Mesoporous carbon nitrides: synthesis, functionalization, and applications
Kripal S.
Lakhi
a,
Dae-Hwan
Park
a,
Khalid
Al-Bahily
b,
Wangsoo
Cha
a,
Balasubramanian
Viswanathan
c,
Jin-Ho
Choy
d and
Ajayan
Vinu
*ab
aFuture Industries Institute, Division of Information Technology, Engineering and Environment, University of South Australia, Mawson Lakes 5095, South Australia, Australia. E-mail: ajayan.vinu@unisa.edu.au; Tel: +61-8-8302-5384
bSABIC Corporate Research and Development Center at KAUST, Saudi Basic Industries Corporation, Thuwal 23955, Saudi Arabia
cNational Centre for Catalysis Research (NCCR), Department of Chemistry, Indian Institute of Technology Madras, Chennai-600036, India
dCenter for Intelligent Nano-Bio Materials (CINBM), Department of Chemistry and Nano Science, Ewha Womans University, Seoul 03760, Republic of Korea
First published on 3rd November 2016
Abstract
Mesoporous carbon nitrides (MCNs) with large surface areas and uniform pore diameters are unique semiconducting materials and exhibit highly versatile structural and excellent physicochemical properties, which promote their application in diverse fields such as metal free catalysis, photocatalytic water splitting, energy storage and conversion, gas adsorption, separation, and even sensing. These fascinating MCN materials can be obtained through the polymerization of different aromatic and/or aliphatic carbons and high nitrogen containing molecular precursors via hard and/or soft templating approaches. One of the unique characteristics of these materials is that they exhibit both semiconducting and basic properties, which make them excellent platforms for the photoelectrochemical conversion and sensing of molecules such as CO2, and the selective sensing of toxic organic acids. The semiconducting features of these materials are finely controlled by varying the nitrogen content or local electronic structure of the MCNs. The incorporation of different functionalities including metal nanoparticles or organic molecules is further achieved in various ways to develop new electronic, semiconducting, catalytic, and energy harvesting materials. Dual functionalities including acidic and basic groups are also introduced in the wall structure of MCNs through simple UV-light irradiation, which offers enzyme-like properties in a single MCN system. In this review article, we summarize and highlight the existing literature covering every aspect of MCNs including their templating synthesis, modification and functionalization, and potential applications of these MCN materials with an overview of the key and relevant results. A special emphasis is given on the catalytic applications of MCNs including hydrogenation, oxidation, photocatalysis, and CO2 activation.
1. Introduction
Carbon nitrides (CNs) are fascinating materials with unique properties including semiconductivity, basicity, high hardness, chemical robustness, and high chemical and mechanical stability. The incorporation of nitrogen in the carbon matrix of CNs not only offers a unique electronic structure with a band gap less than 2.7 eV but also helps to enhance their field emission, photo and basic catalytic, carbon capture, and energy storage properties. The properties of the CN materials are mainly controlled by the structure, composition and crystallinity of the CN framework. As well documented in the literatures, typical CN materials with five different structures have been predicted so far: one is two dimensional graphitic carbon nitrides C3N4 (g-C3N4) and four are three-dimensional CNs, namely α-C3N4, β-C3N4, cubic-C3N4, and pseudocubic-C3N4.1–6 Among these CNs, g-C3N4, a stable layered material made of a CN framework with a structure similar to a graphitic nanostructure, has attracted a lot of attention because of the mild conditions required for the synthesis, its stability and highly versatile physical and chemical properties, and its wide range of application possibilities in various fields including catalysis, hydrogen storage, capture and storage of carbon dioxide, water decontamination, solar energy conversion, and even sensing of toxic molecules. This triggered the researchers to put considerable efforts into the investigation of g-C3N4, which has resulted in in-depth fundamental knowledge acquisition and a series of reports covering topics from the history of CNs to their synthesis, structure and multipurpose applications.There are several methods available for the synthesis of CNs including pyrolysis or polymerization of nitrogen containing molecules including melamine, urea, thiourea, cyanamide (CA), etc. Recently, exfoliation routes have also been successfully developed for the preparation of a single nanosheet of g-C3N4 materials.7–9 However, CN materials with a perfect C3N4 stoichiometry have not been achieved because of the incomplete de-amination or polymerization of the nitrogen precursor. The experimental conditions, nature of the nitrogen–carbon precursors and the degree of polymerization of the nitrogen precursors mainly dictate the crystallinity and the nitrogen content of the CN materials, which have a direct relation with the final properties of these materials. Although five structures of CNs with a C/N stoichiometric ratio of 0.75 have been theoretically predicted, it has been very difficult to prepare such materials without any defect sites or hydrogen contents under mild reaction conditions. This brings about huge debate on the naming of CN materials as the C/N ratio varies from 4.3 to 3 depending on the nature of the precursors and the reaction temperatures used in the synthesis. The definition of CN materials has changed significantly from the ideal C3N4 stoichiometry and now the same has been extended to include compounds such as C3N, C3N2, C10N3, C5N, C3N5, C3N6, C3N7etc., having lower or higher nitrogen content than the ideal C3N4 configuration.10 The higher and lower nitrogen content with respect to the ideal C3N4 is attributed to the presence of structural defects which are affected by the reaction conditions, degree of condensation and relative reactivities of the precursors and nitrogen rich or low cyclic rings in the CN framework. In this review, the CN materials with a C/N ratio higher than 0.75 are called nitrogen low CN materials, whereas the materials with a C/N ratio lower than 0.75 are presented as nitrogen rich CN materials.
Although CN materials exhibit unique electronic and mechanical properties, the non-porous nature together with a relatively low surface area of those CN materials prepared either from molecular or chemical precursors at high temperatures limits the exploitation of these materials for extended applications. Applications of these fascinating materials mainly in the fields of catalysis and adsorption where surface area and pore volume play an important role in controlling their performance are quite limited because of the unusually low surface area (<10 m2 g−1) and specific pore volume due to their nonporous structure. The problems of low surface area and specific pore volume can be overcome by the introduction of porosity in the CN matrix through templating strategies while retaining the carbon and nitrogen chemistry and the basic building blocks of the bulk g-C3N4. The field of CNs has experienced a revolution in the last decade because of the increasing interest in developing porosity in the CN materials as the presence of the porous structure results in higher specific surface areas expanding the scope of application of these materials.
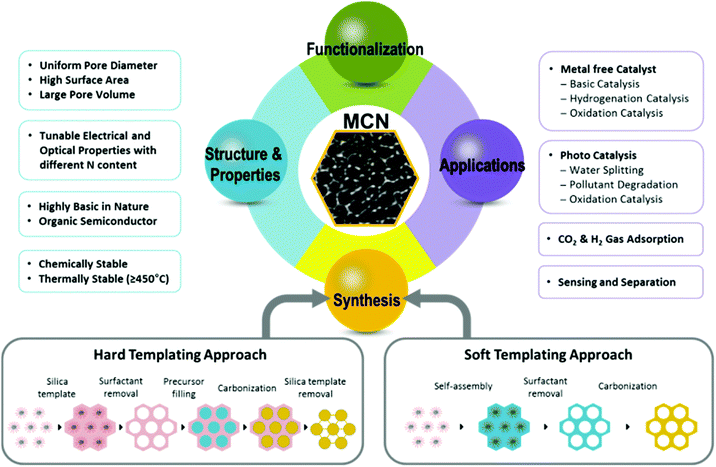
The first report of mesoporous carbon nitrides (MCNs) by Vinu et al. in 2005,11 led to a new class of MCNs, with large specific surface areas and different pore diameters between 2 and 50 nm, which has been considered for a large spectrum of potential applications in the fields of electro, basic and photocatalysis, energy conversion, gas storage, adsorption, and selective sensing and separation owing to their unique electronic, optical, basic, and mechanical properties.11–45 The synthesis of MCNs mainly involves the templating approaches, in which hard or soft sacrificial templates have been utilized to create ordered porosity and a high surface area in the MCN nanostructures.46–47 Over the last decade, intensive research has been carried out to exploit the myriad of inherent structural and textural properties such as ordered structure, extremely high surface areas, narrow pore size distribution, tuneable pore size, uniform particle size, controllable shape and morphology, and also the crystallinity and semiconducting features of the MCNs, and the surface engineering and functionalities including organic and inorganic nanostructures inside the mesochannels of the MCNs.
MCNs prepared through a nanohard templating approach using mesoporous silicas as hard-templates can have specific surface areas of up to 1125 m2 g−1, a higher pore volume of up to 1.8 cm3 g−1 and tuneable pore diameters along with a large number of basic active sites.11,12,29 Furthermore, the MCN materials also exhibit tuneable band gaps, a high basicity along with high chemical and thermal stabilities, and surface functionalities in addition to the high surface area, large pore volume, uniform pore diameter, and controlled morphology, which are critical for obtaining higher performances in various applications including catalysis, sensing and adsorption.
Controlling the valence and conduction band edge of MCNs by simply tuning the quantity of nitrogen in the MCN framework is one of the exciting and challenging research areas that has received a lot of attention in recent years.22,23 Nitrogen content of the MCNs can also be controlled by using precursors with different aromatic or aliphatic molecules with different nitrogen content.11,12,22,23 Recently, MCNs with a nitrogen content higher than the theoretically predicted stable g-C3N4 have also been prepared, which have unique but quite different semiconducting properties than g-C3N4 and are found to be attractive materials for photocatalysis.22,23 These materials are referred to as nitrogen rich MCNs, which opens a new platform that can be used for the manipulation of CN based materials.
In this review, we give an overview of the synthesis, structural and physicochemical properties, as well as the prospects for application of original and functionalized MCNs with different nitrogen contents, structures and pore diameters. As shown in Fig. 1 and summarized in Table 1, the main focus of this review will be on the synthetic strategies for MCNs through templating approaches with various molecular precursors of carbon and nitrogen sources. The fundamental mechanism and principles for the preparation of MCNs are introduced together with a discussion of the respective advantages and disadvantages of synthetic routes, the resulting structural properties, and the key features of these materials. Modifications and functionalization of MCN materials with metals, inorganic metal complexes or organic molecules are also considered in the following section. The selected key examples of MCNs with different functional groups and post-functionalized ones, as well as a comprehensive overview of the state of research with their potential applications are discussed. This review article specifically covers metal free and photo-catalytic applications involving basic catalysis, hydrogenation, oxidation, and water splitting, as well as CO2 uptake for clean energy and environment. It is expected that this review would help the readers to know and understand the pioneering work in the field of MCNs and the current developments spanning the various aspects of MCNs, which may open the door for large-scale applications.
| Name | Template | Precursor | C/N ratio | Structure & morphology | D pore (nm) | S BET (m2 g−1) | V Total (cm3 g−1) | Key feature & application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| MCN-1 | SBA-15 |
• Carbon tetrachloride
• Ethylenediamine |
3.3–4.5 |
• 2D hexagonal
• p6mm symmetry • Turbostratic • Rod |
4.2–6.4 | 505–830 | 0.55–1.25 |
• First 2D MCN
• Tunable textural properties • Highest adsorption capacity • Bifunctional basic and acidic sites • Controlled rod shape • CO2 adsorption capacity |
11–15 and 17 |
| MCN-2 | SBA-16 |
• Carbon tetrachloride
• Ethylenediamine |
4–4.15 | 3D cage | 0.8 | 3.45 | 0.81 | First 3D MCN | 20 |
| MCN-3 | IBN-4 |
• Carbon tetrachloride
• Ethylenediamine |
2.3 |
• p6mm symmetry
• Rod-like |
3.8 | 645 | 0.67 |
• Nano-sized MCN
• Transesterification |
21 |
| MCN-4 | SBA-15 | Amino guanidine | 0.56–0.64 |
• 2D hexagonal
• p6mm symmetry |
3.1–5.8 | 152–321 | 0.30–0.66 | Friedel–Crafts acylation of benzene | 22 |
| MCN-5 MCN-ATN | KIT-6 | 3-Amino-1,2,4-triazine | 0.92 |
• 3D cubic
• Ia3d group |
5.5–6.0 | 472–635 | 0.71–0.99 | Highly selective sensing performance for acidic molecules | 23 |
| MCN-6 | KIT-6 |
• Carbon tetrachloride
• Ethylenediamine |
4.3–4.7 |
• 3D cubic
• Ia3d group • Irregular |
3.5–5.0 | 558–637 | 0.81–0.90 |
• High basicity
• Knoevenagel condensation reaction |
24 |
| MCN-7 | FDU-12 |
• Carbon tetrachloride
• Ethylenediamine |
2.15–2.99 | • 3D cage | 3.4–8.3 | 632–901 | 0.40–1.10 | Large mesopore and high surface area for CO2 capture | 16 |
| UF-MCN | INC-2 |
• Urea
• Formaldehyde |
2.28–2.30 |
• 2D hexagonal
• P6mm group • Graphitic • Disk shape |
2.5–3.5 | 350–500 | 0.30–0.60 | Metal-free oxidation of cyclic olefins with H2O2 | 25 |
| Meso CN spheres | Cellular silica foams |
• Carbon tetrachloride
• Ethylenediamine |
4.55 | Spheres with hierarchical 3D mesostructures | 4.0–43 | ∼550 | ∼0.9 | CO2 uptake | 61 |
| mpg-C3N4 | SiO2 12 nm | Cyanamide | 0.71 | Disordered spherical pore | 12 | 86–439 | — | Cyclotrimerisation of nitriles into triazine and cyclisation of alkynes | 26 and 38 |
| g-CN | Colloidal silica | Cyanamide | 0.72–0.74 | Highly uniform ordered pore | ∼260 | — | — | C3N4 network with tri-s-triazine rings (C6N7) by trigonal N atoms | 63 |
| C3N4-G-r | SiO2 12 nm | Guanidinium chloride | 0.73 | Graphitic | 1.8–13.4 | 146–215 | 0.57–0.84 | Friedel–Crafts acylation reaction | 27 |
| CN-H-SBA15 | SBA-15 | Hexamethylenetetramine | 4.04 |
• 2D hexagonal
• p6mm symmetry |
3.76 | 788 | 0.68 | Transesterification of β-keto esters | 28 |
| DUT-1 | SBA-15 | Hexamethylenetetramine | — |
• 2D hexagonal
• p6mm symmetry |
4.8–11 | 971–1124 | 1.31–1.79 | Superior dehydrogenation catalyst at 750 °C of pyrolysis temperature | 29 |
| 2D-meso-CN | SBA-15 | Cyanamide | 0.88 | 2D hexagonal | 2.78 | 361 | 0.50 | H2 adsorbent | 65 |
| 3D-meso-CN | FDU-15 | 3D cubic | 2.45 | 343 | 0.67 | ||||
| mpg-CN | Silica | Ammonium thiocyanate | 0.75 | g-C3N4 | 11.9 | 129 | 0.49 | Photocatalytic hydrogen evolution | 67 |
| ompg-CN | SBA-15 | 5.3 | 171 | 0.24 | |||||
| mpg-CxNy | Triton X100 | Dicyandiamide | 0.69–0.89 | Graphite-like packing | 3.1–3.4 | 16–116 | 0.05–0.28 | First report on mpg-CxNy by a soft-template | 30 |
| P123 | 0.82–2.06 | 3.8–4.3 | 10–299 | 0.03–0.13 | |||||
| CNBF | BmimBF4 | Dicyandiamide | 0.65 | Sponge-like mesopore | >9.5 | 444 | 0.32 | Metal-free catalysts for cyclohexane oxidation | 31 |
| g-C3N4 | P123 | Melamine | 0.69 | Worm-like pore | 11.0 | 27–90 | — | Photocatalytic H2 evolution | 43 |
| CN-T | Triton X100 |
• Melamine
• Glutaraldehyde |
— | Graphite-like structure | 3.8 40 | 172–221 | 0.02–0.21 | Lipase immobilization support | 72 |
2. Syntheses of MCNs
The introduction of well-ordered porosity in the CN framework is quite challenging. A templating strategy is generally used for creating well-ordered porosity in the CN materials.11–13,47 In this section, we will discuss the synthesis methods and templating strategies as applicable to MCNs, as reported in the literature to date. Two well-established techniques are employed for the synthesis of porous materials including siliceous as well as non-siliceous techniques. These are hard-templating (nanocasting or replication) and soft-templating (self-assembly) approaches. Both approaches are illustrated in Fig. 1. Soft templating involves the use of organic structure directing agents, which are self-assembled with C and N precursors to form an ordered structure. In this case, porosity is generated upon removing the surfactants through ethanol washing or a calcination process. On the other hand, the hard templating strategy uses highly ordered 2D or 3D porous solid materials to replicate their structure into CN nanostructures. Generally, mesoporous silica materials such as SBA-15, FDU-12, MCM-41, and MCM-48 are used as the hard templates while organic molecules and/or supramolecules, such as amphiphilic surfactants and block copolymers, are used as soft templates. In the following section, we will discuss the processes involved in these templating strategies.2.1 Hard templating approach
The nanocasting technique using a hard template is the most widely reported and successfully applied method used for the introduction of mesoporosity in solid materials such as carbons, nitrides, polymers and ceramics.48,49 Casting as a process to make industrial goods and household items has been known to humans for several centuries. In the casting process, a rigid mold made of a suitable material such as wax, metal or plaster is filled with the material that is to be cast. After the necessary processing time, the mold is removed and a negative replica of the mold bearing all the features of the mold is obtained. Large concrete underground drainage pipes, plaster, toys, sculptures, and pots are typically made using this casting process. Nanocasting is conceptually identical to the casting process described above. However, the two differ in terms of the length scale involved. While casting is predominantly done on a macroscopic scale, nanocasting on the other hand is done on the nanoscale and hence the prefix “nano” is used while referring to the casting process as applied to the synthesis of materials with nanodimensions. The word “template” is widely accepted by the material science community as a substitute for the word mold when referring to casting in the nanoscale. Based on the physical state of the template, a template could be called a hard template if it is a solid or a soft template if it is a liquid. Nanocasting as a synthetic route for nanomaterials involves the following three key steps: (a) synthesis of the ordered mesophase silica template; (b) infiltration of the template with the necessary precursors, including the conversion of precursors into a solid; and (c) removal of the template.50The discovery of the mesoporous silica family M41S51,52 by Mobil scientists ushered in a new area of synthesis of non-siliceous mesoporous materials with a large surface area such as ordered mesoporous carbons (OMC) with different structures (CMK-1 and CMK-3) via the so-called nanocasting approach where mesoporous silicas such as MCM-48 and SBA-1553–57 are used as the templates.58,59 In 1999, Ryoo et al. and Hyeon et al. realized the potential of nanocasting in the area of mesoporous materials and independently reported the first ordered mesoporous carbon with a 3D structure (CMK-1) using MCM-48 as the hard template employing the nanocasting strategy.57,58 Ryoo et al. used sucrose as the carbon source and mesoporous silica such as MCM-48, SBA-15, and SBA-154,55,57 as templates to produce a series of materials represented as CMK-x. Hyeon et al. also introduced a similar strategy at the same time and reported new well-ordered mesoporous carbons that are represented as SNU-x.59 However, it is worth mentioning here that the nanocasting strategy using zeolite Y as a hard template was already adopted by Kyotani et al. for creating nanoporosity in the carbon materials before Ryoo et al. and Hyeon et al.58,59 Interestingly, these porous carbon materials were microporous in nature but exhibited a high specific surface area of ∼2200 m2 g−1.60
In 2005, this simple nanocasting strategy was first successfully utilized by Vinu et al. for creating mesoporosity in the CN nanostructures, and reported the preparation of the first ever hexagonally-ordered MCN materials (MCN-1) with a uniform pore diameter, a high surface area, and a large pore volume through a simple polymerization reaction between ethylenediamine (EDA) and carbon tetrachloride (CTC) using SBA-15 with various pore diameters as the templates (Fig. 2).11 The structure of MCN-1 exhibited a two-dimensional hexagonal lattice (p6mm), and was also similar to that of the ordered mesoporous carbon, CMK-3, composed of ordered carbon nitride rods. The BET surface area (505 m2 g−1) and the specific pore volume (0.55 cm3 g−1) of MCN-1 are significantly higher than those of non-porous bulk g-C3N4. It should be noted that the pore size of MCN-1 is 4.0 nm, which is much lower than that of the mesoporous silica template SBA-15 (9.1 nm) but similar to the wall thickness, revealing the transformation of silica walls into pores and the pores of the templates into CN walls. This confirms the replication of the structure of the mesoporous template into the MCN nanostructures.
