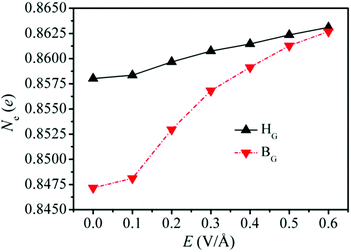 | ||
| Fig. 8 The charge transferred from Li to G/BP for HG and BG sites in the Li/G/BP system with an external electric field. | ||
It is found that the amounts of charge transferred from Li increase due to the presence of an external electric field. The larger charge transfer from Li to G/BP leads to the stronger electron interaction between Li and the G/BP heterostructure, which may be responsible for the increase of adsorption energies of Li in HG sites for the Li/G/BP system when the electric field is applied (see Fig. 6). One can also see that the charge transferred from Li in the HG site almost increases linearly with increasing E, which corresponds to the increase in the mechanism for the adsorption energies of the HG site induced by the electric field in the Li/G/BP system (see Fig. 6). Fig. 8 shows that the charge transferred from Li increases in HG and BG sites with E ranging from 0.0 to 0.6 V Å−1, respectively. When E = 0.0 V Å−1, i.e., without the external electric field, the amount of charge transferred from Li in the HG site is larger than that in the BG site, which can be used to explain the fact that the adsorption energy of Li in the HG site is higher than the value in the BG site, as shown in Table 1.
It is of interest that charge transfer caused by the external electric field in the BG site increases more sharply than that in the HG site. Therefore, the difference in the charge transferred from Li between BG and HG becomes smaller and smaller with increasing E as shown in Fig. 8. For example, the difference between BG and HG is about 0.0004 e when E reaches 0.6 V Å−1, which is much smaller than the value of 0.0109 e without the external electric field, which indicates that the charge transfer of Li in the BG site is more sensitive to the increase of E compared with that in the HG site when E ranges from 0.1 to 0.6 V Å−1. The analysis above may account for the fact that the diffusion energy barrier decreases with increasing E, as shown in Fig. 7.
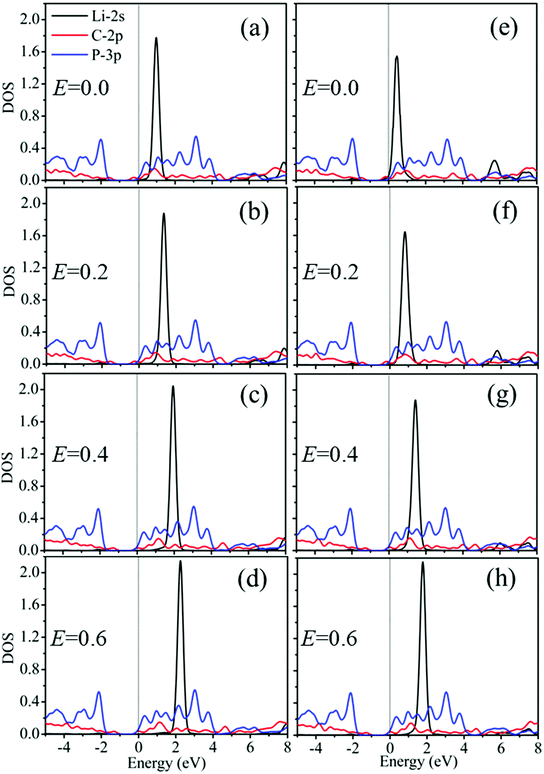
To further explore the interaction between Li and G/BP, we have calculated the DOS of the Li atom and its nearest neighbor C and P atoms in the Li/G/BP system with E of 0.0, 0.2, 0.4 and 0.6 V Å−1, as the representative case studied above. The results on DOS are shown in Fig. 9.
It is shown that since the Li-2s of HG and BG sites is above the Fermi level, Li atoms donate electrons to G/BP, which is in accordance with the conclusion inferred from our Bader charge analysis and similar to Li adsorption in graphene/MoS2.24 It is evident that the DOS of Li-2s shift to the deep conduction band with increasing E, which also supports the conclusion of a large charge transfer inferred from the Bader charge analysis between the Li and G/BP.24,56–58 The above results may account for the stronger interaction between the Li atoms and G/BP. Therefore, the external electric field can enhance the adsorption of Li on the G/BP heterostructure. When E = 0.0 V Å−1, Fig. 9(e) shows that the Li adsorption on the BG site introduces a lower DOS peak of the Li-2s and C-2p states at ∼0.7 eV, which may be attributed to the lighter hybridization between the two states, so, the adsorption energy of Li in the BG site is smaller than that in the HG site, as shown in Table 1. More importantly, the evident difference in the DOS peaks of Li-2s between HG and BG sites is observed when E = 0.0 V Å−1, i.e., without the external electric field. However, the difference in the DOS peaks mentioned above decreases with increasing E, which can also be used to explain the decrease of the diffusion barrier.
The adsorption and diffusion behavior of Li is vital for the electrochemical performance in anode materials for LIBs. The experimental study indicates that the safety and reversibility of LIBs depend on the adsorption behavior.59 The lower diffusion energy barrier improves the mobility of Li in anode materials for LIBs, and the high mobility is beneficial for rapid charging.60 Guo et al.26 have studied the graphene/black-phosphorus heterostructure; they found that the graphene/black-phosphorus heterostructure can greatly improve the binding energy, meanwhile, the high mobility of Li in the graphene/black-phosphorus heterostructure has been maintained in anode materials. Our work shows that the adsorption energy of Li reaches 2.47 eV; however, the energy barriers of Li diffusion decrease in the interlayer of the G/BP heterostructure, which indicates, as well as the graphene/black-phosphorus heterostructure, that the G/BP heterostructure should be regarded as a good electrode material in LIBs. The present investigation on the G/BP heterostructure combined with Li adsorption in the graphene/MoS2,24 graphene/MnO2,25 and graphene/phosphorene26 is beneficial to systematically understand the graphene-based nanosheets. Furthermore, our calculations indicate that the adsorption and diffusion behavior of Li can be modulated by an external electric field.
4. Conclusions
We have investigated the adsorption and diffusion behaviors of Li in the G/BP heterostructure. The effect of an electric field on the adsorption and diffusion behaviors has also been investigated. All of the present calculations are performed using the first principles method based on DFT. The results show that adsorption energies of Li on the graphene side of the G/BP heterostructure are higher than that on monolayer graphene, and Li adsorption on the BP side of the G/BP/Li system is slightly stronger than that on the monolayer BP.The energy barriers of Li diffusion decrease about 0.16 eV, 0.13 eV and 0.12 eV for the three diffusion paths considered in the interlayer of the G/BP heterostructure, respectively, compared to that on the monolayer graphene. At the same time, the mobility of Li on the BP side of the G/BP heterostructure is preserved. The above results suggest that the rate performance of G/BP is significantly better than that of monolayer graphene.
Furthermore, the adsorption energies of Li atoms in the three different most stable sites, i.e., HG, TP and H1 sites, increase about 0.49 eV, 0.26 eV, and 0.13 eV, respectively, as E reaches 0.6 V Å−1. The diffusion energy barrier is significantly decreased by the external electric field. The Bader charge analysis and the DOS calculations indicate that the external electric field is able to urge the charge transfer from Li to G/BP, and can promote strong electronic interaction between Li and G/BP, which demonstrates that the external electric field can not only enhance the adsorption but can also modulate the diffusion barriers of Li in the G/BP heterostructure.
Acknowledgements
This work was financially supported by the Scientific Research Fund of Sichuan Provincial Education Department (No. 14ZB0308, 16ZA0355) and the Scientific Research Fund of Sichuan University of Arts and Science (No. 2014YY001).References
- C. Zhao, X. Wang, J. Kong, J. M. Ang, P. S. Lee, Z. Liu and X. Lu, ACS Appl. Mater. Interfaces, 2016, 8, 2372–2379 CAS.
- J. Shi, Z. Wang and Y. Q. Fu, Electrochim. Acta, 2015, 186, 71–75 CrossRef CAS.
- X. Sun, Z. Wang and Y. Q. Fu, Sci. Rep., 2015, 5, 18712 CrossRef CAS PubMed.
- Z. Wang, X. Niu, J. Xiao, C. Wang, J. Liu and F. Gao, RSC Adv., 2013, 3, 16775–16780 RSC.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- M. S. Balogun, Y. Luo, W. Qiu, P. Liu and Y. Tong, Carbon, 2015, 98, 162–178 CrossRef.