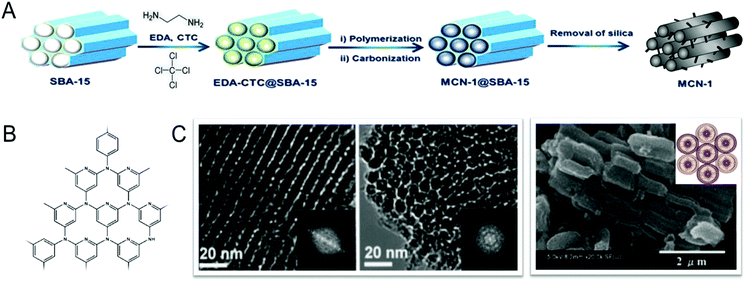 | ||
| Fig. 2 Synthesis of MCNs using a hard templating approach. (A) Preparation of the MCN-1 using SBA-15 with EDA and CTC. Modified and reprinted with permission from ref. 15. Copyright 2015, Nature publishing group. (B) Highly idealized schematic wall structure of the MCN-1, and (C) HR-TEM and HR-SEM images of the MCN-1 samples prepared at different EDA to CTC weight ratios. Modified and reprinted with permission from ref. 11 and 12. Copyright 2005 and 2008, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The pore diameter, specific surface area, nitrogen content and specific pore volumes of the MCNs can also be tuned with a simple adjustment of the pore diameter of the template and the amount of infiltration of the C and N sources into the pores of the templates. Vinu et al. reported the effect of pore tuning of the mesoporous silica template on the surface area, pore diameter and nitrogen content of MCNs. The pore diameter, surface area and nitrogen content of MCN-1 was tuned by synthesizing SBA-15 silica templates at three different temperatures, 100, 130 and 150 °C, and using these to synthesize MCN-1.12 The template materials prepared at different temperatures have different pore diameters, pore connectivity and surface textures which result in a huge difference in the specific surface area and pore volume of the MCN-1 samples. It was reported that the mesoporous silica template with the largest pore diameter can support the formation of MCN-1 with the largest pore diameter. This is mainly due to the incomplete filling of the C and N precursors in the mesoporous silica template with the largest pore diameter. It was also demonstrated that the nitrogen content of MCN-1 can be finely controlled by varying the amount of infiltration of the nitrogen precursor, EDA, as it controls the degree of polymerization of the C and N precursors. When a high amount of EDA was used, MCN-1 with the highest nitrogen content can be prepared. This is due to the fact that the large quantity of EDA can enhance the degree of polymerization between EDA and CTC, which limits the release of nitrogen from the completely polymerized CN matrix upon carbonization.
In 2007, Vinu et al. reported 3D cage type MCNs using SBA-16 as the silica template and CTC and EDA as the carbon and nitrogen sources; the material was called MCN-2 (Fig. 3A).20 In comparison to MCN-1, MCN-2 shows a much higher specific surface area of 810 m2 g−1 and a relatively higher total pore volume of 0.81 cm3 g−1. It is evident that the 3D cage type structure of SBA-16 significantly contributed to the higher specific surface area and pore volume of MCN-2. Based on the chronology of the reports, MCN-2 has better textural properties than MCN-1. However, MCN-1 and MCN-2 exhibit relatively lower nitrogen content due to the low thermodynamic stability of nitrogen in the MCNs, as nitrogen prefers to stay as a nitrogen molecule rather than connecting with a carbon of the CN matrix. In addition, the large size of the particle also does not favour a higher incorporation of nitrogen in the CN matrix as it hinders the complete polymerization of C and N precursors due to the incomplete filling of the deep parts of the pores with the required precursors.
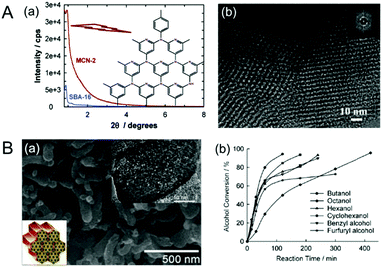 | ||
| Fig. 3 (A) MCN-2. (a) Powder XRD patterns of the SBA-16 and MCN-2 with a highly idealized schematic structure and (b) HR-TEM image of the MCN-2. Reprinted with permission from ref. 20. Copyright 2007, American Chemical Society. (B) MCN-3. (a) HR-SEM and HR-TEM images of the MCN-3, and (b) basic catalytic performance of MCN-3 in the transesterification of β-keto esters of different alcohols. Reprinted with permission from ref. 21. Copyright 2009, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
As the nitrogen content in MCN-1 and MCN-2 was low, Vinu et al. adopted a new “nanoparticle” strategy for increasing the nitrogen content in MCNs by using ultra-small size mesoporous silica nanoparticles through a hard templating nanocasting technique using the EDA and CTC polymerization approach and the material was called MCN-3 (Fig. 3B).21 The template used for the synthesis of MCN-3 was prepared by using a mixture of Pluronic P123 and Fluorocarbon-4 (FC-4) surfactants. The nitrogen content of MCN-3 (C/N ratio = 2.3) is much higher than that of MCN-1 and MCN-2 (C/N = 4.5), which were prepared using an identical polymerization process as discussed earlier. It was surmised that the small nanoparticles of the template helped to retain a huge quantity of nitrogen in MCN-3 as it requires more energy to break the CN bond in the nanoparticles than in the large particles. However, the C/N ratio of MCN-3 is still lower than that of the theoretically predicted carbon nitride (C/N = 0.75).
Vinu et al. overcame the problem of lower nitrogen content in the MCNs by following a twofold strategy: (1) lowering the carbonization temperature and time; (2) using a single carbon nitrogen precursor as a polymerization species inside the pore channels of the mesoporous silica template.22,23 A single molecular precursor with a high nitrogen content namely amino guanidine was used to prepare MCN-4 using SBA-15 as the template (Fig. 4). Interestingly, the C/N ratio of MCN-4 was determined to be 0.64 through electron energy loss spectra (EELS), which is slightly lower than that of the ideal C3N4 (C/N = 0.75). This high nitrogen content in the MCN-4 samples was mainly due to the combination of low reaction temperature (400 °C), and the high nitrogen containing precursor that easily undergoes polymerization and offers a highly thermally stable diamino-s-tetrazine moiety that is linked trigonally with the nitrogen atoms. The reduction of temperature from 600 °C to 400 °C and the change of CN precursors make a huge difference in the CN stoichiometry of the MCN samples.22
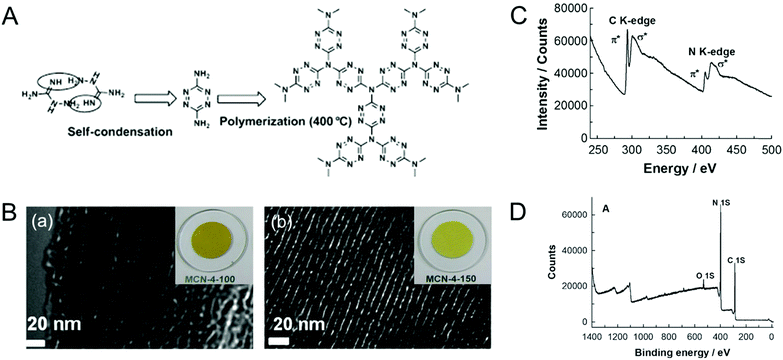 | ||
| Fig. 4 (A) Mechanism of the formation of MCN-4 (highly idealized). (B) HR-TEM images of the (a) MCN-4-100, (b) MCN-4-150 with their color photos, (C) EELS of MCN-4-150, and (D) XPS survey spectra of MCN-4-150. Reprinted with permission from ref. 22. Copyright 2012, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Vinu et al. also tried to make MCNs using 3-amino-1,2,4-triazine (ATN), which is an aromatic precursor, and KIT-6 as the mesoporous silica template at a reaction temperature of 400 °C. Unfortunately, ordered MCNs were not obtained. Therefore, the reaction temperature was increased from 400 °C to 500 °C and a highly ordered MCN-5 sample with a crystalline graphitic CN framework was obtained (Fig. 5A).23 Surprisingly, the C/N ratio of MCN-5 was 0.92, which is almost similar to that of the C3N4 (0.75) but much lower than that of MCN-1, MCN-2 and MCN-3. This can be explained by the fact that the use of a cyclic aromatic single precursor such as ATN would significantly reduce the amount of energy required to frame cyclic triazine units in the wall structure and at the same time, the presence of a large amount of nitrogen in the cyclic structure of the ATN molecule would boost the nitrogen content in the final product. The difference in the nitrogen content of MCN-4 and MCN-5 is mainly attributed to the difference in the reaction temperature and the C/N ratio of the initial precursors. These results reveal that the choice of the reaction temperature and the CN precursor is highly critical, which has the capability to tune the crystal structure and the nitrogen contents of the final products.
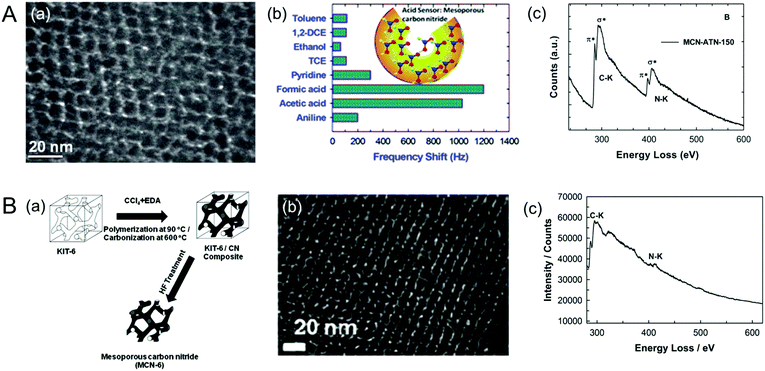 | ||
| Fig. 5 (A) (a) HR-TEM image of MCN-5 (MCN-ATN-150), (b) its highly selective sensing performance for acidic molecules, especially formic acid, and (c) EELS of MCN-5-150. Reprinted with permission from ref. 23. Copyright 2013, Royal Society of Chemistry. (B) (a) Scheme for the synthesis, (b) HR-TEM image of the MCN-6, and (c) EELS of MCN-6-150. Reprinted with permission from ref. 24. Copyright 2012, Royal Society of Chemistry. | ||
It was also attempted to tune the nitrogen content of the MCNs by changing the structure of the template from 2D to 3D. MCN-6 was prepared by using KIT-6, a 3D mesoporous silica with a large pore diameter, prepared using the Pluronic surfactant with a co-solvent and following an identical procedure as for the synthesis of MCN-1, MCN-2, and MCN-3 (Fig. 5B).24 It was found that the C/N ratio of MCN-6 is identical to that of MCN-1 and MCN-2, revealing that the structure of the template does not affect the final nitrogen content of the product.
The morphology of the MCN was also controlled by using porous templates with different morphologies. Park et al. reported the synthesis of MCNs using the nanocasting technique with disk-shaped 2D-hexagonal mesoporous silica (INC-2) as a hard template in a polymerization reaction involving urea and formaldehyde as the double molecular carbon and nitrogen precursors (Fig. 6A).25 This synthesis involved the successful replication of the disk-shape morphology of the silica template in the MCNs. The nitrogen content and the specific surface area of these materials can also be tuned by varying the amount of urea in the synthesis mixture.
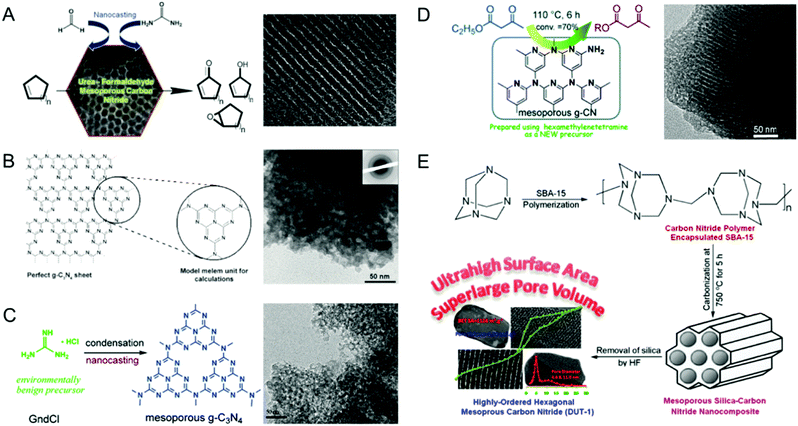 | ||
| Fig. 6 Various MCNs with different hard templates and precursors and their TEM images. (A) UF-MCN synthesized using INC-2 with urea and formaldehyde and metal-free catalytic oxidation of cyclic olefins. Reprinted with permission from ref. 25. Copyright 2013, Elsevier B. V. (B) mpg-C3N4 synthesized using SiO2 nanoparticles with cyanamide (CA). Reprinted with permission from ref. 26. Copyright 2006, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) C3N4-G-r synthesized using SiO2 nanoparticles with guanidinium chloride. Reprinted with permission from ref. 27. Copyright 2013, Royal Society of Chemistry. (D) CN-H-SBA15 synthesized using SBA-15 with hexamethylenetetramine. Reprinted with permission from ref. 28. Copyright 2014, Royal Society of Chemistry. (E) DUT-1 synthesized using SBA-15 with hexamethylenetetramine. Reprinted with permission from ref. 29. Copyright 2014, American Chemical Society. | ||
Zhao and co-workers also used double molecular precursors namely CTC and EDA as the carbon and nitrogen sources and spherical mesoporous cellular silica forms (MCFs) as the hard templates via the nanocasting technique.60 These materials showed spherical morphology with a reasonably high BET surface area of 550 m2 g−1, indicating a successful replication process. They also exhibited 3D mesostructures with bimodal distribution with small and large mesopores with pore diameters of ca. 4.0 and 43 nm, respectively. Interestingly, the nitrogen content of these materials is almost similar to those of MCN-1 and MCN-2, indicating that the morphology of the materials does not affect the nitrogen content of the final product. However, the mesopore volume of these samples was much higher than that of MCN-1, which is due to the large mesopores in the samples. In both of these approaches, the morphology of the templates is replicated in the MCNs, which significantly alters the textural properties of the samples.
Following the first report by Vinu et al. Goettmann and Antonietti co-workers in 2006 also reported the synthesis of disordered MCNs using silica nanoparticles (12 nm, SiO2 particles from commercially available Ludox HS40, Aldrich) as the hard template and liquid CA as a single molecular precursor (Fig. 6B).26 The material possesses a pore size of 12 nm and the surface area varies between 86 and 439 m2 g−1 depending on the precursor to silica weight ratio. Compared to the MCN-x (1, 2, 3, 6) reported by Vinu et al., the surface area of these materials is low. However, the nitrogen content of these materials is much higher than that of MCN-1 and is closer to the theoretically predicted nonporous C3N4 but with a lot of hydrogen in the CN wall structure. This interesting feature made these materials quite attractive for photocatalytic applications. The same group had previously reported the facile synthesis of graphitic C3N4 using dicyanamide (DiCA) as the precursor.62
Not only mesoporsity, but also macroporosity was introduced in the MCN nanostructures. Hwang et al. realized this idea and synthesized macroporous carbon nitride via infiltration of liquid CA in the interstitial voids of colloidal silica crystals which were used as the templates under a static vacuum at 85 °C, followed by annealing of the mixture at 550 °C for 3 h and the removal of the template through treatment with aqueous HF.63 Elemental analysis and EDX measurements showed a C/N ratio of 0.72 and 0.74 respectively, which is similar to the nitrogen content of the theoretically predicated C3N4. It was claimed that the structure of the MCN prepared using this route consists of tri-s-triazine rings (C6N7) which are cross-linked by trigonal N atoms. However, there is a lack of evidence in their assumption as the reported materials were not characterized using the main tools such as XPS or EXAFS. Although the use of CA as a precursor in the preparation of MCNs ensures a high nitrogen content, it suffers from various drawbacks including high cost and an explosive nature besides being toxic. These factors strongly inhibit the use of CA on a large commercial scale for the synthesis of MCNs.
Xu and co-workers proposed a novel and environmentally benign precursor guanidine salt and colloidal silica nanoparticles as hard templates for the synthesis of disordered MCNs.27 The resulting MCNs exhibited a disordered mesoporous structure with bimodal pores (1.8 and 13.4 nm), surface areas between 146 and 215 m2 g−1 and pore volumes in the range 0.57–0.84 cm3 g−1 (Fig. 6C). The disorder in the structure might be attributed to the low periodic mesoporosity in the colloidal silica templates. They further reported that the amount of silica template plays a critical role in controlling the specific surface area and pore volumes of the final product. Interestingly, the bulk C/N molar ratio of these samples was found to be in the range of 0.7 and 0.73, indicating slightly higher nitrogen content than in bulk g-C3N4. In 2014, the same researchers also used hexamethylenetetramine (HMT) as a single molecular precursor for the synthesis of MCNs using SBA-15 as the hard template via the nanocasting technique (Fig. 6D).28 The resulting MCN showed a very high surface area of 788 m2 g−1 and a pore volume of 0.68 cm3 g−1. The nitrogen content of the samples is low and similar to the content in MCN-1 and MCN-2. The prepared materials also showed a highly ordered mesoporous structure which is similar to that of the template, confirming the successful replication of the ordered structure of the silica template into the MCNs.
Zhao et al. also reported the synthesis of highly ordered MCNs with ultra-high surface areas using the same HMT as the C and N precursors and SBA-15 as the hard template in the nanocasting process (Fig. 6E).29 The materials were denoted as DUT-1-T, where T denotes the polymerization pyrolysis temperature 600, 750 and 900 °C whereas DUT denotes the Dalian University of Technology. DUT-1 showed an exceptionally high surface area in the range of 971–1124 m2 g−1, a super large pore volume in the range of 1.31–1.79 cm3 g−1 and high nitrogen content in the range of 9.2–23% besides bimodal pore size distribution. It was reported that the pyrolysis temperature played a key role in controlling the surface area and pore volume of the DUT-1 samples. The nitrogen content was significantly reduced from 23 wt% to 9.3 wt% as the carbonization temperature was increased from 600 to 900 °C. Among the three samples, DUT-1-900, which was prepared at 900 °C, exhibited the highest surface area and pore volume. However, the specific surface area of the samples reported by Xu and his co-workers was quite different although both the research teams used the same HMT as the C and N precursors and SBA-15 as the hard template. This was attributed to the perfect crosslinking of the C and N precursors and the huge release of nitrogen, leaving a lot of microporosity in the CN walls, at a high temperature. It is also surmised that the difference in the pyrolysis temperature and the ratio of SBA-15 and HMT makes a huge difference to the final properties of the MCN. Although the CN source used in this synthesis of MCNs was different from that used for MCN-1,11 both the materials prepared at 600 °C showed almost similar textural and crystal structure properties.
Park and Zhao et al. modified the nanocasting technique slightly by using a controlled pore filling technique and the incipient wetness method (IWM) without using any additional solvent.64 Liquid CA was used as the single molecular precursor and 2D-hexagonal SBA-15 and 3D-cubic mesoporous silica were used as the hard templates. The advantage of the IWM is that only a calculated amount of the precursors is needed to efficiently and effectively fill the meso-channels of the template and there is no accumulation of the precursor outside the meso-channels, thereby avoiding the formation of unwanted amorphous particles. The C/N ratio of the MCN was found to be 0.88 after carbonization at 550 °C for 3 h. The specific surface area for the MCN from SBA-15 and 3D cubic mesoporous silica is 361 and 343 m2 g−1, respectively, which is much lower than that reported by Vinu et al. The pore volumes are 0.5 and 0.67 cm3 g−1 respectively. The pore diameter was 2.78 nm for MCNs prepared from SBA-15 and 2.45 and 8.03 nm for MCNs prepared from 3D-cubic mesoporous silica. Unfortunately, the XRD and nitrogen adsorption data of these samples revealed that the samples prepared using the IWM exhibited a disordered mesoporous structure which is due to the fact that the IWM is not an effective method for the perfect filling of the mesopores, which is critical to obtain well-ordered mesopores.
Lee et al. claimed that MCNs can be prepared using a single molecular precursor, urea through nanocasting using colloidal silica nanoparticles with a size of 7 mm (commercially available Ludox SM-30) as the hard template.65 The samples were annealed for 4 h at 550 °C to obtain yellow coloured MCNs, which were found to be photoactive. The BET specific surface area of these materials was found to be in the range of 41 and 224 m2 g−1 and was related to the ratio of urea to silica. The absence of low angle XRD and CHN analysis data in the report resulted in a failure to make any conclusions on the mesostructural order and the composition of the sample.
Researchers have also tried to prepare MCNs without the aid of mesoporous silica templates and its nanocomposites with inorganic metal oxides such as silica. Normal silica precursors can be used to create the mesoporosity through a simple interpenetration in the condensed form of the CN precursors. Kailasam and co-workers reported the synthesis of a MCN–silica composite following a combination of sol–gel and thermal condensation approaches using silica and CN precursors.66 Interestingly, the procedure involved a simple mixing of CA and the silica precursor tetraethyl orthosilicate (TEOS), and the subsequent evaporation of the solvent at 80 °C following polymerization of the mixture at 550 °C for 4 h to give a silica–carbon nitride composite. The silica–carbon nitride composite could be treated with aqueous NH4HF2 to give a pure disordered MCN and the same composite, if heated to 650 °C for 5 h, could yield pure silica. The interpenetration of the silica within the CN framework generates disordered mesoporosity after heat or silica removal treatment. The composite materials as well as the pure carbon nitride showed disordered mesopores and the specific surface area varied between 77 and 270 m2 g−1 depending on the ratios (3, 6, or 12) of TEOS with CA. However, no porosity was observed when a large amount of the silica source was added in the synthesis mixture.
MCNs with different electronic properties can also be prepared by using a CN precursor containing sulphur. Cui and Antonietti co-workers realized this amazing opportunity for introducing sulphur into the framework of MCNs. A single molecular precursor containing sulphur, ammonium thiocyanate, was used for the synthesis of an ordered mesoporous graphitic carbon nitride (ompg-CN) and a mesoporous graphitic carbon nitride (mpg-CN) using SBA-15 and nano-sized silica (commercially available as 12 nm, Ludox HS40) respectively as the hard template via the nanocasting technique.67 Interestingly, the C/N ratio of ompg-CN was found to vary between 0.74 and 0.77, with the average being very close to the ideally predicted 0.75 for the perfect C3N4 structure. However, the surface area exhibited by these materials was not so impressive lying in the range of 129 to 239 m2 g−1 and with a pore volume varying between 0.24 and 0.82 cm3 g−1. It is believed that the elemental sulphur can escape as H2S and provide accelerated and improved condensation of the intermediates such as dimelamine to form the CN materials through a simple deamination route.
Recently, Yang et al. reported a very simple sol–gel based synthesis of MCNs using single molecular amino cyanide as the C/N precursor and colloidal silica as the hard template.68 It was found that the silica removal process with NH4HF2 is an important step as it can control the morphology and the mesostructure of the final product. The treatment of the MCN–silica nanocomposite with NH4HF2 at different time intervals can convert the MCN into low dimensional nanostructured CN materials, which is attributed to the anisotropy self-decomposition through a possible acidic hydrolysis of the CN frameworks. A longer NH4HF2 treatment time favors the formation of hierarchical mesostructures and pores of more than 150 nm can be obtained. It should be noted that the BET surface of MCNs prepared using this route was not that high (185.5 m2 g−1), but had pore diameters in the range of 10 to 150 nm.
Although different hard templates including silica precursors, mesoporous silica templates, colloidal silicas, and silica nanoparticles were utilized for the synthesis of MCNs using various C and N precursors, this hard templating approach requires the toxic and expensive template removal process that involves hazardous HF or ammonium bifluorides. It is not only considered to be a time consuming and hazardous process, but also a non-environmentally friendly process, which may be a hurdle for the translation of these researches into commercialization of real world products. Therefore, it is imperative to find eco and environmentally friendly approaches to prepare MCNs.
2.2 Soft-templating approach
A soft-templating strategy has been widely applied for the synthesis of various mesoporous materials including inorganic metal oxides and mesoporous carbons. This soft templating strategy was simultaneously realized by Dai et al. and Ikkala et al., and Zhao et al. reported the first synthesis of porous carbon structures and films via the self-assembly of block copolymers (BCPs).69–71 Unlike the widely reported hard template based nanocasting procedures for the synthesis of MCNs, reports on the use of soft templates for the synthesis of MCNs are quite limited. The main reasons for the little success reported using soft templating based synthesis of MCN materials in particular are attributed to the thermodynamics of the synthesis process and the condensation of the C and N precursors in the presence of surfactants, as well as physiochemical factors such as decomposition of the soft template during pyrolysis. This may either contaminate the final products with more carbons or collapse the mesostructure due to the strong interaction between the soft templates and the CN polymerized frameworks. These drawbacks can be overcome by using an extraction procedure involving sulphuric acid as an alternate route to remove the soft template.69The soft templating strategy was realized by Antonietti et al. for the preparation of MCNs via simple self-assembly between the organic structure directing agents and the CA (Fig. 7A).30 A host of ionic and non-ionic surfactants and amphiphilic block polymers such as P123, P127, Triton X-100, Brij30, Brij58, and Brij76 were used as structure directing agents for the preparation of the MCNs. A stepwise calcination program was applied in order to avoid the collapse of the mesoporous structure of the MCNs during the surfactant removal process. It should be noted that when the traditional soft-templating method was used, the yield of the MCN product was almost zero. This low yield is due to the side reactions between the CN polymerized framework and the surfactants, which makes the whole self-assembled unit highly volatile. It was quite interesting to note that only the Triton X-100 based soft template offered mesostructured CNs without any micropores, and the BET surface area and the pore diameter were reported to be 76 m2 g−1 and 3.8 nm, respectively. No micropores were present in the sample but some large pores up to 15 nm were observed. The porous CN prepared using the P123 surfactant yielded only micropores and the BET surface area was found to vary from as low as 8 m2 g−1 to 299 m2 g−1 depending on the template to precursor ratio. This was quite surprising as P123 generally offers a well-ordered mesostructure. It was assumed that the robust self-assembly of the surfactant was not formed when it was added together with the CN precursors and the chemical reaction between the surfactant and the CN polymerized frameworks is also responsible for the collapse of the structure during template removal. It has been observed that the carbon content of the final product is much higher when P123 was used as the soft-template. This confirms the reaction between the surfactant and the CN precursors. Interestingly, the conducting properties of these materials are much higher than that of the samples prepared using the nano hard-templating approach.
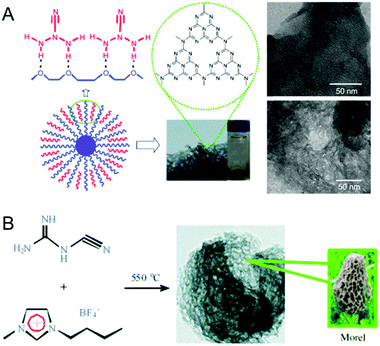 | ||
| Fig. 7 Synthesis of MCNs using the soft templating approach. (A) Facile one-pot synthesis and TEM images of the mpg-CxNy by using P123 (top) or Triton X100 (bottom) with dicyandiamide. Reprinted with permission from ref. 30. Copyright 2010, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Synthesis and TEM images of the boron- and fluorine-containing MCN (CNBF) by using 1-butyl-3-methyl-imidazolium tetra-fluoroborate with dicyandiamide. Reprinted with permission from ref. 31. Copyright 2010, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The same group also demonstrated the use of ionic liquids (IL) as soft templates for the preparation of MCNs, as ILs are thought to be the excellent templates for the synthesis of mesoporous materials (Fig. 7B).31 1-Butyl-3-methylimidazolium tetrafluoroborate was used as the soft template and DiCA as the organic precursor to prepare boron and fluorine containing MCNs with a C/N ratio of around 0.65. The material reportedly exhibited well-pronounced mesopores with a BET surface area as high as 444 m2 g−1 and a total pore volume of 0.32 cm3 g−1. Interestingly, unlike the material prepared from Triton X-100, microspores were completely absent. The surface area could be varied by changing the template to precursor ratio. ILs offered nanoporous textures in MCNs even without using a step-wise calcination step. This may be attributed to the poor volatility of the ILs, which limits the strong reaction between the CN network and the ILs. When an IL with a DiCA counterion was used as the soft-template, the carbon content of the final product was significantly increased due to the polymerization of the anions present in the IL.
Recently, Yan reported MCNs with a worm-like porous structure using Pluronic P123 as the soft template and melamine as the single molecular precursor.43 The BET surface area was found to be 30 m2 g−1 and it increased to 90 m2 g−1 after calcination at 500 °C. It was reported that the nitrogen adsorption–desorption isotherms are type IV, which are typically observed for the mesoporous materials but the capillary condensation step in the isotherm was not steep, revealing the absence of well-ordered mesoporosity in the sample. It may be worth noting here that Antonietti et al. did not find any mesoporosity in the materials synthesized using P123 as the soft template and DiCA as the precursors, and the BET surface area was attributed to the presence of micropores only.31 Interestingly, elemental analysis showed that the C/N ratio of this sample was 0.65, which is lower than the theoretical C/N ratio of ideal C3N4 (0.75). It should also be noted that the carbon content in the MCNs prepared by Yan (C/N = 0.65) is much lower than that of the MCNs prepared by Antonietti et al.30 The main reason for the difference in the carbon content of the samples prepared by these two groups may be due to the nature of the precursors used in the synthesis. This means that the volatility of the self-assembled unit may be controlled by using different CN precursors.
Shen et al. reported the soft template synthesis of MCNs with bimodal pore size distribution using Triton X-100 as the soft template and melamine and glutaraldehyde as the double molecular precursors.72 These materials show type IV isotherms, indicating the presence of mesoporosity in the material. However, the BET surface area for the two samples carbonized at 600 and 800 °C are reported as 172 and 221 m2 g−1, which are much higher than those of the MCNs reported by Zhang and Antonietti et al.30 who also used Triton X-100 as the soft template and DiCA as the precursor. The higher surface area was attributed to the decomposition of Triton X-100 during the carbonization process. The mesopore sizes were found to be 3.8 nm and 10–40 nm, which originated from the removal of the polymeric surfactant and the intraparticle space between the plate-like particles of the MCN, respectively. The FT-IR spectra revealed that the nitrogen atom is present as C–N and N–H chemical groups suggesting a polar nature of the material. The absence of the low angle peak of the samples suggested that the samples possess disordered mesopores. This observation was also consistent with the nitrogen adsorption isotherm of these samples, which did not show any steep capillary condensation step.
The above results reveal that the soft-templating strategy, even though it is an eco and environmentally friendly approach, mostly offers disordered MCNs with a low specific surface area and specific pore volume. In addition, the samples mostly have either small pores that originate from the surfactants, or ultralarge pores originating from the intraparticle space of the aggregated particles, which can be altered with a simple adjustment of the pyrolysis temperature.
2.3 Template-free approach
Generally, soft or hard templates are required to introduce the ordered porosity in CN materials. However, this templating process involves tedious synthesis procedures, long synthesis time and high cost, which put a major hurdle in the way of commercialization. Recently, many efforts have been made to introduce mesoporosity in CNs using a template free approach. The template-free approach as the name implies does not involve any kind of hard or soft template for the synthesis of the MCNs. The process essentially relies on the experimental conditions, especially on the type of energy used in the reactions including thermal, sonochemical and low energy acoustic waves, which dictate the extent of mesoporosity (more precisely nanoporosity) in the MCN materials. This approach has been gaining reasonable momentum recently as it has the potential to reduce the cost of the synthesis of MCNs to a low level by eliminating the expensive templates and the hazardous template removal process which normally forms a key step in the hard and soft templating approaches discussed in the earlier sections. However, the template free approach is not quite as successful as the hard templating or soft-templating approach due to the poor textural properties of the final MCN products, but is undergoing continuous development. In this section, we briefly touch upon the essence of the template free approach with some relevant examples.Lu et al. employed the template free approach for the synthesis of MCNs by directly heating urea at ambient pressure to 600 °C at 10 °C min−1 and holding the temperature at 600 °C for 4 h.73 The resulting powder sample was simply washed with water and anhydrous ethanol followed by filtration and drying at room temperature. The wide angle XRD pattern exhibited a sharp peak suggesting a well pronounced graphitic character but the N2 adsorption–desorption isotherm revealed disordered mesopores in the sample with no sharp capillary condensation step. The BET specific surface area was found to be 51.6 m2 g−1, which is much lower than that of MCNs prepared using hard or soft templates. Mesoporosity can be introduced on the non-porous CN materials with the simple treatment of sonication. Kumar et al. realized this approached and obtained MCNs with a surface area higher than 112 m2 g−1 and a large pore volume of 0.39 cm3 g−1.74 Although these results are impressive, the absence of a low angle XRD pattern raises some concerns about the ordered nature of the material. These results imply that it is still a challenge to prepare highly ordered MCNs with excellent specific surface areas and uniform pore diameters without any templates.