- E. Yoo, J. Kim, E. Hosono, H. S. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277–2282 CrossRef CAS PubMed.
- T. M. Bandhauer, S. Garimella and T. F. Fuller, J. Electrochem. Soc., 2011, 158, R1–R25 CrossRef CAS.
- G. N. Zhu, Y. G. Wang and Y. Y. Xia, Energy Environ. Sci., 2012, 5, 6652–6667 CAS.
- K. Chang and W. Chen, Chem. Commun., 2011, 47, 4252–4254 RSC.
- S. Yang, X. Feng, L. Zhi, Q. Cao, J. Maier and K. Müllen, Adv. Mater., 2010, 22, 838–842 CrossRef CAS PubMed.
- M. Yoshio, H. Wang and K. Fukuda, Angew. Chem., 2003, 115, 4335–4338 CrossRef.
- J. Zhu, R. Duan, S. Zhang, N. Jiang, Y. Zhang and J. Zhu, SpringerPlus, 2014, 3, 1–8 CrossRef PubMed.
- R. Raccichini, A. Varzi, S. Passerini and B. Scrosati, Nat. Mater., 2015, 14, 271–279 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- M. S. Park, J. S. Yu, K. J. Kim, G. Jeong, J. H. Kim, Y. N. Jo, U. Hwang, S. Kang, T. Woo and Y. J. Kim, Phys. Chem. Chem. Phys., 2012, 14, 6796–6804 RSC.
- H. Wang, Y. Yang, Y. Liang, J. T. Robinson, Y. Li, A. Jackson, Y. Cui and H. Dai, Nano Lett., 2011, 11, 2644–2647 CrossRef CAS PubMed.
- K. Chen, S. Song, F. Liu and D. Xue, Chem. Soc. Rev., 2015, 44, 6230–6257 RSC.
- H. K. Chae, D. Y. Siberio-Pérez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, Nature, 2004, 427, 523–527 CrossRef CAS PubMed.
- T. Doi, M. Zhou, L. Zhao, S. Okada and J. I. Yamaki, Electrochem. Commun., 2009, 11, 1405–1408 CrossRef CAS.
- W. Li, Y. Yang, G. Zhang and Y. W. Zhang, Nano Lett., 2015, 15, 1691–1697 CrossRef CAS PubMed.
- Z. Wang, Q. Su, H. Deng and Y. Q. Fu, ChemElectroChem, 2015, 2, 1182–1186 CrossRef CAS.
- Z. Wang, Q. Su, H. Deng, W. He, J. Lin and Y. Q. Fu, J. Mater. Chem. A, 2014, 2, 13976–13982 CAS.
- X. Shao, K. Wang, R. Pang and X. Shi, J. Phys. Chem. C, 2015, 119, 25860–25867 CAS.
- G. Yu, L. Hu, N. Liu, H. Wang, M. Vosgueritchian, Y. Yang, Y. Cui and Z. Bao, Nano Lett., 2011, 11, 4438–4442 CrossRef CAS PubMed.
- G. C. Guo, D. Wang, X. L. Wei, Q. Zhang, H. Liu, W. M. Lau and L. M. Liu, J. Phys. Chem. Lett., 2015, 6, 5002–5008 CrossRef CAS PubMed.
- Y. Cai, G. Zhang and Y.-W. Zhang, J. Phys. Chem. C, 2015, 119, 13929–13936 CAS.
- Z. Zhu and D. Tománek, Phys. Rev. Lett., 2014, 112, 176802 CrossRef PubMed.
- J. Guan, Z. Zhu and D. Tománek, Phys. Rev. Lett., 2014, 113, 046804 CrossRef PubMed.
- Y. Ding and Y. Wang, J. Phys. Chem. C, 2015, 119, 10610–10622 CAS.
- Y. Li and X. Chen, 2D Mater., 2014, 1, 031002 CrossRef.
- Q. F. Li, C. G. Duan, X. G. Wan and J. L. Kuo, J. Phys. Chem. C, 2015, 119, 8662–8670 CAS.
- W. Jin, Z. Wang and Y. Q. Fu, J. Mater. Sci., 2016, 51, 7355–7360 CrossRef CAS.