3. Modification and functionalization of MCNs
The surface functionalization of various mesoporous materials including mesoporous silica, mesoporous carbons or mesoporous organic/inorganic hybrid materials has expanded their applications in many fields through simple modification with organic molecules, inorganic metal or metal oxide nanoparticles, and bio-molecules.15,18,35,75–87 These modifications allow fine tuning of the bulk and interfacial properties of the parent materials via a simple interaction with the guest molecules. As MCNs are fascinating materials with electronic, basic, and photo electrochemical properties, the surface functionalization of these materials could elevate the possibilities of their application in various fields. It is expected that the techniques or approaches used for the functionalization of the existing mesoporous materials including silica or carbons can be utilized to modify the properties of MCNs. However, the mechanical, thermal and chemical stabilities of the CN framework structure of MCNs are quite different from mesoporous carbons or silica, which could pose new challenges in the functionalization and characterization of functionalized MCNs. This is clearly reflected in the minimum number of reports on the functionalization of MCNs. A few techniques including impregnation, adsorption–desorption, and photo-assisted reduction processes have been employed to functionalize MCNs with metal or metal halide nanoparticles inside the mesochannels. In this section, we will review methods for functionalizing MCNs with different metals, dyes and organic molecules.3.1 MCNs doped with metals
The electronic and catalytic properties of MCNs can be altered by the incorporation of metals including transition or noble metals inside the mesochannels or by doping within the CN framework walls. In recent years, a few research groups have reported the incorporation of metal nanoparticles into the nanochannels of MCNs and investigated their performance as potential electro, photo and redox catalysts for energy storage and conversion and catalytic transformations. A selected set of noble metals, namely Pt, Pd, Au and Ag, has been encapsulated in the mesochannels of MCNs. Although the catalytic performance of these metal loaded MCNs has been discussed in the catalytic section that is described in the later part of this review, we briefly discuss the various reports involving metal-doped MCNs in this section.In 2010, Datta et al. reported the incorporation of gold nanoparticles with different sizes on the mesochannels of MCN-1 with different pore diameters.18 The pore diameter of MCN-1 was varied to tune the size of the gold nanoparticles inside the mesopores of the MCN. It was demonstrated that the multifunctionality of MCN-1, which acts as a stabilizing, reducing and size controlling agent, can be effectively used for the encapsulation of highly dispersed and size tuneable ultra-fine gold nanoparticles. The presence of functional groups on the surface of MCN-1 not only helps the formation of gold nanoparticles through an in situ reduction process but also offers a high dispersion of these nanoparticles along the porous channels. Better performance in a three component coupling reaction involving benzaldehyde, piperidine, and phenylacetylene was also observed for the gold functionalized MCN-1 sample. These interesting results led to the suggestion that this simple strategy could be applied for the fabrication of various noble metal nanoparticles inside MCN-1, which could result in promising catalytic materials for various organic transformations (Fig. 8A).18
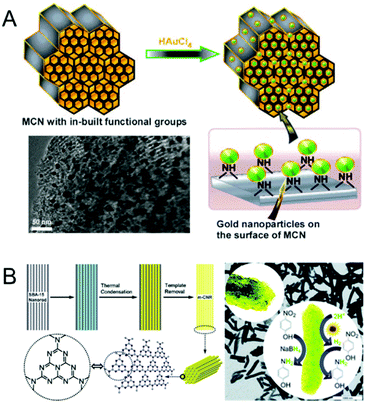 | ||
| Fig. 8 Metal doped MCN. (A) Encapsulation of gold nanoparticles over MCNs with in-built functional groups without any external stabilizing agent and its TEM image. Reprinted with permission from ref. 18. Copyright 2010, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Schematic diagram of the synthesis of m-CNR (yellow) templated from SBA-15 nanorods (gray) using CA (turquoise) as the monomer, its schematic molecular structure (left), and the TEM image with a reaction scheme for hydrogen generation from water and the reduction of nitrophenol using tandem catalysis in one step (bottom). Reprinted with permission from ref. 35. Copyright 2012, Royal Society of Chemistry. | ||
Li et al. reported the use of MCN nanorods as the support for ultra-fine metal nanoparticles such as Au, Pt and Pd (Fig. 8B).35 MCNs were used as effective and robust stabilizers for the growth of highly homogeneously distributed nanoparticles along the mesochannels of rod-shaped particles. It was also demonstrated that the hybrid material with metal nanoparticles decorated inside the mesochannels of the MCN showed enhanced catalytic activity and stability in the chemical reduction of 4-nitrophenol. The presence of a metal also helped to produce hydrogen in situ through the water reduction reaction that was used for the further reduction of 4-nitrophenol into 4-aminophenol. Lu et al. also reported the use of Pt nanoparticle loaded MCNs as bifunctional air electrode materials for rechargeable lithium–air batteries.75 The electrochemical performance of the metal functionalized MCN electrode was found to be impressive with a round trip efficiency of 87%. Zhang et al. and Gong et al. reported the preparation of Pd doped MCNs through a simple impregnation process.76,77 The prepared catalysts showed higher catalytic activities for the chemo-selective hydrogenation of aqueous bromate and quinolone as compared to that of Pd doped activated carbon and mesoporous carbon. Pd doped MCNs were also found to be highly active and chemo-selective for the liquid phase reduction of nitriles as a method for preparing amines using hydrogen as a reducing agent under ambient conditions without adding any additives or solvents.78 These amine compounds are used as intermediates in many pharmaceutical applications.
A photo-assisted reduction process was also used for the introduction of noble metal nanoparticles inside the mesochannels of MCNs as it allows for a uniform and high dispersion of nanoparticles along the mesochannels of the MCNs. Bu et al. realized this simple approach and reported the incorporation of Ag nanoparticles inside the mesochannels of MCNs.79 It was also demonstrated that the modification of MCNs with Ag nanoparticles significantly increases not only the conductivity but also the separation efficiency of photo-generated electron–hole pairs, which enhances its photoelectric conversion performance. The adsorption capacity and photocatalytic degradation of Rhodamine B of MCNs were also significantly improved after modification with Ag nanoparticles. However, the performance of these hybrid materials was directly related to the size of the Ag nanoparticles and small Ag nanoparticles were found to be the best for photocatalytic applications. The electron transfer from the noble metal nanoparticles to the MCNs supported by light has been effectively utilized by Li et al. for the coupling of aryl halides with different coupling molecules at room temperature.80 Interestingly, when the MCN support was not used, the metal particles did not show any activity, revealing the importance of the functional groups and structural properties of MCNs for the effective encapsulation of the metal nanoparticles. In order to improve the photocatalytic properties of MCNs, Yang et al. adopted the adsorption–desorption approach for the incorporation of AgBr nanoparticles inside the mesochannels of MCNs and further demonstrated their enhanced photocatalytic activity in the degradation of Methyl Orange (MO) dye.81 The enhanced photocatalytic activity was attributed to the changes in the electronic properties of MCNs after the incorporation of AgBr. It is expected that these nanocomposites with different amounts of AgBr nanoparticles could also be effectively used for the generation of hydrogen through the photocatalytic pathway.
3.2 MCN functionalized with dyes
By functionalizing the MCN porous surface with organic dye molecules, the electronic properties of the MCN can be easily modified. Moreover, the light absorption, conductivity, and photocatalytic properties of the MCN are significantly enhanced after functionalization with specific dyes. In this section, the methods used for the immobilization of dyes and their effects on the electronic properties and photocatalytic activities of MCNs are described. Takanabe et al. demonstrated that the surface functionalization of MCNs with light sensitive magnesium phthalocyanine (MgPc) can expand the absorption to wavelengths higher than those of pristine MCNs and helps to enhance light absorption from 600 nm for the non-functionalized g-MCN to 750 nm for the MgPc functionalized MCN (Fig. 9).82 The samples prepared with the co-catalyst Pt showed a high photocatalytic evolution of hydrogen from water at wavelengths higher than 600 nm. Interestingly, the monolayer coverage of MgPc inside the mesochannels of MCNs acts as a photocatalytic sensitizer and plays a critical role in controlling the photocatalytic hydrogen evolution as it supports the smooth transfer of the excited electrons from MgPc to the conduction band of MCNs and finally to the Pt co-catalyst.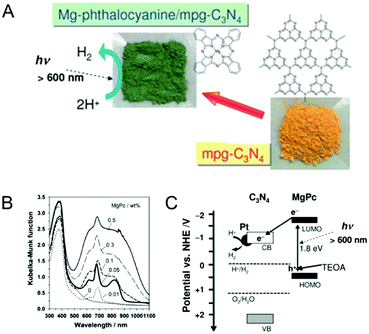 | ||
| Fig. 9 Dye functionalized MCN. (A) Structure of magnesium phthalocyanine (MgPc) and idealized mpg-C3N4. (B) UV-Vis diffuse reflectance spectra of mpg-C3N4 modified with various amounts of MgPc. (C) Scheme of the electron–hole transport in MgPc/Pt/mpg-C3N4 photocatalytic hydrogen evolution on dye-sensitized MCNs. Reprinted with permission from ref. 82. Copyright 2010, Royal Society of Chemistry. | ||
Lee et al. also modified the porous surface of MCNs with zinc phthalocyanine (ZnPc) through a simple impregnation method and observed an extended visible light absorption window.83 The photocatalytic activity of the ZnPc/MCN hybrid material for the removal of phenol was found to be dependent on the amount of ZnPc loading up to a maximum of 0.05 wt%, which supports the electron transfer from dyes to MCNs and limits the electron–hole recombination. However, further loading of dye into the MCNs resulted in a decrease in the photocatalytic activity for the degradation of phenol.
Wu et al. successfully immobilized polyoxometalates inside the MCN to improve the electrical contact between the redox-active centers and the surface of the electrode for the oxidation of water.84 The high specific surface area of the MCN together with the surface functional groups and the high conductivity assist the sequential electron transfer to the electrode, which helps to improve the energy dispersion and protects the catalytic particles from deactivation through surface restructuring. It was demonstrated that the OER of MCN/polyoxometalates is ca. 6 times higher than that of other polyoxometalate supported carbon nanostructures. Chen et al. reported the modification of MCNs with a thiophene motif and observed an enhanced photoactivation of molecular oxygen for the oxidation of alcohols.85 It was suggested that the incorporation of thiophene changes the electronic structure and further narrows down and adjusts the band gap and the positions of the LUMO and HOMO associated with the non-functionalised MCNs, which resulted in an enhanced photocatalytic activity. Moreover, modification with thiophene only altered the chemical composition and electronic structure without significantly affecting the textural parameters associated with MCNs.
3.3 MCNs functionalized with organic molecules
The surface charge and the catalytic properties of the MCN materials can be altered by functionalization with organic molecules. Yu et al. reported the functionalization of MCNs with ammonia in order to introduce more amine groups and basicity to the porous surface.86 The more basic groups on the surface made them effective adsorbents for the selective removal of acidic formaldehyde molecules. Besides enhancing the overall basic character of the MCNs, functionalization also contributed to the overall surface area by generating micropores, which in turn significantly enhances the overall adsorption capacity of ammonia functionalized MCNs. It should be noted that ammonia functionalized MCNs showed a much higher adsorption capacity for formaldehyde as compared to the non-functionalized MCNs.Ye et al. modified the surface of g-MCN with ferrocene functional groups in order to improve the photocatalytic properties.87 The functionalization reaction involved the amidation reaction of ferrocenecarboxylic acid (Fc-COOH) with the –NH2 groups on the surface of the MCNs. Ferrocene modified MCNs exhibited enhanced visible light absorption and also accelerated bulk to surface charge transfer and separation. It was demonstrated that the functionalized MCNs served as efficient photocatalysts for the synthesis of phenol from benzene through a selective oxidation process using hydrogen peroxide as the oxidant under visible light conditions.
Very recently, Vinu et al. reported an interesting modification of MCNs to prepare bifunctional MCNs with both acidic and basic surface functional groups (Fig. 10).15 The MCN with both acidic and basic functional groups exhibited enzyme-like catalysis and reported ca. 100% conversion in a one pot deacetalization-Knoevenagel reaction with 99% selectivity. Acid functional groups were introduced via a mild UV irradiation oxidation process, which maintained a balance between the inherent basic functional groups and the newly induced acid functions. This is a very important result as it sheds light on a totally unexplored aspect of MCNs and also introduces a simple approach to decorate the surface with acid functions, which could be applied for the green synthesis of various fine chemicals in a single step.
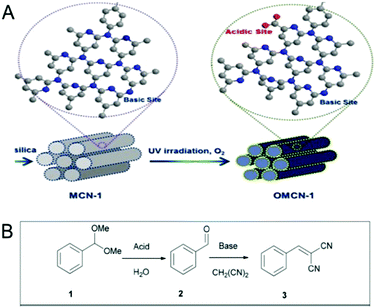 | ||
| Fig. 10 Basic molecule functionalization of the MCNs. (A) Bifunctional MCN-1 induced with acid groups and inherent basic groups through a facile UV light oxidation method and (B) scheme for the enzyme-like catalyst for a one-pot deacetalization-Knoevenagel reaction. Reprinted with permission from ref. 15. Copyright 2015, Nature Publishing Group. | ||
3.4 MCN functionalized with inorganic semiconductors
The role of a co-catalyst is of paramount importance in a photocatalytic reaction as it has the ability not only to tune the conduction and valance band edges of the catalytic system but also to assist the charge separation and the lifetime of the photogenerated species. The co-catalyst also has the capability to lower the activation barrier for the evolution of hydrogen or oxygen from photocatalytic water splitting. Although MCNs have all the hallmarks of an excellent, metal free photocatalyst for visible light water splitting reactions, the photocatalytic performance of MCNs, including selectivity, activity, and stability, is significantly dependent on the nature and type of the surface co-catalyst as it can easily fine tune the conduction and valance band edges of the MCN and support reductive or oxidative catalysis. The synergy between MCNs and the co-catalysts in a photocatalytic reaction finally dictates the overall performance. As a result, mostly expensive noble metals such as gold, silver and platinum have been utilized as co-catalysts which however limit the scalability of water splitting and energy harvesting reactions for industrial scale applications due to their high cost and scarcity. Therefore, researchers tried to functionalize MCNs with semiconducting nanostructures inside the mesoporous channels in order to tune its band structure. In this section, we discuss some of the recent reports where MCNs have been functionalized with inorganic semiconductors in order to replace the expensive noble metal based co-catalysts.Hou and Wang co-workers recently reported an enhanced photocatalytic hydrogen evolution activity of MCNs functionalized with a layer of MoS2 which serves as a co-catalyst.88 The functionalization was performed by impregnating MCNs with a solution of (NH4)2MoS4, followed by sulfidation with H2S at 350 °C. Interestingly, the photocatalytic activity of the MoS2/MCN hybrid material was comparable to that of the Pt loaded MCN. The high activity was related to the minimal lattice mismatch of the MCN and MoS2 which facilitated the uniform growth of the co-catalyst on the surface of the MCN. This unique structure offered a higher accessible surface area which significantly improved light utilization and enhanced the electron transfer across the interface through the electron tunnelling effect. More importantly, the unique structure of the MoS2 supported MCN makes room for the directional migration of electrons from the MCN to MoS2, which then react with protons to generate a high amount of hydrogen. The group also studied the photocatalytic activity of H2 evolution by loading MoS2 on a silica support. Surprisingly, there was no H2 evolution. Likewise, MCNs alone did not show any significant H2 evolution. These results highlight that the synergy between MCNs and MoS2 is absolutely critical for an appreciable H2 evolution reaction.
WO3, an active semiconducting oxide material that is typically used for promoting oxygen evolution, was encapsulated inside the MCN materials.89 Surprisingly, MCN/WO3 significantly enhanced the H2 evolution reaction. The functionalized MCN showed almost a twofold increase in efficiency when a very small quantity of WO3 (ca. 1 wt%) was added to the MCN support material. The superior performance was attributed to the high surface area and the synergy between MCN and WO3, which results in improved charge separation, thereby increasing the lifetime of the electron–hole pair generated during the reaction. The electrons in the conduction band of the MCNs are available for the reduction of the protons to produce hydrogen whereas the holes in the valance band of WO3 are effectively utilized for the oxidation of sacrificial agents. It was also assumed that the addition of WO3 also promotes the photorecombination of the electrons from the conduction band of WO3 with the electron holes from the valance band edge of the MCN. These results reveal the importance of co-catalysts for the selective production of hydrogen through the photocatalytic process assisted by MCNs.
4. Applications of MCNs
MCNs are interesting materials with unique basic, semiconducting, intercalating and adsorption properties, and have excellent chemical and mechanical stability, which makes them attractive for a range of applications including sensing, catalysis, energy storage and adsorption. It is also expected that these fascinating materials can also offer highly ordered porous structures, high specific surface areas, large pore volumes and a uniform pore size distribution. Unique properties can also be introduced into MCNs by modifying their porous structure or walls, which also has significant effects on improving their performance in various applications. In this section, we will highlight the application of MCN materials as reactive hard templates for the generation of new nanostructures, photo, basic, hydrogenation and oxidation catalysts, adsorbents and sensors.4.1 MCNs as reactive hard templates
Recent reports have indicated the use of MCNs as templates for the synthesis of metal nitrides and carbides where MCNs play the role of hard reactive templates and also the nitrogen source for the metal nitrides.32–34,90–92 The term “reactive template” suggests that MCNs act both as a nanoreactor and as a source of nitrogen/carbon for the synthesis of metal nitrides/carbides.In 2007, Antonietti and co-workers first reported the synthesis of binary metal nitride nanoparticles using MCNs as a reactive template (Fig. 11A).32,90 MCNs play a dual role of a nanoreactor as well as an in situ source of nitrogen in the synthesis. The formation mechanism of metal nitrides using MCNs as a reactive template involves a number of steps: (1) synthesis of MCNs using the established methods; (2) infiltration of the pores of the MCNs by a solution of metal precursors via a sol–gel process; (3) removal of the solvent results in the formation of amorphous metal oxide nanoparticles in the pore channels of the MCNs; (4) thermal decomposition of the composite obtained in step 3 at 800 °C under an inert or sealed environment results in the conversion of metal oxides into metal nitrides due to donation of nitrogen from the carbon nitride nanoreactor to the preformed metal oxide. It is important to note that thermal treatment of the carbon nitride/precursor composite is a dangerous step and must be done carefully as it involves the release of hydrogen cyanide and cyanogen gases.
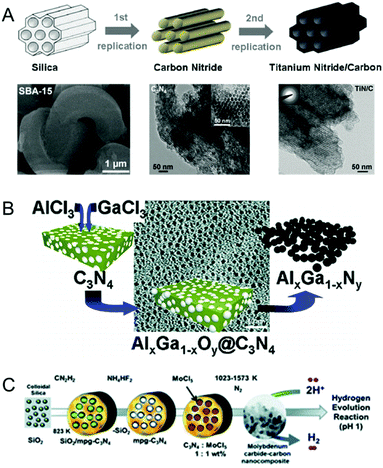 | ||
| Fig. 11 MCNs as the reactive hard templates. (A) Reaction pathway for the synthesis of 2D hexagonal mesoporous carbon and metal nitrides and the SEM/TEM images. Reprinted with permission from ref. 32. Copyright 2009, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Ternary metal nitride nanoparticles using MCNs and the TEM image. Reprinted with permission from ref. 33. Copyright 2008, American Chemical Society. (C) Molybdenum carbide–carbon nanocomposites. Reprinted with permission from ref. 34. Copyright 2013, Royal Society of Chemistry. | ||
It is interesting to note that the final morphology of the metal nitride obtained is a copy of the silica template used to prepare the MCNs. This means that the morphology of the metal nitride prepared using a hard reactive template could be easily varied by varying the morphology of the template used for the synthesis of the MCNs. The aforementioned method achieves two goals with ease: (a) the use of a high decomposition temperature automatically removes the template material; (b) the template MCN material serves as a nitrogen source, converting the infiltrated metal precursor into its corresponding metal nitride. The same group extended the above method for the synthesis of ternary metal nitride such as Al–Ga–nitride and Ti–V–nitride following a similar procedure to above (Fig. 11B).33 Metal sources such as GaCl3, AlCl3, TiCl3 and VOCl3 were used as suitable metal precursors for the synthesis of ternary metal nitrides. Furthermore, it is believed that the size of the metal nanoparticles could be easily controlled by changing the concentration of the metal precursor solution used to infiltrate the MCN. This method of synthesis provides another alternative to the well-known post-synthetic approach for the synthesis of metal nitrides and could be extended to develop metal nitride nanoparticles with different morphologies.
Alhajri et al. reported the synthesis of tantalum carbides/nitrides and molybdenum carbide/carbon nanocomposites with a hexagonal structure and small crystal sizes in the range of 3–20 nm using the reactive template method (Fig. 11C).34,91 The formation of tantalum carbide/nitride was controlled by varying the weight ratio of the reactive template to the tantalum precursor, the nature of the carrier gas (Ar, N2 and NH3) and the decomposition temperature. The formation of tantalum carbide as one of the products suggests that carbon nitride acts not only as the reactive template but also as a source of carbon and nitrogen. Under an Ar/N2 flow, TaN/C was obtained whereas Ta3N5 was obtained under a NH3 flow. Interestingly, molybdenum carbide/carbon nanocomposites showed a much higher surface area as compared to that of tantalum carbide/nitride. Tantalum carbides/nitrides and molybdenum carbide/carbon nanocomposites were used as electrocatalysts for hydrogen evolution reactions (HER) and it was found that these samples were highly stable and showed the highest HER current in acidic media. The excellent electrochemical performance was attributed to the small particle size and the highest specific surface area. Yuliati et al. also reported the synthesis of tantalum nitride (Ta3N5) nanoparticles with different sizes using MCNs as the reactive template and ammonia as the nitrogen source.92 Ta3N5 nanoparticles exhibited a high specific surface area and less defect sites and showed higher photocatalytic hydrogen evolution activity under visible light than bulk Ta3N5.
4.2 Metal free catalyst
MCN materials as metal free catalysts have attracted a lot of attention in the last few decades as witnessed by a large number of reports in the literature.10,12,14,18,21,22,24,26–29,37–39,77,93–96 The presence of inherent basic groups attributes strong basic catalytic properties to the CNs, which can be easily further enhanced through suitable functionalization as discussed further in the sections below. The mesoporous nature of the CN materials is an added advantage as it directly affects the transport and adsorption properties of the catalyst besides providing a large surface area for the ease of reaction. In this section, we will review the recent developments in the field of catalysis centred on non-functionalized MCN materials.Friedel Craft reactions when catalyzed by the standard AlCl3 type of acid generates nearly about 80% waste by mass.26 Goettamann et al. reported the use of MCNs, which were prepared using colloidal silica nanoparticles as the templates, as a metal free Lewis base catalyst for alkylation and acylation of benzene with a number of electrophiles such as alcohols and carboxylic acids and with a quaternary ammonium salt such as tetramethylammonium bromide or urea as the electrophiles (Fig. 12A).37 The conversion and product distribution were found to be dependent on the reaction conditions, and the nature of the solvent. It was further demonstrated that the MCN showed 100% conversion of formic acid to benzaldehyde, which is definitely a promising result and could pave the path for an alternate and greener process for the industrial production of benzaldehyde. In a standard AlCl3 catalysed Friedel crafts reaction involving a benzene ring and an electrophile, the reaction involves activation of the electrophile; however, Goettamann and co-workers were able to show that the activation of benzene in the alkylation and acylation reactions can be done with MCNs without the addition of the electrophile.26,38 It was also demonstrated that MCNs showed an excellent catalytic performance for the production of benzonitrile via the reaction between benzene and urea. It was assumed that the reaction involved the addition of urea to benzene with the removal of both water and ammonia molecules. In this case, MCNs activated both benzene and urea to achieve a high yield of benzonitrile. These results reveal that MCNs can be used as an excellent Lewis basic catalyst for the Friedel Craft aromatic substitution reactions for the synthesis of various value added organic products. However, the detailed mechanism or evidence was not provided to confirm the unique role of MCN in activating the benzene and urea molecules and further studies are needed to understand the exact mechanism of the reaction over MCNs.
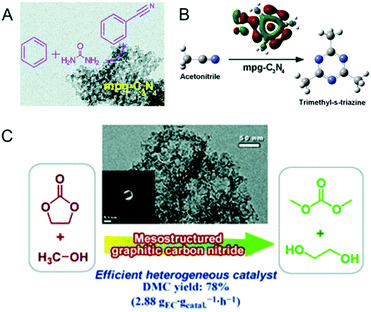 | ||
| Fig. 12 Metal free catalyst for basic catalysis. (A) Sustainable Friedel–Crafts reactions for direct activation of benzene. Reprinted with permission from ref. 37. Copyright 2006, Royal Society of Chemistry. (B) Cyclisation of functional nitriles and alkynes. Reprinted with permission from ref. 38. Copyright 2007, Royal Society of Chemistry. (C) Production of DMC via transesterification of EC with methanol. Reprinted with permission from ref. 39. Copyright 2013, Royal Society of Chemistry. | ||
Goettmann and co-workers further extended the application of MCNs as a metal free catalyst for cyclotrimerization of triple bonds of functional nitriles and alkynes (Fig. 12B).38 A number of alkyl and aryl cyano compounds and alkynes were selected as reactants under relatively mild reaction conditions with reaction temperatures varying between 120 to 180 °C and using hexane and xylene as the solvent. The reaction time was however varied from 24 to 80 h. On the other hand, Ansari et al. demonstrated the use of MCNs as a base catalyst for a popular C–C coupling reaction called the Knoevenagel condensation involving the synthesis of α,β-unsaturated carbonyl compounds via condensation of ethylcyanoacetate with a host of substituted aromatic aldehydes.93 These reactions were carried out in a microwave and were found to have very short run time of 12 minutes with a maximum conversion reported as high as 93% and a selectivity of >90% and a high recyclability. The effect of the nature of the groups on the aldehyde component (electron withdrawing or releasing) was clearly manifested in the % yield of the Knoevenagel product. While electron withdrawing groups (− inductive effect) resulted in an increase of yield, the presence of electron releasing groups (+ inductive and + mesomeric effects) decreased the yield. The catalytic nature of the MCNs was further corroborated when no significant conversion was reported in the absence of the catalyst.
The basicity of the MCN can be significantly enhanced through de-protonation via treatment with different basic aqueous solutions such as K2CO3, KOH, and tBuOK. The structure of the MCN was not at all affected after de-protonation but the basicity was significantly enhanced.94 Su et al. demonstrated the multifunctional and highly basic catalytic nature of the MCN by using a de-protonated MCN for Knoevenagel condensation and transesterification reactions.94 Two different de-protonation strategies were utilized, namely treatment of MCNs at high temperatures (200, 300 and 400 °C) and treatment with different base solutions. Heat treatment resulted in reduced conversion due to structural etching and pore shrinkage. Base treatment, on the other hand, resulted in a marked increase in conversion especially when treated with t-BuOK. No change in the structure or mesoporosity of the MCNs was observed after base treatment and the catalyst could be recycled and used several times without much loss of activity. The reaction time was found to be reasonable between 4.5 and 5 h for reactions performed at 70 °C. A longer reaction time was reported when the reaction was conducted at room temperature. The conversions were reportedly high for both Knoevenagel and transesterification reactions. The MCN catalysts were found to be highly stable and showed excellent activity when the reactions were carried out between various aldehydes and malonic derivatives, alcohols and ketoesters. Interestingly, the amount of catalyst directly affects the conversion and selectivity.
Vinu et al. also reported the use of a physically and chemical modified MCN as a metal free catalyst for Friedel Crafts, transesterification and Knoevenagel types of reactions with impressive conversion/yields.12,14,21,22,24 Vinu and co-workers first reported the use of MCN nanoparticles as a metal free base catalyst for the transesterification reaction of β-keto esters. A host of long chain primary, aryl and cyclic alcohols were used as substrates for the transesterification reaction. Interestingly, the less reactive long chain primary alcohols showed good conversion although the reaction time was higher than that of short alkyl chains. The high catalytic activity was attributed to the presence of a huge number of basic sites due to functional groups such as –NH2, –NH and nitrogen bonded to the carbon matrix, in the wall structure of the MCNs besides excellent textural parameters. From a commercial standpoint, the catalyst showed high recyclability and stability. No reaction product was observed in the absence of the MCN catalyst under identical conditions, revealing the importance of the basic active sites of MCNs in the Knoevenagel type of organic reactions.
Talapaneni and Vinu co-workers also reported base catalysed Knoevenagel and transesterification reactions using MCN-1, MCN-4 and MCN-6.24 The Knoevenagel reaction between benzaldeyde and malononitrile proceeded with ca. 95% conversion and 100% selectivity to yield the final α,β-unsaturated nitrile. Interestingly, the reaction was carried out at room temperature and the reaction run time was only 4 h. The group further studied the effect of the pore diameters of MCN-6 samples on the Knoevenagel reaction. MCN-6 was prepared with different pore diameters and surface areas and the resulting samples were denoted as MCN-6-T (T = 100, 130 and 150 °C are the synthesis temperatures of the silica template KIT-6). Among the MCN-6-T samples, MCN-6-150 showed the highest activity, maximum conversion with 100% selectivity to α,β-unsaturated nitrile. This best activity was attributed to the combination of better textural parameters and basicity of the MCN-6-150 sample compared to MCN-6-100 and MCN-6-130.
Talapaneni and co-workers also reported the use of highly ordered and pore tuned MCN-4 as the base catalysts for the Friedel Craft's acylation reaction between benzene and hexonyl chloride (HC) to yield hexanophenone.22 These reactions were carried out at 90 °C and the reaction run time was 9 h. MCN-4 samples with different pore diameters and surface areas denoted as MCN-4-T (where T = 100, 130 and 150 °C are the synthesis temperatures of the silica template SBA-15) were also used as the catalysts. Furthermore, the group compared the activity of pore tuned MCN-4 with MCN-1 and non-porous CN. MCN-1 showed an HC conversion of 65% while non-porous CN showed a conversion of only 38% under identical conditions. Among the MCN-4-T samples, MCN-4-130 showed the highest activity with an HC conversion of 90% and 100% selectivity to hexanophenone while MCN-4-100 showed the lowest activity with an HC conversion of 85%. The exceptionally high activity of MCN-4-130 was attributed to the fact that MCN-4-130 has the highest surface area and a highly ordered porous structure that provides a large number of reaction sites.
Chokkalingam et al. studied the metal free transesterification ethylacetoacetate as a fixed substrate and a variety of alcohols such as a 1-butanol, 1-octanol, cyclohexanol, benzyl alcohol and furfuryl alcohol as the second reactants using MCN-1 as the catalyst.14 The conversion of all the studied alcohols increased progressively with increasing reaction time. Furthermore, the MCN-1 catalyst showed the highest conversion for 1-butanol and, interestingly, its activity was higher for aliphatic alcohols as compared to cyclic and aromatic alcohols. The effect of temperature on the catalysis by MCN-1 was also investigated with 1-butanol and ethylacetoacetate as the reactants. A dramatic increase in the conversion was observed when the reaction temperature was increased from 90 to 150 °C, clearly manifesting the strong temperature dependence of the reaction. However, with the increase in reaction temperature, selectivity was found to decrease substantially, producing a large number of side products. The catalytic activity of MCN-1 for the transesterification reaction was attributed to the presence of NH- and NH2 groups which offer strong Lewis basic sites.
Xu et al. reported the synthesis of dimethylcarbonate (DMC) via a transesterification reaction between ethylenecarbonate (EC) and methanol (MeOH) (Fig. 12C) using MCN catalysts.39 The yield of DMC was found to be reasonable with an EC conversion of 76% for a reaction run time of 6 h and a reaction temperature of 160 °C. The group also studied the effect of reaction run time and temperature on the conversion and selectivity. The impact of the molar ratios of MeOH/EC was also studied. As the ratio of MeOH/EC was increased from 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the EC conversion first increased and then decreased. The same group also reported the transesterification of β-keto esters using a high surface area MCN, synthesized from hexamethylenetetramine as the C/N precursor.28 The transesterification reaction of 1-butanol with ethylacetoacetate was carried out at 110 °C with a 70% yield. It is interesting to note that the same reaction was also studied by Chokkalingam et al. who reported 69.4% yield at 110 °C for a reaction run time of 6 h.
1, the EC conversion first increased and then decreased. The same group also reported the transesterification of β-keto esters using a high surface area MCN, synthesized from hexamethylenetetramine as the C/N precursor.28 The transesterification reaction of 1-butanol with ethylacetoacetate was carried out at 110 °C with a 70% yield. It is interesting to note that the same reaction was also studied by Chokkalingam et al. who reported 69.4% yield at 110 °C for a reaction run time of 6 h.
The chemo-selective catalytic hydrogenation of quinoline to 1,2,3,4-tetrahydroquinoline using Pd supported MCNs with an ordered porous structure under extremely mild reaction conditions of 1 bar and a temperature of 30–50 °C using molecular hydrogen as a reducing agent was demonstrated by Gong et al.77 An ultrasonication method was used to obtain a high dispersion of the nanoparticles on the surface of the MCNs. Interestingly, previous reports for the same reaction employed vigorous conditions such as a high temperature of up to 200 °C and a pressure of between 2 and 4 MPa. In that respect, the results are remarkable. It is worth mentioning that the N2 adsorption isotherm did not change significantly after Pd was loaded on the support material MCN. However, Pd loading did result in a slight shrinkage of the BET area from 212 to 197 m2 g−1, the pore volume from 0.48 to 0.32 cm3 g−1 and the pore diameter from 7.42 to 5.68 nm.
Although the selectivity to the final product remained nearly unchanged, conversion however varied depending on the solvent. It is important to note here that the specific catalytic activity did not show a clear correlation with the dielectric constant, polarity or dipole moment of the solvent. Furthermore, the high catalytic activity of Pd@ompg-C3N4 is attributed to the high nitrogen content in the support ompg-C3N4 materials which anchor a large quantity of the Pd materials which in turn act as the active sites for hydrogen activation. In addition to the above factors, the cylindrical mesoporous structure of the support materials further facilitates the reaction by reducing mass transfer related limitation. From a commercial standpoint, the Pd@ompg-C3N4 material showed a high recyclability without much loss of activity for the hydrogenation of quinoline. The activity of Pd@ompg-C3N4 was much higher than that of Pd supported activated carbon. The high activity was attributed to the synergetic interaction between the nanoparticles and the MCN support and the graphitic C–N–C layers and the mesoporous structure that facilitates mass transfer. The presence of incompletely condensed amino groups that help to anchor or reduce the Pd particles also supported the high activity.