- T. Ramireddy, T. Xing, M. Rahman, Y. Chen, Q. Dutercq, D. Gunzelmann and A. Glushenkov, J. Mater. Chem. A, 2015, 3, 5572–5584 CAS.
- Z. Yu, J. Song, M. L. Gordin, R. Yi, D. Tang and D. Wang, Adv. Sci., 2015, 2, 1400020 CrossRef.
- B. S. Lee, J. Yoon, C. Jung, K. Y. Kim, S. Y. Jeon, K. H. Kim, J. H. Park, H. Park, K. H. Lee, Y. S. Kang, J. H. Park, H. Jung, W. R. Yu and S. G. Doo, ACS Nano, 2016, 10, 2617–2627 CrossRef CAS PubMed.
- J. M. Soler, E. Artacho, J. D. Gale, A. Garcia, J. Junquera, P. Ordejon and D. Sanchez-Portal, J. Phys.: Condens. Matter, 2002, 14, 2745–2799 CrossRef CAS.
- J. A. White and D. M. Bird, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 4954–4957 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- N. Troullier and J. L. Martins, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 1993–2006 CrossRef CAS.
- M. Hammouri, S. K. Jha and I. Vasiliev, J. Phys. Chem. C, 2015, 119, 18719–18728 CAS.
- D. W. Drumm, A. Budi, M. C. Per, S. P. Russo and L. C. L. Hollenberg, Nanoscale Res. Lett., 2012, 8, 1–11 Search PubMed.
- W. Shi, Z. Wang, Z. Li and Y. Q. Fu, Mater. Chem. Phys., 2016, 183, 392–397 CrossRef CAS.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Butterworth-Heinemann Ltd, Oxford, 2nd edn, 1997, vol. 15 Search PubMed.
- T. Schwabe and S. Grimme, Phys. Chem. Chem. Phys., 2007, 9, 3397–3406 RSC.
- K. T. Chan, J. B. Neaton and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235430 CrossRef.
- Y. Li, X. Wang, W. Shi and Z. Yan, J. Appl. Phys., 2014, 116, 084308 CrossRef.
- V. V. Kulish, O. I. Malyi, C. Persson and P. Wu, Phys. Chem. Chem. Phys., 2015, 17, 992–1000 RSC.
- V. V. Kulish, O. I. Malyi, C. Persson and P. Wu, Phys. Chem. Chem. Phys., 2015, 17, 13921–13928 RSC.
- A. H. Castro Neto, F. Guinea, N. M. R. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81, 109–162 CrossRef CAS.
- J. Sun, H. W. Lee, M. Pasta, H. Yuan, G. Zheng, Y. Sun, Y. Li and Y. Cui, Nat. Nanotechnol., 2015, 10, 980–985 CrossRef CAS PubMed.
- C.-C. Fu, F. Willaime and P. Ordejón, Phys. Rev. Lett., 2004, 92, 175503 CrossRef PubMed.
- J. Zheng, Z. Ren, P. Guo, L. Fang and J. Fan, Appl. Surf. Sci., 2011, 258, 1651–1655 CrossRef CAS.
- J. Ortiz-Medina, F. López-Urías, H. Terrones, F. J. Rodríguez-Macías, M. Endo and M. Terrones, J. Phys. Chem. C, 2015, 119, 13972–13978 CAS.
- H. Guo, W. Zhang, N. Lu, Z. Zhuo, X. C. Zeng, X. Wu and J. Yang, J. Phys. Chem. C, 2015, 119, 6912–6917 CAS.
- Q. Yue, Z. Shao, S. Chang and J. Li, Nanoscale Res. Lett., 2013, 8, 425 CrossRef PubMed.
- S. Meng and S. Gao, J. Chem. Phys., 2006, 125, 014708 CrossRef PubMed.
- F. Valencia, A. H. Romero, F. Ancilotto and P. L. Silvestrelli, J. Phys. Chem. B, 2006, 110, 14832–14841 CrossRef CAS PubMed.
- C. M. Park and H. J. Sohn, Adv. Mater., 2007, 19, 2465–2468 CrossRef CAS.
- D. Datta, J. Li, N. Koratkar and V. B. Shenoy, Carbon, 2014, 80, 305–310 CrossRef CAS.
| This journal is © the Owner Societies 2017 |