The production of amines through a simple liquid phase hydrogenation of nitriles has been receiving a lot of attention as these amines are widely used as intermediates for the synthesis of various pharmaceutical compounds. Li et al. employed Pd@mpg-C3N4 for the chemo-selective catalytic hydrogenation of nitriles to amines.96 A number of aliphatic and aromatic nitriles were used and the reaction conditions were slightly varied. The highest conversion of 99% with >99% selectivity and a high recyclability was reported at 70 °C without any additives and solvents. In comparison to the commercially available Pd@C, Pd supported on mpg-C3N4 showed better activity and selectivity. Zhao et al. reported the role of highly ordered MCN denoted as DUT-1 with an ultra-high surface area (1124 m2 g−1) and a high pore volume (1.79 cm3 g−1) as a dehydrogenation catalyst for the dehydrogenation of ethyl benzene.29 The catalytic performance of DUT-1 was compared with those of MCN-1 and CMK-3 and nanodiamonds. Among the materials, DUT-1 showed the highest catalytic activity. Although the overall conversion for all these materials as dehydrogenation catalysts was not high, DUT-1 showed a high selectivity towards styrene. The catalytic activity for this reaction followed the order DUT-1 > ND > MCN-1 > CMK-3. It is interesting to note that although DUT-1 and MCN-1 had nearly the same nitrogen content, still DUT-1 showed a superior catalytic activity than MCN-1 towards the dehydrogenation reaction which was attributed to the ultra-high surface area, super high pore volume and the microstructures including bulk structure and surface chemical properties. In addition, the surface nitrogen in DUT-1 that offered a high electron density and basicity not only promoted dehydrogenation but also suppressed the formation of cracking side-products such as benzene and toluene. These results revealed that the combination of excellent textural parameters together with the nitrogen functionalities on the surface of DUT-1 makes them excellent catalysts for the dehydrogenation of aromatics.
Ansari et al. reported the catalytic activation of CO2 over MCNs and the role of MCNs in the catalytic oxidation of cyclic olefins (Fig. 13A).40 CO2 activation was successfully tested using a carbonation reaction of propylene oxide to propylene carbonate. Although the yields of these reactions were in the range of 25–37, the selectivity was quite high in the range of 92–98. The catalytic activity of the MCNs was also tested in the oxidation of cyclohexene with molecular oxygen in the absence and/or presence of CO2. The oxidation reactions were also carried out in the presence of nitrogen to see the synergistic effect of CO2. Interestingly, a conversion in the presence of O2/CO2 was higher than in the presence of N2/CO2 indicating the role of CO2 as a promotional agent. Earlier, Antonietti et al. reported the basic catalysis of MCN in the conversion of benzene to phenol, mediated using CO2, wherein CO2 was used as an oxygen source.98
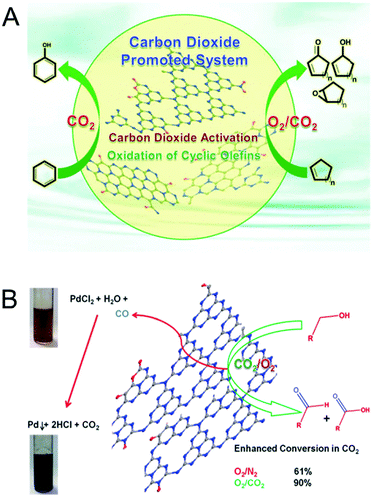 | ||
| Fig. 13 Metal free catalyst for oxidation catalysis. (A) CO2 activation and promotional effect in the oxidation of cyclic olefins. Reprinted with permission from ref. 40. Copyright 2011, Royal Society of Chemistry. (B) CO2 oxidation of aromatic alcohols. Reprinted with permission from ref. 41. Copyright 2013, Royal Society of Chemistry. | ||
The addition of heteroatoms in the MCN nanostructure makes a huge difference in the final oxidation catalytic activity of the MCNs. Wang et al. reported the role of boron and fluorine containing MCN polymers with a high nitrogen content for the highly selective oxidation of cyclohexane using hydrogen peroxide as an oxidant.31 Although the overall conversion of cyclohexane to cyclohexanone is not so high, the selectivity to cyclohexanone is quite good (>91%). Interestingly, no other oxidation product was observed in the GC-MS analysis of the products, which indicates the highly selective and controlled nature of the reaction. Also, the MCN polymeric materials exhibited a high stability in the absence of light and could be recycled multiple times without any significant loss of activity.
The catalytic activity in both the above reactions, i.e., activation of CO2 and oxidation, was attributed to the presence of a large quantity of nitrogen atoms which act as Lewis base sites. The oxidation reactions were also carried out with 5, 6, 8 and 12 membered olefins and the O2/CO2 mixture and the promotional effect of CO2 was reported in all cases. The maximum conversion was reported to be less than 40% with up to 37% selectivity for the epoxide product. Interestingly, the small ring size showed higher conversion compared to larger ring sizes under identical reaction conditions. This could be due to the fact that larger membered rings offer greater diffusional resistance and cannot access the active sites. The MCN catalysts also showed good recyclability and could be recycled three times without a significant loss of activity.
In 2013, the same group also reported the CO2 assisted oxidation of aromatic alcohols using MCNs (Fig. 13B).41 Like in the case of cyclic olefins, the aromatic alcohols registered higher conversion in the presence of CO2/O2 when compared to O2 alone. This result is another example of the synergistic effect of CO2 and molecular oxygen for reactions catalysed using high nitrogen containing MCNs. The CO2 molecules were activated with the help of the MCNs and were also acting as an oxygen source in this particular reaction. This was confirmed by the fact that the carbamate was formed on the surface of the MCNs. In another report, Min et al. also tested the surface oxygen functionalities of MCNs for the oxidation of cyclic olefins such as cyclopentene, cyclohexene, and cis-clyclooctene using H2O2 as the oxidant under mild reaction conditions.25 Surprisingly, the conversion in this case was found to be much higher and in the range 65–80% depending on the ring size whereas selectivity towards epoxide was found to be higher for larger rings and varied in the range 41–92%, which was attributed to the existence of surface oxygen species in the MCN nanostructures. These outstanding results developed a new area of research and the CO2 activation capability of MCNs could be effectively applied for various organic transformations.
4.3 Photocatalysis using MCNs
Heterogeneous photocatalysis has been gaining a lot of attention in recent years owing to their power to replicate the photosynthetic processes including the oxidation of water and the reduction of CO2via up-hill reaction and decomposition of toxic molecules through down-hill reactions.99 As hydrogen is considered to be a clean fuel and the raw material for many chemical transformations, many research groups have been actively working on developing new processes for the production of hydrogen especially using environmentally friendly approaches. One of them is the production of hydrogen from water splitting using renewable sources such as water. Inorganic semiconducting metal oxides such as titania nanoparticles, metals, sulphides and phosphides have been widely used as photocatalysts for water splitting.100 However, these materials need to be modified to match up the valence band or conduction band edge of the catalyst for the splitting of water molecules under both UV and visible light. MCNs are metal free semiconducting photocatalysts which exhibit not only the appropriate band gap (2.7 eV) for water splitting but also crystalline semiconducting structures and surface functional groups. The highly ordered mesoporous structure and the high crystallinity of MCNs are expected to support the reactions of the photogenerated electrons and the holes that are in the conduction and valence bands of the MCNs, respectively, with the water molecules to produce hydrogen and oxygen but limit the recombination of the electron and holes. These excellent characteristics of MCN materials make them powerful photocatalysts for water splitting and other photocatalytic reactions. In this section, we will review the photocatalytic applications of MCN materials with different structures and nitrogen contents.Wang et al. reported the photocatalytic hydrogen evolution using MCNs prepared from CA and found that the hydrogen production from the photochemical reduction of water increased by one order of magnitude when using MCNs as opposed to a polymeric non-porous carbon nitride (Fig. 14A).42 However, it was found that large surface area does not necessarily mean greater hydrogen evolution. In fact, the sample with the highest surface area showed the lowest hydrogen evolution rate which was still higher when compared to non-porous carbon nitride. These results are strong evidence that MCN is a better candidate than its non-porous analogue. The porous nature of the MCN can be clearly seen in the TEM image and the variation of the hydrogen evolution rate with wavelength (Fig. 14A). A significant improvement in the photocatalytic water splitting was observed when MCNs prepared from the soft-templating approach was used (Fig. 14B).43 The prepared materials exhibited worm-like pores with an adsorption edge of up to 800 nm and showed excellent photocatalytic activity with a high hydrogen evolution under visible light. However, the quantum efficiency (QE) of MCN measured at 420 nm was ca. 1.8% which is still low.
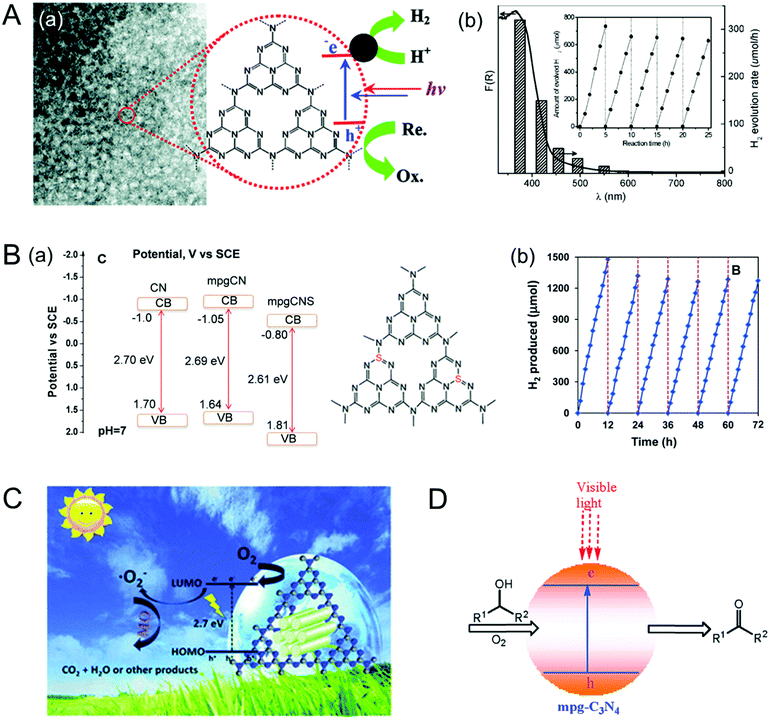 | ||
| Fig. 14 MCNs as the photocatalysts for water splitting (H2 evolution) under visible light. (A) (a) TEM image of 3D porous mpg-C3N4 with semiconducting properties and (b) wavelength dependence of the H2 evolution rate on Pt/mpg-C3N4. Reprinted with permission from ref. 42. Copyright 2009, American Chemical Society. (B) (a) Proposed formation mechanism of sulfur-doped g-C3N4 with a band structure of CNs, mpgCN and mpgCNS and (b) H2 evolution stability test for mpgCNS. Reprinted with permission from ref. 43. Copyright 2012, Royal Society of Chemistry. (C) Cosolvent-free nanocasting synthesis of ordered MCN and its remarkable photocatalytic activity for MO degradation. Reprinted with permission from ref. 44. Copyright 2015, Royal Society of Chemistry. (D) Molecular and textural engineering of conjugated MCN catalysts for the selective oxidation of alcohols under visible light. Reprinted with permission from ref. 45. Copyright 2010, American Chemical Society. | ||
Chen and co-workers investigated the photocatalytic activity of MCNs with hexagonally ordered pores for the photochemical reduction of water using visible light.101 The group studied the effect of light and MCNs on the H2 evolution reaction and found that there was no reaction when the system was irradiated with light in the absence of MCNs or in the presence of MCNs without irradiation. These results confirm that MCNs indeed catalysed the photochemical reduction of H2O in the presence of light. Furthermore, time resolved photo-luminescence showed that the charge separation could last for 11 ns which was slightly shorter than that for bulk carbon nitride (non-porous). The shorter charge separation time was attributed to the presence of a nanoporous structure in the material. In terms of H2 evolution, ordered MCNs showed about 5 times higher activity than bulk carbon nitride.
The doping of MCNs with sulphur results in significant changes to the band edge and the crystal structure of MCNs. Hong et al. reported photocatalytic hydrogen evolution from water using MCNs doped with sulfur. The MCNs showed a surface area of 128 m2 g−1 and mesopores in the range 10–20 nm.102 Sulfur doping was performed in situ by using thiourea as a nitrogen and carbon precursor. The optical investigation suggests that sulfur doped MCNs exhibited enhanced and extended light absorbance as compared to the non-doped non-porous carbon nitride. It is believed that sulfur doping causes a lower density of defects resulting in almost 30 times higher hydrogen evolution activity compared to the non-porous carbon nitride. The QE was significantly improved after doping and it registered ca. 5.8% at 440 nm. This was regarded as the highest ever QE reported for the doped MCN.
Lee et al.65 successfully demonstrated the photocatalytic degradation of phenol using MCNs under visible light. The group prepared MCNs with different initial mass ratios of urea to silica (3, 4, 5, 6) and found that the textural parameters including specific surface area, pore volume and pore size, morphology and structure depend on the amount of urea added onto the silica template. The MCN sample prepared with a mass ratio of urea to silica of 5 showed the highest photocatalytic degradation efficiency of 74% for phenol and a high photocatalytic stability. It should be noted that when non-porous carbon nitride was used, only 20% degradation of phenol was observed. The highest photocatalytic degradation of phenol by MCNs prepared with a mass ratio of urea to silica of 5 was attributed to the excellent textural properties such as a high surface area and the presence of mesopores with a uniform pore size distribution of 7 nm.
Chen et al. reported the light assisted oxidation of benzene to phenol catalysed by Fe loaded MCNs in the presence of H2O2.103 In view of the industrial importance of this reaction, this is a remarkable result and can be scaled up to replace the current three step cumene process used in the industrial production of phenol. The group studied the photocatalyzed oxidation of benzene to phenol co-catalyzed with H2O2 both in the presence and absence of MCNs and found that there was no conversion in the absence of MCNs. This result confirms that MCNs are indeed responsible for the direct oxidation of benzene to phenol, however the yield is low. Chen and co-workers further improved the photocatalytic performance by doping the MCNs with different metals and found that Fe has the best promotional effect among the different metals studied. This study also highlighted the importance of a high surface area as a large surface area enhances mass transfer, improves the light harvesting capacity for improved photocatalytic performances in organic transformation reactions.
Zhan et al. utilized MCNs as photocatalysts for the catalytic oxidation of α-hydroxy ketones to synthesize benzoic acids under visible light.104 This reaction is particularly important as it represents an environmentally friendly and facile route to synthesize an industrially important chemical, benzoic acid. The MCNs facilitated the oxidative cleavage of the carbon–carbon bond of benzils via C–H activation to form carbonyl compounds with the help of visible light. The MCNs also showed excellent catalytic performances, even for the substituted α-hydroxy ketones, to yield the desired products and exhibited appreciable recyclability without much loss of catalytic activity even after 3 cycles.
Earlier, Su et al. reported the photocatalytic activation of O2 for the selective oxidation of benzyl alcohols to the corresponding benzaldehydes (Fig. 14D).45 In this case, the research group demonstrated excellent catalytic selectivity achieved by combining the surface basicity and inherent semiconducting properties of the MCNs. It was also demonstrated that MCNs can also be effectively used for the selective conversion of various other alcohol substrates to their corresponding aldehydes or ketones using visible light. This result is quite encouraging as it provides an alternate, mild and environmentally friendly metal-free photocatalytic system for the synthesis of various fine chemicals under visible light.
4.4 Adsorption of gases
MCNs owing to their mesoporous structure, crystalline graphitic CN framework, and the presence of a large number of basic surface functionalities in the form of –NH or –NH2 functions have been reported as adsorbents for a certain set of molecules including CO2 methane, and hydrogen and are also used as a separation medium. In this section, we will present some of the recent reports that deal with MCNs for adsorption and separation applications.The inherent basic nature of MCNs coupled with encouraging reports about the basic catalytic properties of MCNs have prompted the scientific community to exploit MCNs as potential adsorbents for the capture of an acidic molecule such as CO2. In recent years, the reports on CO2 adsorption using porous silica materials, pure carbon and amine functionalized silica and carbon materials have been significantly increased owing to the awareness of the greenhouse effect and global warming that mainly results from the release of gases such as CO2 and methane. However, CO2 adsorption on MCNs is a relatively untouched area with not many reports even though they exhibit a large number of basic sites with a high specific surface area and a large pore volume. Li et al. reported the CO2 adsorption capabilities of spherical MCNs prepared using mesoporous cellular silica foams (MCF) as hard templates via a nanocasting approach.61 It was demonstrated that the adsorption temperature has a telling effect on the quantity of CO2 adsorption as adsorption at 25 °C (2.9 mmol g−1) is higher than at 75 °C (0.97 mmol g−1).
CO2 adsorption on MCNs progressively increased with increasing adsorption time and reached a saturation level after a certain time. In comparison to MCNs, the adsorption capacity of pristine carbon prepared using MCF as the hard template via the nanocasting process was much lower at 25 °C (2.5 mmol g−1) and 75 °C (0.3 mmol g−1), which indicates the superior CO2 adsorption properties of MCNs over pure carbon. It is generally believed that the surface area plays a critical role in deciding the overall adsorption capacity of a material; however, in this case, pristine carbon with a higher surface area of 737 m2 g−1 showed a lower adsorption capacity compared to MCNs with a much lower surface area of 550 m2 g−1. The difference in the performance of these two materials could be attributed to the presence of a large number of basic sites due to the high nitrogen content and oxygen containing basic sites in the MCN material. Furthermore, in this case, MCNs have a large number of micropores in addition to the hierarchical mesostructure, mesopores and stable frameworks, which further enhanced its overall CO2 adsorption capacity. It can be said that the overall adsorption capacity of the materials is a cumulative effect of its structural, morphological, textural and basic properties.
Recently, Deng et al. also reported CO2 adsorption isotherms for MCNs and the carbon nitride–carbon composite material (MCN/C) prepared from SBA-15 as the hard template and EDA and CTC as the carbon and nitrogen precursors, respectively.105 Additionally, N2 adsorption isotherms were also reported to show the ability of MCN and MCN/C composite materials to selectively adsorb CO2 from a mixture of CO2/N2, thereby extending its use as a separation medium for the N2/CO2 mixture. MCNs showed a CO2 adsorption capacity of 2.16 and 1.76 mmol g−1 at 0 °C and 25 °C and 1 atm. On the other hand, the MCN/C composite material showed a higher CO2 adsorption capacity compared to the MCNs under identical conditions of temperature and pressure. The superiority of the composite material for CO2 adsorption was attributed to a large number of micropores donated by the extra carbon layer in the composite material. It was the cumulative effect of a large surface area, higher nitrogen content and higher micropore volume that dictated the overall CO2 adsorption capacity of the MCN/C composite materials. Both the MCNs and the MCN/C composite materials showed a strong adsorbate–adsorbent interaction manifested through the reasonable isosteric heat of adsorption in the range of 15.8–19.7 and 20.5–26.2 kJ mol−1 respectively for the MCNs and the MCN/C composite.
Very recently, Lakhi et al. reported high pressure adsorption on a large pore and cage type MCN known as MCN-7-T (where T is the synthesis temperature of the silica template FDU-12) (Fig. 15A).16 Among the materials, it is the MCN-7-130 sample which showed the highest CO2 adsorption of 13.5 mmol g−1 whereas MCN-7-100 and MCN-7-150 exhibited an adsorption capacity of 10.5 and 11.3 mmol g−1 respectively at 0 °C and 30 bar. Furthermore, Lakhi et al. showed the effect of adsorption temperature on the CO2 adsorption capacity of the materials. CO2 adsorption was found to be low at a higher temperature. In this case, the highest adsorption capacity of MCN-7-130 was found to be dependent on the BET surface area. For the three samples, the BET surface area and CO2 adsorption capacity followed the same order of MCN-7-130 > MCN-7-150 > MCN-7-100. The same group also demonstrated how adsorption pressure could be used to increase the adsorption capacity of a MCN. It also highlighted the importance of textural parameters such as a high BET surface area, besides the strength of the basic functional groups. The MCN-7-130 sample showed a strong adsorbate–adsorbent interaction as demonstrated by a high isosteric heat of adsorption in the range 34.95–24.3 kJ mol−1 which was much higher than that reported by Deng et al.105
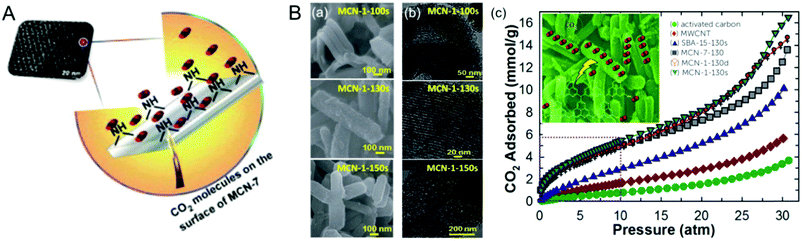 | ||
| Fig. 15 (A) Cage type MCN-7 with large mesopores for CO2 capture. Reprinted with permission from ref. 16. Copyright 2010, Elsevier B. V. (B) MCN-1-Ts with controlled rod shape morphologies and their excellent CO2 adsorption capacity. (a) SEM, (b) TEM images and (c) comparison of CO2 adsorption capacities of MCN-1-130s measured at 0 °C and at a pressure up to 30 bar. Reprinted with permission from ref. 17. Copyright 2015, Royal Society of Chemistry. | ||
Most recently, Lakhi et al. demonstrated the synthesis of highly ordered MCNs with rod shaped morphologies and different pore diameters and reported enhanced CO2 adsorption capacity (Fig. 15B).17 The morphology of the MCNs was controlled by the morphology of the silica template. The group utilized a very simple and cost effective “static” technique to control the morphology of the silica template SBA-15-Ts (T denotes hydrothermal synthesis temperature and s denotes static conditions). The static synthesis approach offered a uniform rod shaped morphology for the mesoporous silica SBA-15 templates, which were replicated into the MCN. The resulting silicas were used as hard templates in a nanocasting process and the morphology was replicated from the template to the MCN (labelled as MCN-1-Ts). Among the samples studied, the sample (MCN-1-130s) prepared at 130 °C registered the highest CO2 adsorption of 16.6 mmol g−1 at 0 °C and 30 bar and it was found to be higher than MCN-1 (14.6 mmol g−1) with an irregular morphology (MCN-1-130d, d = dynamic condition) and 3D cage type MCN-7-130 (13.5 mmol g−1). These results reveal that the morphology of the MCNs plays a critical role in controlling the CO2 adsorption properties of the MCN nanostructures.
The development of porous solid materials that can accommodate large volumes of gas and also have the ability to dispense the same is being pursued with a lot of interest and scientific rigor. Among the gases of interest is hydrogen (H2) gas. The versatile and porous nature of the MCNs offers a green and environmentally benign option of storing large volumes of hydrogen gas. The application of MCNs as hydrogen storage materials is still an area of ongoing scientific activity.
There are a lot of reports on hydrogen adsorption using porous silica106 and porous carbon107 materials, however, only one report involving hydrogen adsorption on MCNs is available. Park et al. reported for the first time the hydrogen storage capabilities of MCNs.64 The group used 2D and 3D high nitrogen containing MCNs prepared using IWM as adsorbents for hydrogen and compared its hydrogen capacity with pure mesoporous carbon FDU-15. The hydrogen uptake of the materials was recorded at 77 K and 298 K and up to 50 bar pressure. It is evident that the adsorption temperature is a critical factor affecting the quantity of H2 adsorbed, as adsorption for both the samples is higher at 77 K than at 298 K. At higher pressures (under 50 bar), the 2D MCNs showed a higher adsorption than the 3D MCNs at 77 K and 298 K. This observation could be attributed to the higher surface area of the 2D MCNs (361 m2 g−1) compared to that of the 3D MCNs (343 m2 g−1), although there is not much difference in the surface areas. However, at lower pressures under 25 bar at 77 K and under 9 bar at 298 K, the 3D MCNs showed a slightly higher adsorption capacity than the 2D MCNs. This observation could be ascribed to the 3D mesostructure and bimodal mesopore distribution of the 3D MCNs. In contrast, pure mesoporous carbon FDU-15 showed a much lower H2 uptake compared to the 2D and 3D MCNs, which suggested superior hydrogen adsorption properties of the MCNs over pure carbon materials and scope for further research in this area.
4.5 Sensing and separation
Environmental remediation and treatment of hazardous waste is another key area of activity involving chemists, material scientists and engineers. Recently, a lot of effort has been put into find environmentally benign solutions for the detection, degradation and clean-up of toxic chemicals and hazardous waste from life supporting sources such as ground drinking water, soil and food.108 In view of the pressing need to find cheaper and efficient solutions, MCNs owing to their semiconducting and inherent basic properties have been used for the detection and separation or removal of toxic molecules and hazardous pollutants. Here we discuss some of the recent reports discussing the use of MCNs for sensing and separation applications.Haque and Vinu co-workers demonstrated for the first time the adsorption capacity of MCNs for the selective removal of phenol and its derivatives from waste water (Fig. 16).13 They used MCN-1 as an adsorbent and compared its capacity with the commercially available adsorbents such as activated carbon and CMK-3-150 (CMK-carbon prepared using SBA-15 at 150 °C as the hard template). The adsorption process followed pseudo-second order kinetics and the maximum adsorption quantity was obtained by fitting the data to Langmuir's equation. The adsorption quantity increased progressively with time for all the three materials, however, MCN-1 showed a much higher adsorption of phenol as compared to the activated carbon and CMK-3-150 under the same adsorption conditions. MCN-1 exhibited the highest phenol adsorption capacity of 609 mg g−1 and it is 2.37 and 1.29 times the adsorption capacity registered at 25 °C when using activated carbon and CMK-3-150, respectively.
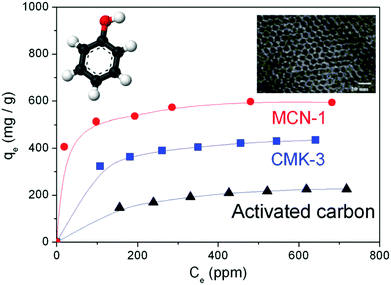 | ||
| Fig. 16 Superior adsorption capacity of MCN-1 for phenol. Reprinted with permission from ref. 13. Copyright 2010, Royal Society of Chemistry. | ||
Furthermore, the endothermic nature of the adsorption process could be attributed to a stronger interaction between the pre-adsorbed water and the adsorbent as compared to the interaction between phenol and adsorbent MCN-1. The positive value of enthalpy ΔH in this case is also suggestive of the chemisorption of the acidic phenol molecule on MCN-1 with basic functional groups such as NH or NH2. The adsorption process was thermodynamically spontaneous and proceeded in the direction of increasing entropy as indicated by the negative Gibbs free energy ΔG and positive entropy change ΔS values. The effect of solution pH on the adsorption quantity was also studied. The amount of phenol adsorption over MCN-1 decreased significantly as the solution pH was increased. This was due to the fact that phenol was converted into phenolate at a higher pH, which poorly interacts with the highly basic surface of MCN-1 because of the strong electrostatic repulsion between the adsorbent and the adsorbate. This finding also supported the fact that the basic sites of MCN-1 really influenced the adsorption of acidic organic molecules such as phenol. It should also be noted that the MCN-1 adsorbent was quite stable even after several adsorption experiments with the phenolic materials from waste water and the adsorption properties were not affected. As mentioned in the previous section, the adsorption properties can be significantly improved after increasing the number of basic groups via treatment with ammonia. It was found that the ammonia treated samples showed a much higher adsorption capacity for formaldehyde as compared to the CMK-3 and non-functionalized MCN-1 samples. This high adsorption capacity was attributed to the highest number of nitrogen functional groups on the surface of MCN-1 together with the micropores which were generated during the treatment with ammonia gas.86
Yan and co-workers109 demonstrated the selective adsorption of perfluoroctane sulfonate (PFOS) and pefluorooctanoic acid (PFOA) using a magnetic MCN. They also found that under the identical conditions, the maximum adsorption capacities for PFOS and PFOA over magnetic MCNs was found to be 454.55 and 370.37 mg g−1 respectively, which indicates that the magnetic MCN has a stronger affinity for PFOS compared to PFOA. The adsorption of PFOS and PFOA by the magnetic MCN was attributed to electrostatic and hydrophobic interactions.
Jia et al. recently reported the selective detection of acidic/basic molecules using highly ordered macro–mesoporous carbon nitride films.110 The macro–mesoporous carbon nitride films showed a strong affinity for acetic acid in preference to aniline. However, when the macro–mesoporous carbon nitride films were treated with UV light and oxygen, the sample showed a stronger affinity for aniline over acetic acid molecules. Therefore, these MCNs are called photo-switch sensors. This result is quite interesting as the photo-switch phenomenon can be applied for the selective sensing of both acidic and basic molecules with the help of MCN nanostructures after the appropriate modification.
Chen et al. also compared the Cr(IV) adsorption capacity of MCN-1 with activated carbon and CMK-3.111 The adsorption capacity of MCN-1 increased with increasing reaction time and reached a constant value. However, CMK-3 showed a lower adsorption capacity than MCN-1 when the same adsorption conditions were used. Interestingly, no change in the adsorption amount was observed for the activated carbon and the adsorption capacity was nearly constant with adsorption time. From the Langmuir isotherm, the maximum Cr(IV) adsorption capacity for MCN-1 was found to be 48.31 mg g−1 whereas activated carbon and CMK-3 registered 35.34 and 35.09 mg g−1 respectively. Although not a huge difference in the Cr(IV) adsorption capacities of these materials was observed, MCN-1 nonetheless showed the highest among the porous materials studied in this work. The effect of temperature on the Cr(IV) adsorption capacity over MCN-1 was studied. It was found that as the solution temperature was increased from 15 to 25 °C, the adsorption capacity was also increased, indicating that the adsorption process was endothermic in nature.
Recently, Mane and Vinu23 co-workers reported the selective sensing capabilities of MCNs for the detection of acidic toxic molecules using a quartz crystal microbalance (QCM). The group prepared MCNs with different pore diameters and textural properties and found that the sample with the largest pore diameter and well-pronounced mesoporosity showed the highest selective sensing performance especially for acidic formic acid molecules in a QCM experiment. The sensing performance of the MCNs was attributed to the acid–base interaction between the acidic molecule and the basic functional groups on the wall structure of the MCNs. However, the MCNs also registered a considerable amount of adsorption of pyridine, which is a basic molecule. It was demonstrated that the uneven distribution of electron density on the surface of pyridine results in a strong interaction between the highly polar pyridine and the amine groups on the surface of the MCNs, which contributed to the adsorption of pyridine molecules. These results reveal that the surface charge of the adsorbent and the adsorbate is important as it dictates the final adsorption capacity of the adsorbent.
5. Conclusions and future outlook
The multifunctional characteristics of MCNs including semi-conductivity, basicity, intercalation and mechanical properties present them as preferred candidates for various applications including catalysis, sensing, adsorption and separation and energy. In this review, we have reviewed the current research trends in the area of MCN materials and considered different synthesis procedures, different C and N precursors, surface modifications and functionalization with organic and inorganic molecules and nanostructures, and the application of MCNs in catalysis (basic, oxidation–reduction and photo catalysis), adsorption, sensing and separation. From the unique structure, textural parameters, semiconducting and catalytic properties and various application possibilities, it was clear that MCNs have a tremendous potential and further high impact discoveries are yet to be made. It is hoped that this review article will serve as a good starting point for beginners in this field and seasoned researchers alike.In view of the plethora of research articles on new materials and technology for energy generation, storage and conversion, it stands to reason that a significant proportion of the scientific research and development activities is aimed at developing alternative, economical and sustainable energy resources before the world runs out of conventional fossil fuel based energy sources. Earlier in this article, we discussed the application of MCNs as a semiconductor based photo catalyst for H2 production (water splitting). Although MCN materials offer excellent performances in the hydrogen evolution reaction through the photocatalytic pathway, they still need expensive co-catalysts and sacrificial agents which make the whole process quite expensive. The future challenge here is to design MCNs with a tunable band structure, with inbuilt functional moieties including organic or inorganic nanostructures in the mesochannels that should be able to split the water molecules without any co-catalysts and sacrificial agents. Photocatalytic water splitting without sacrificial agents has been realized by functionalizing either a hydrogen evolution co-catalyst or a water oxidation cocatalyst onto the surface of non-porous CN materials.112 It is highly anticipated that this methodology will be successful in designing new MCNs functionalized with inorganic nanostructures for water splitting in the future. As presented in this review, MCNs were found to be the best materials not only for splitting of water but also for the capturing of CO2. However, the future challenge would be to utilize both the in-built semiconducting and basic properties in the MCN system to convert the adsorbed CO2 into fuels and chemicals with water as an electron source through the photocatalytic pathway. As both H2O and CO2 are thermodynamically stable molecules, it calls for the careful design of semiconducting properties namely tuning of the band gap to achieve the visible light assisted splitting of water into protons and electrons which should be used for the reduction of CO2 to fuels and chemicals. This approach is similar to photosynthesis where nature uses sunlight and water to reduce CO2 into a number of organic products and every attempt is being made to mimic this naturally occurring photosynthesis process. However, at the moment, limited success has been achieved as far as the artificial photosynthesis process is concerned. Although MCNs present an alternate photoactive semiconducting material for CO2 reduction, only a few reports are available in the literature discussing the possibility of using carbon nitride as a photocatalyst for CO2 reduction using water and sunlight.113,114 These reports suggest that the conversion of CO2 with water using MCNs is a scientifically challenging task and we need to do more research on the fundamental aspects of MCNs including the adsorption and diffusion of both water and CO2 molecules in the mesoporous system, band structure calculations and the role of defect sites. More research on the theoretical understanding of MCNs is also needed to fine tune the band edges, textural parameters and crystal structure of the MCN as this information is highly critical to activate both CO2 and water molecules.
Although MCNs have been effectively used as catalysts for various applications, they still suffer from a lot of defect sites and poor surface areas. Some catalyses require MCNs with defect sites but light assisted catalysis would need MCNs with a defect free CN framework. However, only little attention has been given to control the defect sites of MCNs and their role on the MCNs. More fundamental studies involving theoretical calculations are required to predict and understand the synthesis mechanisms and their role in controlling the defect sites in MCNs. The specific surface area of MCNs is a major concern. Only a hard templating approach offered MCNs with a high surface area but it is not a cost-effective process as it requires multiple steps involving sacrificial templates and high temperature calcination processes. It is also important to put more efforts on synthesising MCN using a template free approach through a soft templating approach which will pave the path for commercialization of these materials.
Another challenge is to prepare carbon nitrides with different C and N stoichiometric ratios such as C3N5, C3N6, C3N7 and C3N8 and different dopants to control the band edges of the materials. At the moment, a vast majority of reports are focused on the C3N4 or C4N type of configuration. In order to prepare MCNs with different nitrogen contents, a careful selection of carbon and nitrogen precursors together with a suitable carbonization temperature and the choice of hard template are required to achieve MCNs with different nitrogen contents. The textural parameters of the reported C3N4 type of MCNs are not so impressive, which further opens opportunities to devise a new methodology for the synthesis of MCNs with a higher surface area and pore volume. It would also be interesting to prepare MCNs with controlled morphologies, crystal structures, and porous structures, and to study the effects of morphology and band structure on the photo-catalytic applications.
Another important aspect of MCNs that has not been explored sufficiently is to use them in energy storage and conversion applications. It is expected that these metal free MCNs have the capabilities to replace the so-called “golden photocatalyst” titania for dye sensitised quantum dots and organic solar cells. These materials could also be used as electrodes for supercapacitors, fuel cells, and battery applications and it is quite surprising that MCNs have not been effectively utilized for supercapacitors, batteries and fuel cell applications. It is also expected that MCNs, after proper functionalization with metal or metal oxide nanoparticles or different functional groups on the surface will further expand their application possibilities in catalysis. For example, the introduction of acid functionalities on the surface of MCNs, which already have basic functionalities together with semiconducting features, would enable MCNs to be used as enzyme-like catalysts for efficient and highly specific organic transformations with the help of light radiation.
Acknowledgements
One of the authors A. Vinu is grateful to ARC for the award of future fellowship and the University of South Australia and the Future Industries Institute for the start-up grants and Alexander von Humboldt foundation for the Friedrich Wilhelm Bessel Research Award. The authors are also grateful to SABIC Pty Ltd for the award of the research project with the University of South Australia (PG 083037).Notes and references
- Y. Liu and M. L. Cohen, Science, 1989, 245, 841 Search PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76 CrossRef CAS PubMed.
- Y. Zheng, J. Liu, J. Liang, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2012, 5, 6717 CAS.
- Y. Zheng, Y. Jiao, M. Jaroniec, Y. Jin and S. Z. Qiao, Small, 2012, 8, 3550 CrossRef CAS PubMed.
- G. Dong, K. Zhao and L. Zhang, Chem. Commun., 2012, 48, 6178 RSC.
- G. Liu, P. Niu, C. Sun, S. C. Smith, Z. Chen, G. Q. Smith and H. M. Cheng, J. Am. Chem. Soc., 2010, 132, 11642 CrossRef CAS PubMed.
- J. Xu, L. Zhang, R. Shi and Y. Zhu, J. Mater. Chem. A, 2013, 1, 14766–14772 CAS.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452 CrossRef CAS PubMed.
- K. Schwinghammer, M. B. Mesch, V. Duppel, C. Ziegler, J. Senker and B. V. Lotsch, J. Am. Chem. Soc., 2014, 136, 1730 CrossRef CAS PubMed.
- A. Thomas, A. Fisher, F. Goettmann, M. Antonietti, J. O. Muller, R. Schlogl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893 RSC.
- A. Vinu, K. Ariga, T. Mori, T. Nakanishi, S. Hishita, D. Golberg and Y. Bando, Adv. Mater., 2005, 17, 1648 CrossRef CAS.
- A. Vinu, Adv. Funct. Mater., 2008, 18, 816 CrossRef CAS.
- E. Haque, J. W. Jun, S. N. Talapaneni, A. Vinu and S. H. Jhung, J. Mater. Chem., 2010, 20, 10801 RSC.
- C. Anand, S. V. Priya, G. Lawrence, G. P. Mane, D. S. Dhawale, K. S. Prasad, V. V. Balasubramanian, M. A. Wahab and A. Vinu, Catal. Today, 2013, 204, 164 CrossRef CAS.
- L. Zhong, C. Anand, K. S. Lakhi, G. Lawrence and A. Vinu, Sci. Rep., 2015, 5, 12901 CrossRef PubMed.
- K. S. Lakhi, W. S. Cha, S. Joseph, B. J. Wood, S. S. Aldeyab, G. Lawrence, J. H. Choy and A. Vinu, Catal. Today, 2015, 243, 209 CrossRef CAS.
- K. S. Lakhi, A. V. Baskar, J. S. M. Zaidi, S. S. Al-Deyab, M. El-Newehy, J. H. Choy and A. Vinu, RSC Adv., 2015, 5, 40183 RSC.
- K. K. R. Datta, B. V. S. Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961 CrossRef CAS PubMed.
- A. Vinu, T. Mori and K. Arig, Sci. Technol. Adv. Mater., 2006, 7, 753 CrossRef CAS.
- A. Vinu, P. Srinivasu, D. P. Sawant, T. Mori, K. Ariga, J. S. Chang, S. H. Jhung, V. V. Balasubramanian and Y. K. Hwang, Chem. Mater., 2007, 19, 4367 CrossRef CAS.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. Q. Lu and A. Vinu, Angew. Chem., Int. Ed., 2009, 48, 7884 CrossRef CAS PubMed.
- S. N. Talapaneni, G. P. Mane, A. Mano, C. Anand, D. S. Dhawale, T. Mori and A. Vinu, ChemSusChem, 2012, 5, 700 CrossRef CAS PubMed.
- G. P. Mane, D. S. Dhawale, C. Anand, K. Ariga, Q. Ji, M. A. Wahab, T. Mori and A. Vinu, J. Mater. Chem. A, 2013, 1, 2913 CAS.
- S. N. Talapaneni, S. Anandan, G. P. Mane, C. Anand, D. S. Dhawale, S. Varghese, A. Mano, T. Mori and A. Vinu, J. Mater. Chem., 2012, 22, 9831 RSC.
- H. Min, M. B. Ansari, Y. H. Mo and S. E. Park, Catal. Today, 2013, 204, 156 CrossRef.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467 CrossRef CAS PubMed.
- J. Xu, H. T. Wu, X. Wang, B. Xue, Y. X. Lin and Y. Cao, Phys. Chem. Chem. Phys., 2013, 15, 4510 RSC.
- J. Xu, T. Chen, X. Wang, B. Xue and Y. X. Li, Catal. Sci. Technol., 2014, 4, 2126 CAS.
- Z. Zhao, Y. Dai, J. Lin and G. Wang, Chem. Mater., 2014, 26, 3151 CrossRef CAS.
- Y. Wang, X. Wang, M. Antonietti and Y. Zhang, ChemSusChem, 2010, 3, 435 CrossRef CAS PubMed.
- Y. Wang, J. Zhang, X. Wang, M. Antonietti and H. Li, Angew. Chem., Int. Ed., 2010, 49, 3356 CrossRef CAS PubMed.
- Y. S. Jun, W. H. Hong, M. Antonietti and A. Thomas, Adv. Mater., 2009, 21, 4270 CrossRef CAS.
- A. Fischer, J. O. Muller, M. Antonietti and A. Thomas, ACS Nano, 2008, 2, 2489 CrossRef CAS PubMed.
- N. S. Alhajri, H. Yoshida, D. H. Anjum, A. T. G. Esparza, J. Kubota, K. Domen and K. Takanabe, J. Mater. Chem. A, 2013, 1, 12606 CAS.
- X. H. Li, X. Wang and M. Antonietti, Chem. Sci., 2012, 3, 2170 RSC.
- L. Yuliati, J. H. Yang, X. Wang, K. Maeda, T. Takata, M. Antonietti and K. Domen, J. Mater. Chem., 2010, 20, 4295 RSC.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Chem. Commun., 2006, 4530 RSC.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, New J. Chem., 2007, 31, 1455 RSC.
- J. Xu, K. Z. Long, T. Chen, B. Xue, Y. X. Li and Y. Cao, Catal. Sci. Technol., 2013, 3, 3192 CAS.
- M. B. Ansari, B. H. Min, Y. H. Mo and S. E. Park, Green Chem., 2011, 13, 1416 RSC.
- M. B. Ansari, H. Jin and S. E. Park, Catal. Sci. Technol., 2013, 3, 1261 CAS.
- X. Wang, K. Maeda, X. F. Chen, K. Takanabe, K. Domen, Y. D. Hou, X. Z. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680 CrossRef CAS PubMed.
- H. Yan, Chem. Commun., 2012, 48, 3430 RSC.
- X. Gao, X. Jiao, L. Zhang, W. Zhu, X. Xu, H. Ma and T. Chen, RSC Adv., 2015, 5, 76963 RSC.
- F. Su, S. C. Mathew, G. Lipner, X. Fu, M. Antonietti, S. Blechert and X. Wang, J. Am. Chem. Soc., 2010, 132, 16299 CrossRef CAS PubMed.
- K. Ariga, Q. Ji, J. P. Hill and A. Vinu, Soft Matter, 2009, 5, 3562 RSC.
- Z. Yang, Y. Zhang and Z. Schnepp, J. Mater. Chem. A, 2015, 3, 14081–14092 CAS.
- S. Polarz and M. Antonietti, Chem. Commun., 2002, 2593 RSC.
- T. Ma, L. Liu and Z. Y. Yuan, Chem. Soc. Rev., 2013, 42, 3977 RSC.
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828 CrossRef CAS PubMed.
- T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T.-W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- Q. Huo, D. I. Margolese, U. Ciesla, P. Feng, T. E. Gier, P. Sieger, R. Leon, P. M. Petroff, F. Schüth and G. D. Stucky, Nature, 1994, 368, 317 CrossRef CAS.
- Q. Huo, R. Leon, P. M. Petroff and G. D. Stucky, Science, 1995, 268, 1324 CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS PubMed.
- M. Hartmann, A. Vinu, S. P. Elangovan, V. Murugesan and W. Böhlmann, Chem. Commun., 2002, 1238 RSC.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS.
- J. Lee, S. Yoon, T. Hyeon, S. M. Oh and K. B. Kim, Chem. Commun., 1999, 2177 RSC.
- T. Kyotani, T. Nagai, S. Inoue and A. Tomita, Chem. Mater., 1997, 9, 609 CrossRef CAS.
- Q. Li, J. Yang, D. Feng, Z. Wu, Q. Wu, S. S. Park, C. S. Ha and D. Zhao, Nano Res., 2010, 3, 632 CrossRef CAS.
- M. J. Bojdys, J. O. Muller, M. Antonietti and A. Thomas, Chem. – Eur. J., 2008, 14, 8177 CrossRef CAS PubMed.
- S. Hwang, S. Lee and J. S. Yu, Appl. Surf. Sci., 2007, 253, 5656 CrossRef CAS.
- S. S. Park, S. W. Chu, C. Xue, D. Zhao and C. S. Ha, J. Mater. Chem., 2011, 21, 10801 RSC.
- S. C. Lee, H. O. Lintang and L. Yuliati, Chem. – Asian J., 2012, 7, 2139 CrossRef CAS PubMed.
- K. Kailasam, J. D. Epping, A. Thomas, S. Losse and H. Junge, Energy Environ. Sci., 2011, 4, 4668 CAS.
- Y. Cui, J. Zhang, G. Zhang, J. Huang, P. Liu, M. Antonietti and X. Wang, J. Mater. Chem., 2011, 21, 13032 RSC.
- S. Yang, W. Zhou, C. Ge, X. Liu, Y. Fang and Z. Li, RSC Adv., 2013, 3, 5631 RSC.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, L. Cheng, D. Feng, Z. Wu, Z. Chen, Y. Wan, A. Stein and D. Zhao, Chem. Mater., 2006, 18, 4447 CrossRef CAS.
- H. Kosonen, S. Valkama, A. Nykanen, M. Toivanen, G. T. Breinke, J. Ruokolainen and O. Ikkala, Adv. Mater., 2006, 18, 201 CrossRef CAS.
- C. Liang, K. Hong, G. A. Guiochon, J. W. Mays and S. Dai, Angew. Chem., 2004, 116, 5909 CrossRef.
- W. Shen, L. Ren, H. Zhou, S. Zhang and W. Fan, J. Mater. Chem., 2011, 21, 3890 RSC.
- S. Min and G. Lu, J. Phys. Chem. C, 2012, 116, 19644 CAS.
- S. Kumar, T. Surendar, B. Kumar, A. Baruah and V. Shanker, RSC Adv., 2014, 4, 8132 RSC.
- Y. Lu, Z. Wen, J. Jin, Y. Cui, M. Wu and S. Sun, J. Solid State Electrochem., 2012, 16, 1863 CrossRef CAS.
- P. Zhang, F. Jiang and H. Chen, Chem. Eng. J., 2013, 234, 195 CrossRef CAS.
- Y. Gong, P. Zhang, X. Xu, Y. Li, H. Li and Y. Wang, J. Catal., 2013, 297, 272 CrossRef CAS.
- Y. Li, Y. Gong, X. Xu, P. Zhang, H. Li and Y. Wang, Catal. Commun., 2012, 28, 9 CrossRef CAS.
- Y. Bu, Z. Chen and W. Li, Appl. Catal., B, 2014, 144, 622 CrossRef CAS.
- X. H. Li, M. Baar, S. Blechert and M. Antonietti, Sci. Rep., 2013, 3, 1743 Search PubMed.
- S. Yang, W. Zhou, C. Ge, X. Liu, Y. Fang and Z. Li, RSC Adv., 2013, 3, 5631 RSC.
- K. Takanabe, K. Kamata, X. Wang, M. Antonietti, J. Kubota and K. Domen, Phys. Chem. Chem. Phys., 2010, 12, 13020 RSC.
- S. C. Lee, H. O. Lintang and L. Yuliati, J. Exp. Nanosci., 2014, 9, 78 CrossRef CAS.
- J. Wu, L. Lio, W. Yan, Y. Xue, Y. Sun, X. Yan, X. Yan, Y. Chen and Y. Xie, ChemSusChem, 2012, 5, 1207 CrossRef CAS PubMed.
- Y. Chen, J. Zhang, M. Zhang and X. Wang, Chem. Sci., 2013, 4, 3244 RSC.
- M. J. Yu, A. Vinu, S. H. Park, J.-K. Jeon, S. H. Jhung and Y.-K. Park, Sci. Adv. Mater., 2014, 6, 1511 CrossRef CAS.
- X. Ye, Y. Zheng and X. Wang, Chin. J. Chem., 2014, 32, 498 CrossRef CAS.
- Y. Hou, A. B. Laursen, J. Zhang, G. Zhang, Y. Zhu, X. Wang, S. Dahl and I. Chorkendorff, Angew. Chem., Int. Ed., 2013, 52, 3621 CrossRef CAS PubMed.
- K. Kailasam, A. Fischer, G. Zhang, J. Zhang, M. Schwarze, M. Schroder, X. Wang, R. Schomacker and A. Thomas, ChemSusChem, 2015, 8, 1404 CrossRef CAS PubMed.
- A. Fischer, M. Antonietti and A. Thomas, Adv. Mater., 2007, 19, 264 CrossRef CAS.
- N. S. Alhajri, D. H. Anjum and K. Takanabe, J. Mater. Chem. A, 2014, 2, 10548 CAS.
- L. Yuliati, J.-H. Yang, X. Wang, K. Maeda, T. Takata, M. Antonietti and K. Domen, J. Mater. Chem., 2010, 20, 4295 RSC.
- M. B. Ansari, H. Jin, M. N. Parvin and S. E. Park, Catal. Today, 2012, 185, 211 CrossRef CAS.
- F. Su, M. Antonietti and X. Wang, Catal. Sci. Technol., 2012, 2, 1005 CAS.
- P. Zhang, F. Jiang and H. Chen, Chem. Eng. J., 2013, 234, 195 CrossRef CAS.
- Y. Li, Y. Gong, X. Xu, P. Zhang, H. Li and Y. Wang, Catal. Commun., 2012, 28, 9 CrossRef CAS.
- A. Corma and H. Garcia, Chem. Rev., 2002, 102, 3837 CrossRef CAS PubMed.
- F. Goettmann, A. Thomas and M. Antonietti, Angew. Chem., Int. Ed., 2007, 46, 2717 CrossRef CAS PubMed.
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- F. E. Osterloh, Chem. Mater., 2008, 20, 35 CrossRef CAS.
- X. Chen, Y.-S. Jun, K. Takanabe, K. Maeda, K. Domen, X. Fu, M. Antonietti and X. Wang, Chem. Mater., 2009, 21, 4093 CrossRef CAS.
- J. Hong, X. Xia, Y. Wang and R. Xu, J. Mater. Chem., 2012, 22, 15006 RSC.
- X. Chen, J. Zhang, X. Fu, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 11658 CrossRef CAS PubMed.
- H. Zhan, W. Liu, M. Fu, J. Cen, J. Lin and H. Cao, Appl. Catal., A, 2013, 468, 184 CrossRef CAS.
- Q. F. Deng, L. Liu, X.-Z. Lin, G. Du, Y. Liu and Z.-Y. Yuan, Chem. Eng. J., 2012, 203, 63 CrossRef CAS.
- N. Z. Logar and V. Kaucic, Acta Chim. Slov., 2006, 53, 117 CAS.
- Y. Xia, Z. Yang and Y. Zhu, J. Mater. Chem. A, 2013, 1, 9365 CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, Int. J. Hydrogen Energy, 2007, 32, 2664 CrossRef CAS.
- T. Yan, H. Chen, F. Jiang and X. Wang, J. Chem. Eng. Data, 2014, 59, 508 CrossRef CAS.
- L. Jia, H. Wang, D. Dhawale, C. Anand, M. A. Wahab, Q. Ji, K. Ariga and A. Vinu, Chem. Commun., 2014, 50, 5976 RSC.
- H. Chen, T. Yan, X. Wang and F. Jiang, J. Taiwan Inst. Chem. Eng., 2014, 45, 1842 CrossRef CAS.
- G. Zhang, Z.-A. Lan, L. Lin, S. Lin and X. Wang, Chem. Sci., 2016, 7, 3062 RSC.
- R. Kuriki, H. Matsunaga, T. Nakashima, K. Wada, A. Yamakata, O. Ishitani and K. Maeda, J. Am. Chem. Soc., 2016, 138, 5159 CrossRef CAS PubMed.
- Y. Zheng, L. Lin, X. Ye, F. Guo and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 11926 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |