Exceptional energy and new insight with a sodium–selenium battery based on a carbon nanosheet cathode and a pseudographite anode†
Jia
Ding
*ab,
Hui
Zhou
b,
Hanlei
Zhang
b,
Tyler
Stephenson
a,
Zhi
Li
a,
Dimitre
Karpuzov
c and
David
Mitlin
*d
aChemical and Materials Engineering, University of Alberta, Edmonton, Alberta T6G 2V4, Canada. E-mail: dingjiasiom@gmail.com
bNorthEast Center for Chemical Energy Storage, State University of New York, Binghamton, New York 13902, USA
cAlberta Center for Surface Engineering and Science (ACSES), University of Alberta, Edmonton, AB CA T6G 2G6, Canada
dChemical & Biomolecular Engineering, Clarkson University, Potsdam, NY 13699, USA. E-mail: dmitlin@clarkson.edu
First published on 29th September 2016
Abstract
We created a unique sodium ion battery (NIB, SIB) cathode based on selenium in cellulose-derived carbon nanosheets (CCNs), termed Se-CCN. The elastically compliant two-dimensional CCN host incorporates a high mass loading of amorphous Se (53 wt%), which is primarily impregnated into 1 cm3 g−1 nanopores. The results in facile sodiation kinetics due to short solid-state diffusion distances and a large charge transfer area of the nanosheets were established. The architecture also leads to an intrinsic resistance to polyselenide shuttle and to disintegration/coarsening. As a Na half-cell, the Se-CCN cathode delivers a reversible capacity of 613 mA h g−1 with 88% retention over 500 cycles. The exceptional stability is achieved by using a standard electrolyte (1 M NaClO4 EC-DMC) without secondary additives or high salt concentrations. The rate capability is also superb, achieving 300 mA h g−1 at 10C. Compared to recent state-of-the-art literature, the Se-CCN is the most cyclically stable and offers the highest rate performance. As a Se–Na battery, the system achieves 992 W h kg−1 at 68 W kg−1 and 384 W h kg−1 at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 W kg−1 (by active mass in a cathode). We are the first to fabricate and test a Se-based full NIB, which is based on Se-CCN coupled to a Na intercalating pseudographitic carbon (PGC) anode. It is demonstrated that the PGC anode increases its structural order in addition to dilating as a result of Na intercalation at voltages below 0.2 V vs. Na/Na+. The {110} Na reflections are distinctly absent from the XRD patterns of PGC sodiated down to 0.001 V, indicating that the Na metal pore filling is not significant for pseudographitic carbons. The battery delivers highly promising Ragone chart characteristics, for example yielding 203 and 50 W h kg−1 at 70 and 14
144 W kg−1 (by active mass in a cathode). We are the first to fabricate and test a Se-based full NIB, which is based on Se-CCN coupled to a Na intercalating pseudographitic carbon (PGC) anode. It is demonstrated that the PGC anode increases its structural order in addition to dilating as a result of Na intercalation at voltages below 0.2 V vs. Na/Na+. The {110} Na reflections are distinctly absent from the XRD patterns of PGC sodiated down to 0.001 V, indicating that the Na metal pore filling is not significant for pseudographitic carbons. The battery delivers highly promising Ragone chart characteristics, for example yielding 203 and 50 W h kg−1 at 70 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1 (via total material mass in the anode and cathode).
000 W kg−1 (via total material mass in the anode and cathode).
Introduction
Lithium-ion batteries (LIBs) are rapidly advancing as an essential green energy technology powering electric vehicles, plug-in hybrid vehicles, portable electronics and grid energy storage.1,2 Recently sodium-ion batteries (NIBs, SIBs) have also garnered increasing interest because of the low cost and geographically wide-spread terrestrial reserves of sodium.3–9 Unfortunately, insufficient energy density of the devices becomes a critical limiting factor for the wider applications of both systems.10,11 To address this energy-density deficit, researchers are developing the next generation of electrode materials with a higher specific capacity. Certain success has been achieved on the anode side by replacing traditional graphite with Si-based electrodes for LIBs12 and Sn-based electrodes for NIBs13,14 (among many other candidate materials). However the gravimetric capacity of both LIB and NIB cathodes remains lower than that of graphite or other anode materials. For instance, most of the Li-ion intercalation-based cathodes (e.g. LCO, NCA, and LFP)15–18 yield capacities below 200 mA h g−1. For NIBs the cathode capacities are generally even less, being in the 150 mA h g−1 range at most.19,20One approach for achieving a significantly higher cathode capacity, albeit at the expense of a narrower voltage window, is to utilize the reaction between lithium and sulfur. S-based conversion cathodes have the potential to provide 2–5 times the energy density of traditional intercalation LIB cathodes.21,22 However these systems also face major challenges in terms of Coulombic efficiency, rate performance and cycling stability. Sulfur is both ionically and electrically insulating, with the lithium polysulfide discharge products being electrically insulating as well. This leads to low utilization of the active sulfur.23 Moreover, significant capacity decay is endemic, being in part caused by the shuttle mechanisms of the intermediate discharge products.24 To reduce the polysulfide shuttle effect, researchers have employed low-order sulfur species, which yield polysulfides with reduced solubility in the carbonate electrolytes.25,26 It has also been possible to restrict the motion of the active sulfur by physically trapping it within various matrices (e.g. carbons27,28 and metal oxides29,30) and/or promoting stronger chemical bonding between sulfur species and the matrix by changing its surface chemistry.31–34 Early work on Na–S showed its utility for high temperature battery applications.35,36 Recently, several exciting studies have shown a potential path toward ambient temperature Na–S systems as well.37–39 For instance, the authors of ref. 40 created a membrane-electrode-assembly (MEA) comprised of a carbon-coated, presodiated Nafion membrane and a sodium sulfide cathode. A 2016 work utilised a microporous carbon–sulfur composite cathode, and a custom electrolyte based on ionic liquid 1-methyl-3-propylimidazolium-chlorate tethered to SiO2 nanoparticles.41
Selenium has similar chemical properties to sulfur but much higher electrical conductivity (1 × 10−3vs. 0.5 × 10−27 S m−1). Although selenium has a lower gravimetric capacity with Na/Li than sulfur, it has comparable volumetric capacity; an important factor for devices where space is limited.42 Similar to sulfur cathodes, the shuttle effect also exists for selenium cathodes, leading to cycling deterioration.43 Selenium also possesses a much higher reaction activity with Na at room temperature,44 making it a desirable choice for ambient applications. Therefore Se has been regarded as another promising cathode material for both sodium ion batteries45–49 and lithium ion batteries e.g.ref. 50–59.
To date the number of Na–Se publications is limited to five (the above references) all employing fundamentally different approaches than what is employed here. It is useful to summarize and contrast the previous state-of-the-art on Na–Se versus the current work. The authors in ref. 45 created a Se in a carbon system through in situ carbonization of perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) and selenium (Se) in a sealed vacuum glass tube. The ultimate mass loading of Se was 54%, the highest reported for a Na–Se system. The resultant “in situ formed Se/C composite” consisted of relatively coarse particulates with a blocky morphology resembling that of activated carbon. The Se/C particles were on the scale of 1 micron. The carbon host was graphitic and the Se was at least in part crystalline. The authors in ref. 46 began with mesoporous carbon spheres obtained by carbonizing resorcinol combined with a triblock copolymer and formaldehyde, followed by activation. The spheres were several microns in diameter, possessed a BET surface area of 462 m2 g−1, and a pore volume of 0.2 cm3 g−1. The Se was impregnated into the carbon with a mass loading of 30% via vacuum annealing. The authors report that in the impregnated state the Se remained crystalline and the carbon host was partially graphitized.
The authors of ref. 47 used Pluronic F127/N,N-dimethylformamide (PAN-F127/DMF) as the carbon precursor which was electrospun into fibers that were 400 nm thick and were tens of microns in length. The fibers were annealed at 280 °C, then carbonized in nitrogen at 800 °C and finally chemically activated with KOH. The final carbon host possessed analogous length dimensions to the as-spun fibers but appeared somewhat thinner, in the 300 nm range according to the micrographs provided. Se was then infiltrated through a co-heating process in a sealed environment. Before infiltration the fibers possessed a BET surface area of 936 m2 g−1. Following infiltration the fibers still had unfilled porosity, the surface area being 85 m2 g−1. The starting fibers were mesoporous, with channel-like porosity, which were partially filled with Se. The Se loading was 52% and 50%. The same research group utilised carbonized electrospun polyacrylonitrile (PAN)–CNT nanofibers as the Se host, along with carbon nanotubes as a supporting phase.48 The morphology and structure of the Se-in-fiber electrodes (35% Se loading), as well as the resultant electrochemical performance in a half-cell, are analogous to their other study. In ref. 49 the authors carbonized electrospun polyacrylonitrile/selenium (CPAN/Se) and then co-annealed it with Se in a vacuum. The fibers were approximately 1000 nm thick, while the Se mass loading was 36%. The fibers were not activated and there is no porosity reported, implying that the Se coats the fiber’s macroscopic surface directly.
Our study uses a renewable tree-derived nanocrystalline cellulose (NCC) precursor for the carbon matrix. To our knowledge, researchers have not previously employed NCC to create a Se (or any Na-active material) host. To create the carbon nanosheets, NCC was hydrothermally treated followed by KOH activation. Selenium impregnation is a multi-step process involving high-energy planetary co-milling, and a two-stage co-annealing process inside an argon filled sealed glass tube. The resultant selenium in the cellulose-derived carbon nanosheet (Se-CCN) microstructure is unique: the macroscopically open electrode architecture consists of large carbon sheets arranged in three-dimensions, with the Se impregnated into the nanopores. The pore volume in the carbon nanosheets is high relative to other classes of host carbons, being measured as 1 cm3 g−1 and with a surface area of 1610 m2 g−1. The maximum carbon nanosheet thickness is 100 nm, while its horizontal dimensions are in microns, making the architecture highly elastically compliant. The impregnated Se is fully X-ray amorphous, with there is no evidence that an equilibrium hexagonal phase is present. As will be demonstrated, amorphous Se is key to electrochemical cycling stability, due to inhibition of polyselenide formation and first cycle sodiation-induced pulverization. The carbon nanosheets themselves are also fully X-ray amorphous, containing minimal graphitic ordering. This prevents intercalation of Na into the carbon matrix, which would also lead to cycling-induced electrode disintegration. The mass loading of Se is 53%, which is among the highest reported, being second only to the 54% reported in ref. 45. Importantly, we are the first to create and test a full Se-based Na ion battery (NIB), which consists of a Se-CCN cathode and a pseudographitic carbon anode.
Results and discussion
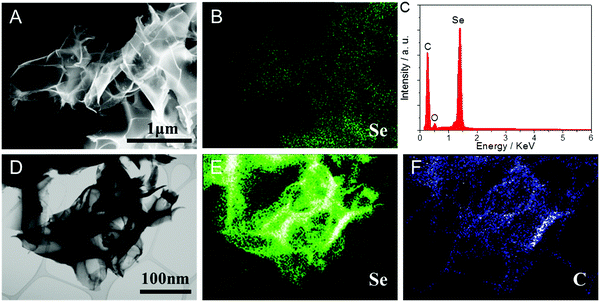
The carbon nanosheet framework was synthesized from a nanocrystalline cellulose (NCC) precursor. The detailed synthesis strategy is described in the Experimental section. To summarize, we combined hydrothermal processing and KOH heat treatment to exfoliate, carbonize and activate the NCC into highly porous sheet-like carbons. Selenium was then loaded into the pores of the carbon nanosheets through a two-step impregnation-annealing process. The first step is a selenium infiltration process at a temperature of 260 °C, which is above its melting point of 221 °C. The second step is a 600 °C soak that removes the residual selenium not wetted into the pores. It also likely enhances the strength of the carbon–selenium bonds inside the pores. The final nanocomposite is termed “Se-CCN”, i.e. Se-cellulose derived carbon nanosheets. As baselines, we also prepared a “Se/C mixture” specimen by physically mixing activated carbon and selenium powder using a mortar and pestle. Pristine selenium powder, termed “Se”, was also tested as a baseline.Fig. 1A shows a scanning electron microscopy (SEM) image of Se-CCN. The material displays a macroscopically open architecture of large carbon sheets arranged in three-dimensions. The surface of the materials is quite smooth, without individual selenium particles being detectable. Fig. 1B gives an energy-dispersive X-ray (EDX) Se map of the region shown in Fig. 1A. Through comparison of the SEM micrograph and EDX map, it may be concluded that there are no isolated islands of Se on the CCN surface and that its distribution is quite uniform. Fig. 1D shows a field emission (FE) TEM bright field micrograph of another region in the Se-CCN material. Again, there is no detectable evidence of “stray” Se particles/islands/crystallites attached on or near the CCN surfaces. The TEM EDX maps of Se and C, shown in Fig. 1E and F, indicate uniform loading of Se throughout the carbon matrix. According to both EDX and XPS analysis, the only detectable impurity in Se-CCN is oxygen. As will be demonstrated by the BET results (Fig. 2), Se fills the micropores in the CCNs, eliminating the vast majority of nitrogen access to their inner structure. We argue that such uniform Se distribution is due to the sheet-like morphology and sub 100 nm thickness (estimated from edge-on sections) of the CCN matrix. During synthesis, the nanoporosity is sufficiently shallow as to permit impregnation of molten Se within a reasonable time frame. It will be demonstrated that such an intimate Se–carbon architecture will result in excellent electrode stability and rate performance. If any Se remains present outside the pores, it is uniformly wetted on the CCN surface, while being mechanically/chemically anchored at the numerous nanopore–surface terminations. The beneficial effect of mechanical anchoring has been reported for S and for MnO in various carbons, e.g.ref. 60–62. It is expected that nanopore-anchored Se would be likewise electrochemically stable and quick to sodiate/desodiate. Overall, however, the terminal high temperature heat treatment should eliminate most of the non-impregnated Se phase from the microstructure.
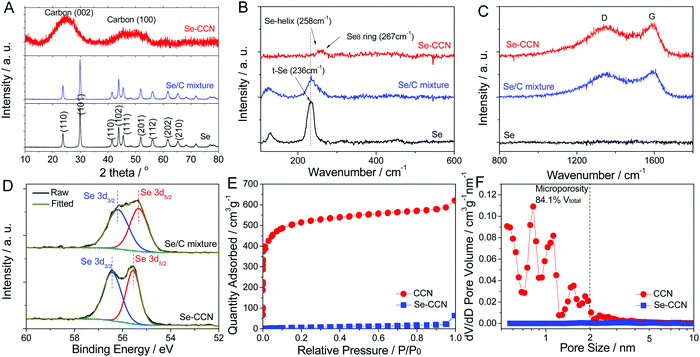
The microstructure of the Se-CCN materials was further analyzed using X-ray diffraction (XRD). The bottom of Fig. 2A shows the XRD pattern of the pristine selenium powders and the Se/C mixture employed as a baseline. In both cases the Se is highly crystalline with a well-defined sharp Bragg reflection indexed to the equilibrium hexagonal lattice (trigonal crystal system P3221). Conversely the Se in Se-CCN is X-ray amorphous, with the only observed reflection being the broad (002) and (100) reflections associated with the short-range ordering in the amorphous carbon matrix. The amorphous CCN carbon matrix is structurally different from the carbon nanosheets obtained from coconut shells, hemp fiber or peanut shells. The coconut-derived carbon is entirely composed of layered graphene planes,63 while carbon from hemp fiber and peanut shells consists of multilayer graphene separated by regions of disorder.64,65 This difference may be attributed to the higher content of lignin in coconut shells, hemp fiber and peanut shells, which promotes graphitic ordering at high temperatures. The amorphous structure of the CCN is highly useful for promoting enhanced cycling as it will not allow for Na intercalation at any voltage. This in turn prevents Na insertion-induced dilation, which may lead to pulverization of the carbon over extended cycling.66–68 As demonstrated by the TGA curve of Se-CCN, shown in Fig. S1 (ESI†), the mass loading of Se is 53 wt%. This mass loading is high enough that if crystalline Se were to be present in Se-CCN, its Bragg reflections would be distinct from the amorphous carbon signal in the X-ray pattern. However since Se Bragg peaks are not discernible, it is safe to conclude that crystalline Se is absent from the composite. This agrees with previous Se-into-carbon-host impregnation studies, where only an amorphous phase is detected once Se is fully penetrated into the pores.55,56
Raman spectroscopy was used to further investigate the structure of Se-CCN. As shown in Fig. 2B, pristine selenium powder exhibits a pronounced band at 236 cm−1, which corresponds to trigonal Se.69 This main band is likewise observed for the Se/C specimen indicating that the Se structure is unchanged after physical mixing, in accordance with the XRD pattern. However, for Se-CCN, the trigonal Se band disappeared. Instead a weak broad band located around 260 cm−1 is present. This band is attributed to the combination of the bond stretching modes of a Se single helix and a Se8 ring, both being short-range structures.46,70 Thus these results of Raman spectroscopy indicate the existence of only short-range ordered selenium in Se-CCN, with no observable long-range order in the material, in accordance with the XRD results. Low-order Se is expected to be beneficial for electrochemical cycling stability, due to inhibition of the polyselenide formation that is known to be severe in crystalline Se and S electrodes against Li or Na.46,50,55,56 The two broad bands observed in Se-CCN at 1347 and 1592 cm−1 are ascribed to the D- and G-bands of the amorphous CCN matrix (Fig. 2C). The intensity ratio of IG to ID is 0.80, confirming the highly disordered structure. Fig. S2 (ESI†) shows a TEM selected area electron diffraction pattern (SAED) for the Se-CCN material. Due to the amorphous structure of both selenium and carbon, the pattern does not show any Bragg diffraction spots, displaying only an amorphous halo associated with short-range near neighbor positions in both materials.
High resolution X-ray photoelectron spectroscopy (XPS) was utilized to investigate the chemical state of Se. According to the XPS survey spectrum, O is detectable in Se-CCN at the level of 6.3 wt% (Fig. S3, ESI†). As shown in Fig. 2D, the standard binding energy of elemental Se is 55.3 and 56.2 eV for Se 3d5/2 and Se 3d3/2 respectively, which are also the values for the Se/C specimen. For the Se-CCN specimen, however, a shift of ∼0.6 eV towards a higher binding energy is observed for both peaks. This shift is caused by the electron cloud density change of Se and indicates that the selenium atoms contacting CCNs have formed some chemical bonds with it.71 A similar shift has been observed by others who have successfully impregnated Se into a carbon host, achieving some chemical interaction between the two.45,48,49,72 The bonding between the selenium and the carbon matrix in Se-CCN is further supported by the Se–C stretch73 and bend74 characteristic peaks observed in the FTIR spectrum. For the Se/C mixture, these peaks were absent. Both plots are displayed in Fig. S4 (ESI†). We also observed some degree of Se–O bonding in the Se 3p XPS spectrum (Fig. S5, ESI†).75
Fig. 2E shows the nitrogen adsorption–desorption isotherms of the bare as-synthesized CCNs and of Se-CCN. Fig. 2F shows their resultant pore size distributions, obtained using the density functional theory (DFT). The adsorption curve of the CCNs display type I isotherms, associated with microporous materials. The calculated BET surface area is 1610 m2 g−1 and the total pore volume is 1.01 cm3 g−1. This high surface area and large pore volume resulted from the known role of KOH as a highly efficient chemical activation agent when combined with a pre-hydrothermal treatment.64 As shown in Fig. 2F, the porosity in bare CCNs is heavily concentrated in the micropore region, i.e. pore sizes less than 2 nm. Based on the pore size distribution analysis, it is calculated that the volume of microporosity (<2 mm) is 84.1% of the total pore volume. The microporous structure of the CCNs is fundamentally different from the highly mesoporous structure of the carbon nanosheets derived from hemp or from peanut shells,64,65,76 and may be attributed to differences both in the structure of the precursor and in the details of the activation process. After Se impregnation, the surface area dropped significantly, being only 87 m2 g−1 in Se-CCN. This is effectively the geometric surface area of the impregnated carbon nanosheets, as there is negligible porosity available for N2 adsorption. In turn this indicates that the available pore surface terminations in the CCNs are plugged by the selenium phase. It is very difficult to confirm full impregnation of Se inside the pore cavities, and attempts at detailed HRTEM were thwarted by the instability of Se under the localized electron beam. However a quick calculation does indicate that there is plenty of pore volume in the CCNs to incorporate the Se phase: the pore volume of the CCNs is 1 cm3 g−1. The density of amorphous Se is 4.28 g cm−3. One gram of CCN will have 1 cm3 of pore volume, which hosts 4.28 g of Se. This means that at 53 wt% Se the pores are roughly ¼ filled.
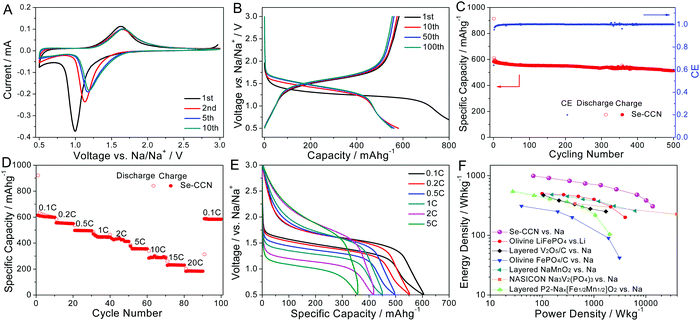
The electrochemical performance of the Se-CCN cathode was first investigated in a half-cell configuration, using sodium metal as the anode. A practical organic carbonate (i.e. EC, DMC) based electrolyte was used without any additives. We performed cyclic voltammetry (CV) and galvanostatic discharge/charge cycling for the Se-CCN cathode. The electrodes were tested between 0.5–3 V vs. Na/Na+. Fig. 3A shows the CV curves of the cathode at a scan rate of 0.1 mV s−1. In the first scan cycle, there is a pair of cathodic and anodic peaks located at 1.00 and 1.62 V, respectively. The peaks on the CV represent the redox reaction between selenium and sodium. These two peaks shifted towards a higher voltage in the following cycles, more significantly for the cathodic peak. They shifted to 1.17 and 1.66 V from the 5th cycle and stabilized thereafter. Similar voltage shifts were reported by the authors in ref. 46, and may be attributed to a change in the overpotential after several cycles associated with sodiating Se in a confining but elastically giving carbon matrix. A single redox peak pair feature indicates that the electrode undergoes a direct phase change between selenium and Na2Se without the formation of soluble sodium polyselenide Na2Sen (n ≥ 4).44,46,77 A direct reaction sequence is promoted by the Se-CCN structure, i.e. amorphous selenium incorporated into the nanopores. As indicated earlier, low order Se is known to be less liable to form intermediates with Na and Li than their crystalline allotropes. Reversible side reactions involving the shuttle of soluble polyselenides will lead to cycling-induced capacity loss as progressively more Se gets consumed.77 Since Se-CCN half-cell cycling capacity is quite stable over 500 cycles (88% retention), the shuttle effect is minimized. Over extended cycling there are also minimal irreversible electrochemical side reactions, e.g. SEI formation on the Se-CCN surface. Irreversible electrochemical parasitic reactions will result in poor Coulombic efficiency (CE). In Se-CCN, the CE increased from 99% at the 10th cycle to 100% ± 0.5% in the subsequent cycles, confirming the stability of the initially formed SEI.
Fig. 3B shows the galvanostatic charge/discharge profiles of Se-CCN cathodes at a current density of 0.2C (1C equals to 678 mA g−1 based on the mass of selenium). The voltage plateaus in the profile agree well with the peak positions in the CV curves. The cathode delivered an initial discharge capacity of 913 mA h g−1 in the first cycle, with 583 mA h g−1 being reversible. The cycle 1 irreversible capacity may be attributed to solid electrolyte interface (SEI) formation, as well as some irreversible trapping of Na within the carbon lattice at high potentials.66 The reversible capacity of 583 mA h g−1 is among the highest values reported for Se-based NIB cathodes.45–47 It indicates excellent utilization of selenium, which we argue is due to its dense distribution primarily within the nanopores of the open carbon nanosheets. The profiles of the 10th, 50th and 100th cycles nearly overlap each other, showing that the activation process is complete by cycle 10. As shown in Table S1 (ESI†), although the plateau voltage of Se-CCN is lower than those of “traditional” Na ceramic cathodes such as layered Na0.85Li0.17Ni0.21Mn0.64O2,78 bilayer V2O5,79 olivine FePO4,80 and NASICONS,81etc.,82–84 the reversible capacity is two and a half times higher or more.
The Se-CCN electrode was also tested for stability during extended cycling, employing a current density of 0.2C. As shown in Fig. 3C, the Se-CCN exhibited exceptional capacity retention: a reversible capacity of 514 mA h g−1 is obtained after 500 deep cycles at 0.2C, which is equivalent to 88% capacity retention. The Coulombic efficiency (CE) increased from 99% at the 10th cycle to 100% ± 0.5% in the subsequent cycles. Fig. S6 (ESI†) displays the cycling results of the Se/C mixture specimen tested under the same conditions. The capacity decayed quickly upon cycling (35% retention after 100 cycles) and the CE was much lower, i.e. ca. 96%. Fig. S7 (ESI†) show the cycling performance and the rate capability of the Se-CCN specimens when the capacity is normalized by the combined mass of Se and the CCN matrix. Since the mass loading of Se is 53%, these capacities are roughly one half of the data shown in Fig. 3. It should be pointed out that the CCN matrix is inactive at the relevant cathodic voltages, i.e. above 1 V, especially since Na access to the pores is blocked by the impregnated Se phase. It should also be pointed out that in all previous Se–carbon vs. Li/Li+e.g.ref. 43, 50, 53, 57, 58 and 75 and Se–carbon vs. Na/Na+ studies,45–49 the reported capacity values are based on Se mass only.
Fig. S8 (ESI†) shows the Nyquist plots of the Se-CCN half-cells prior to cycling, after 200 cycles and after 500 cycles. Table 1 lists the electrochemical performance of state-of-the-art selenium based Na ion cathodes from the literature.45–49 Employing Se as a Na cathode is a relatively new concept and there are not numerous studies published on the subject. Se-CCN displays among the highest selenium loading and is by far the most cyclically stable. Table S2 (ESI†) lists the resistance parameters obtained by fitting the plots. The pristine cell has an equivalent series resistance (Rel) of 7.1 Ω and a charge transfer resistance (Rct) of 57.1 Ω. After 200 cycles these values increased to 13.6 Ω and 157.4 Ω, respectively. Since the Rel involves a combination of electrode and electrolyte impedances, its increase may be due to a range of factors, including changes at the Na counter electrode. The proportionally larger increase in the charge transfer resistance may be more straightforwardly attributed to SEI formation during cycling. After 500 cycles, the charge transfer resistance is minimally altered from the 300 cycle value, growing only by 7% to 168.3 Ω. This indicates a highly stable SEI layer with prolonged cycling. With both Li and Na, SEI is known to be catalytically formed at every cycle on the fracture surfaces of active materials that are newly exposed to the electrolyte, e.g.ref. 85–88. It grows at much slower rates on the pre-existing SEI which is unperturbed. A stable Rct is indicative of an electrode structure that is stable during cycling and does not exfoliate, fracture, decrepitate, etc. thereby exposing unpassivated carbon and Se to the carbonates. The elastically forgiving CCN matrix will be responsible for such stability, buffering the inevitable volume expansion associated with the 2Na+ + 2e− + Se ⇒ Na2Se reaction within its pores.
| Material | Selenium content | Reversible specific capacity | Cyclability | Rate performance | Ref. |
|---|---|---|---|---|---|
| Se-CCN | 53% | 613 mA h g−1 at 67.8 mA g−1 | 88% retention over 500 cycles | 355 mA h g−1 at 3.4 A g−1 (5C) | This work |
| 290 mA h g−1 at 6.8 A g−1 (10C) | |||||
| 184 mA h g−1 at 13.6 A g−1 (20C) | |||||
| Carbon–selenium composites | 54% | ca. 595 mA h g−1 at 50 mA g−1 | 42.6% retention over 50 cycles | ca. 200 mA h g−1 at 0.8 A g−1 | 45 |
| ca. 130 mA h g−1 at 1.2 A g−1 | |||||
| Se@PAN derived carbon nanofibers | 52% | ca. 595 mA h g−1 at 100 mA g−1 | 87% retention over 80 cycles | 230 mA h g−1 at 1 A g−1 | 47 |
| Se@mesoporous carbon | 30% | ca. 485 mA h g−1 at 169.5 mA g−1 | 70% retention over 380 cycles | ca. 265 mA h g−1 at 2.04 A g−1 (3C) | 46 |
| ca. 205 mA h g−1 at 2.72 A g−1 (4C) | |||||
| ca. 168 mA h g−1 at 3.4 A g−1 (5C) | |||||
| Se@CNF–CNT | 35% | 590 mA h g−1 at 50 mA g−1 | 98% retention over 80 cycles | 300 mA h g−1 at 1 A g−1 | 48 |
| CPAN/Se fibers | 36% | ca. 490 mA h g−1 at 67.8 mA g−1 | 81% retention over 300 cycles | ca. 310 mA h g−1 at 1.36 A g−1 (2C) | 49 |
| 245 mA h g−1 at 2.04 A g−1 (3C) |
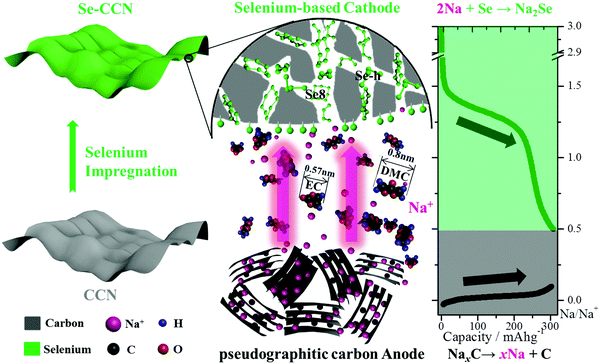
Scheme 1 illustrates the origin of the enhanced stability and CE of Se-CCN. As indicated earlier, crystalline Se is absent from the structure, minimizing polyselenide formation and the shuttle effect. The EC and DMC molecules have approximate diameters of 0.6 and 0.8 nm, with dynamic sizes under microthermal motion being larger.89 Therefore it is expected that neither would be able to penetrate between the impregnated Se and the CCN matrix. The electrode is also structurally quite stable, with an open sheet-like morphology that is elastically forgiving both macroscopically and at the nm-scale of the pores.
The rate capability of Se-CCN was also investigated against sodium. Fig. 3D shows the reversible capacities of the Se-CCN cathodes at current densities of 0.1C to 20C. Reversible capacities of 613, 447, 355, 290, and 184 mA h g−1 are obtained at rates of 0.1C, 1C, 5C, 10C and 20C (i.e. 0.068, 0.68, 3.4, 6.8 and 13.6 A g−1). A capacity of 613 mA h g−1 is calculated to be 90.4% of the theoretical value for the Se to Na2Se conversion reaction. After the rate was reduced from 20C back to 0.1C, the capacity recovered to 587 mA h g−1, indicating minimal high rate degradation. This rate performance is likewise leading the field as per Table 1. As displayed in Scheme 1, due to the sheet-like morphology of the carbon matrix, the Na ion solid-state diffusion distances needed to fully sodiate/desodiate the Se are nanometers in size. The open sheet-like morphology of Se-CCN also provides a large reaction surface allowing for facile charge transfer. According to Fick's first law, diffusion time is proportional to the diffusion distance squared. The time needed to fully sodiate/desodiate a 50 nm “deep” selenium phase inside a 100 nm CCN sheet would be 400 times less than if the selenium was similarly loaded into the pores of a 1 μm activated carbon particle. Fig. 3E compares the voltage profiles of the Se-CCN electrodes at different C-rates. All the discharge/charge curves exhibit a pronounced plateau. The voltage hysteresis grows with higher C-rates, with the cathodic (sodiation) branch showing a proportionally larger hysteresis with increasing C. This may be ascribed to the origin of the sodiation overpotential, which we believe to be primarily related to the stress associated with the volume expansion of Se inside the pores. At shorter charge times, i.e. higher C rates, there less diffusion permitted to relax this stress.
To elaborate the utility of the Se-CCN as a NIB cathode, we generated a Ragone chart for the half cell, i.e. for a Se–Na metal battery. These data are compared with some advanced NIB and LIB cathodes from the literature tested as half cells as well. The shown literature materials are diverse, being a range of ceramic cathodes,90–92 including the successfully commercialized LiFePO4,93 layered P2 type compound Na2/3[Fe1/2Mn1/2]O2,20 layered transition-metal oxides NaMnO2,91 olivine compound FePO4,90 layered oxide V2O5,92 and NASICON Na3V2(PO4)3.94 The advantage of looking at the Ragone chart versus just the absolute reversible capacity of a cathode is that it also accounts for the differences in the operating voltage. This eliminates falsely perceived advantages of high capacity albeit low voltage materials. All cathodes plotted were tested as half-cells vs. Na metal or Li metal. As shown in Fig. 3F, the Se-CCN system is quite favorable, achieving 992 W h kg−1 at 68 W kg−1 and 384 W h kg−1 at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 W kg−1 based on the active selenium mass. Even if the energy–power values were halved to account for the weight of the Na inactive carbon matrix, the Se-CCN electrode would still be among the best for both Na and Li.
144 W kg−1 based on the active selenium mass. Even if the energy–power values were halved to account for the weight of the Na inactive carbon matrix, the Se-CCN electrode would still be among the best for both Na and Li.
The Se-CCN may be an ideal cathode for high energy sodium ion batteries when coupled with a proper anode. For the sodium ion battery anode, we employed an in-house fabricated pseudographitic carbon (PGC).65 PGC is composed of partially ordered domains of graphene, with an interlayer spacing substantially larger than that of graphite. The dilated intergraphene layer spacing allows for reversible Na intercalation/deintercalation at low voltages in conventional electrolyte solutions, which is impossible with graphite due to Na ion's size and electronic structure.3,95 Fig. S9A (ESI†) shows the XRD pattern of the as-synthesized PGC. In the XRD pattern, there are two discernible reflections that are ascribed to (002) and (100) of the ordered graphene domains within the carbon. However the equilibrium reflections for graphite are distinctly absent. Based on the center position of the (002) reflection, the mean intergraphene layer spacing for PGC is 0.379 nm, versus the 0.335 nm spacing for equilibrium graphite. This 13% dilation allows for reversible Na intercalation between the graphene planes, qualitatively akin to Li with graphite. The Raman spectrum of PGC shown in Fig. S9B (ESI†) also demonstrates a degree of ordering in the structure, with a G-band to D-band intensity ratio of 0.98. This is distinct from a typical 0.33–0.4 IG/ID ratio for fully amorphous carbons that do not intercalate Na.96
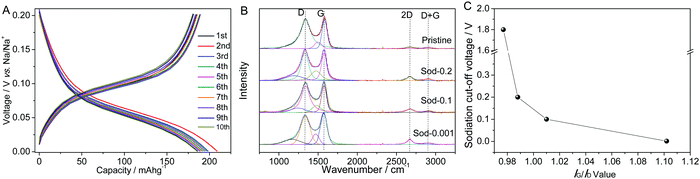
Fig. 4A shows the galvanostatic charge–discharge characteristics of PGC tested between 0.001–0.2 V vs. Na/Na+. Within this narrow voltage window, the anode delivers 185–200 mA h g−1 of reversible capacity when tested at 100 mA g−1. At this current the total reversible capacity is ∼300 mA h g−1, with a highly stable cyclability. After 300 charge–discharge cycles at 100 mA g−1, 75% of the initial capacity is retained. At a current of 800 mA g−1 the reversible capacity is 177 mA h g−1, indicating good rate capability as well. Those results are shown in Fig. S10 and S11 (ESI†). Between 0.001–0.2 V the electrode also displays a relatively flat voltage plateau and minor hysteresis. These features are qualitatively akin to Li in graphite. The PGC low voltage plateau is useful for full cell applications, as it results in the widest and most flat voltage window for the device.
For the following XRD and Raman analyses, the PGC electrodes were galvanostatically discharged to the target cut-off voltages at a current density of 50 mA g−1. The electrodes were then collected from the disassembled coin cells, and thoroughly washed with DMC while inside the argon-filled glove box. In order to minimize the effects of air and moisture exposure, the cleaned electrodes were kept in the glovebox all the way up to the time of analysis. The samples were transferred using argon filled vials to the XRD/Raman equipment, where they were analyzed without further preparation. Fig. S12A (ESI†) shows the resultant XRD patterns of the PGC electrodes at different states of sodiation: pristine, sodiated to 0.2 V vs. Na/Na+, to 0.1 V vs. Na/Na+, and to 0.001 V vs. Na/Na+. It may be observed that with deeper sodiation the (002) peak shifts towards lower 2-theta values, the majority of the change occurring below 0.1 V. The change in the peak position and the corresponding calculated dilation of the intergraphene layer spacing are shown in Fig. S12B (ESI†). This phenomenon is due to the intercalation of Na into the dilated graphene interlayers of PGC. Reversible intercalation of Na into ordered but not fully graphitic carbons was discovered in 2013 and confirmed shortly afterwards.9,66,68 The flat low voltage plateau in Fig. 4A is then due to the coexistence of a two phase region, although the Na staging reactions are not fully understood at the moment. Fig. 4(B) shows the Raman spectra of the PGC electrodes analyzed at the same cutoff voltages. The spectra are fitted and the values of IG/ID were plotted in Fig. 4C. As the sodiation proceeds, the IG/ID ratio increases and the second order 2D band became more prominent. Likewise with XRD, the largest increase in the degree of order in the carbons occurs below 0.1 V. Such intercalation induced ordering is a newly reported phenomenon for PGC carbons, and may serve as the first step toward understanding the staging reactions that may occur at every sodiation cycle.
Importantly, the XRD pattern shown in Fig. S12 (ESI†) shows no evidence of metallic Na plating into the pores of the carbon at any of the voltages. The most intense Na metal reflection would correspond to the {110} family, and would be located at 2-theta = 29.3° in the pattern. Underpotential deposition into the nanopores is expected to occur close to the half-cell potential of the metal in that electrolyte, be it Na or Li. For the case of Li plating into the pores of hard carbons the phenomenon has been confirmed using analogous X-ray analysis.68 Thus if the Na pore filling mechanism was an important contributor to the total reversible capacity of PGC, the {110} Na XRD peak would show up below 0.2 V. As it is distinctly absent from the 0.2, 0.1 and 0.001 V vs. Na/Na+ diffraction patterns, we argue that Na metal pore filling is not important in pseudographitic systems. This is reasonable as pore filling and intercalation occur in approximately the same voltage regime, and thus may be expected to compete in terms of the net contribution to the overall capacity. In fact the hard carbons that were tested against Li, which demonstrated minimal intercalation at low potentials, displayed highly prominent {110} Li peaks instead.68
To introduce Na into the full cell, the individual electrodes were presodiated to set voltages. The Se-CCN cathode was presodiated to the cut-off voltage of 0.5 V vs. Na/Na+. According to the charge/discharge profile of Se-CCN, this puts it in a nearly fully sodiated state. The PGC anode was only partially sodiated to 0.2 V, leaving the vast majority of the useful low voltage capacity untapped. Thus the sodiation states of the cathode and of the anode mimicked closely what is expected in a conventional battery: a sodiated (or lithiated) cathode and an anode with low voltage capacity that is uncharged. As indicated earlier, utilizing the low voltage plateau capacity of the anode maximized the voltage window and yielded a relatively flat profile in the full cell. Both electrodes were cycled twice prior to setting the target cut-off voltages, which was done to minimize the first cycle irreversible capacity loss in the full cell.
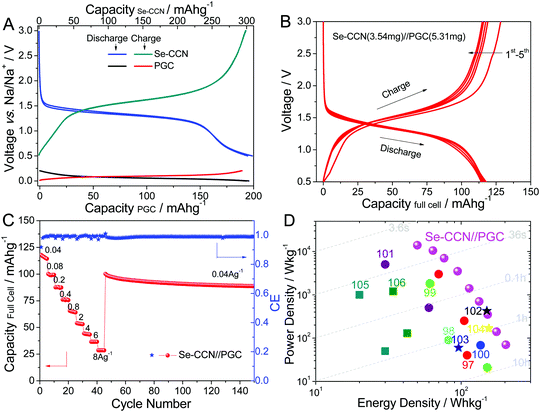
Fig. 5A displays the 3rd and 4th half-cell charge/discharge voltage profiles for the Se-CCN cathode and PGC anode. The discharging profiles are also illustrated on the right column of Scheme 1. During battery discharge, Na+ deintercalates from the pseudographitic domains (i.e. NaxC → xNa + C) in the anode and reacts with the Se to form Na2Se. The PGC exhibits a pronounced flat plateau, with 196 mA h g−1 of capacity within the voltage window of 0–0.2 V vs. Na/Na+ at 100 mA g−1. On the cathode side, Se-CCN displays 300 mA h g−1 of capacity between 0.5–3 V vs. Na/Na+ when normalized by the total mass of the material. In a full cell, the respective mass of the anode and the cathode may be adjusted for capacity matching. Based on the half-cell specific capacity values, the mass ratio of the cathode to the anode in the battery was set to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The typical mass loading for the full cell electrodes is 2 mg cm−2 for the Se-CCN cathode and 3 mg cm−2 for the anode. With an area of 1.77 cm2 for both electrodes, the absolute mass applied in the full cell is 3.54 mg and 5.31 mg for the cathode and anode, respectively. As shown in Fig. S10 (ESI†), the total reversible capability of PGC within a voltage window of 0–3 V is around 300 mA h g−1. Thus in effect we employed a 50% percent oversized anode in the full cell. As the energy and power density calculations counted the total mass of both electrodes (see the Experimental section), the oversized anode was fully accounted for.
3. The typical mass loading for the full cell electrodes is 2 mg cm−2 for the Se-CCN cathode and 3 mg cm−2 for the anode. With an area of 1.77 cm2 for both electrodes, the absolute mass applied in the full cell is 3.54 mg and 5.31 mg for the cathode and anode, respectively. As shown in Fig. S10 (ESI†), the total reversible capability of PGC within a voltage window of 0–3 V is around 300 mA h g−1. Thus in effect we employed a 50% percent oversized anode in the full cell. As the energy and power density calculations counted the total mass of both electrodes (see the Experimental section), the oversized anode was fully accounted for.
Fig. 5B shows the first five cycle discharge/charge profiles of the Se-CCN//PGC sodium full cell tested in a voltage window of 0.5–3 V. It can be seen that the plateaus display only a minor shift towards a lower voltage for the full cell as compared to the Se-CCN half cell. The full cell delivers a capacity of 117 mA h g−1 at a current density of 40 mA g−1. Based on the absolute mass of the electrodes (i.e. 8.85 mg in total), the absolute capacity of a typical full cell is 1.04 mA h. Fig. 5C shows the capacity obtained at various current densities. Since both electrodes offer fast kinetics, the battery delivers excellent capacity even at very high current densities, e.g. 4, 6, and 8 A g−1. Such rate performance is quite exceptional for Na-based battery devices, which are normally relatively low in power. After the high rate tests, when the current is turned back to 40 mA g−1, the capacity recovers to 101 mA h g−1 (89% retention). After 150 cycles at various rates, the final battery cell capacity is still 89 mA h g−1.
The advantage of our Se-CCN//PGC NIB is illustrated using a “global” Ragone chart shown in Fig. 5D, which compares our device to the state-of-the-art Na ion batteries reported in the literature. The energy–power values for Se-CCN//PGC were obtained based on the total mass of both electrodes. The systems plotted for comparison include configurations of P2-Na2/3Ni1/3Mn2/3O2//Sb nanorods,97 NaxNiyMn1−y[Fe(CN)6]//hard carbon,98 Na3V2(PO4)3–C//Sb@TiO2−x,99 NaxFe[Fe(CN)6]//FeOx–CNT,100 Na0.7CoO2//graphite,101 symmetric Na3V2(PO4)3–rGO–CNT//Na3V2(PO4)3–rGO–CNT,102 Na0.8Ni0.4Ti0.6O2//Na0.8Ni0.4Ti0.6O2,103 Na3V2(PO4)3@C//Na3V2(PO4)3@C,104 aqueous NIBs with configurations of NaMnO2//NaTi2(PO4)3,105 and Na1.94Ni1.03Fe(CN)6//NaTi2(PO4)3.106Table 2 provides the critical electrochemical parameters of both the cathodes and full cells of these systems. Although the Se-CCN//PGC full cell exhibited a mid-range output voltage of ∼1.5 V, the high specific capacity/rate capability of the Se-CCN cathode (∼3–6 times of the other cathode capacities) still enabled the full device to display very competitive energy/power densities. Our sodium full cell has a specific energy density of 203 and 175 W h kg−1 at power densities of 70 and 139 W kg−1. Such energy–power values are favorable in the broader context of the existing Na ion systems.
| Full cell configuration (cathode//anode) | Cathode type | Cathode capacity (mA h g−1) | Cell voltage (V) | Energy density (W h kg−1) | Power density (W kg−1) | Ref. |
|---|---|---|---|---|---|---|
| a Calculated values based on the published graphs and values. TM = transition metal. | ||||||
| Se-CCN//PGC | Selenium based cathode | ∼300 | ∼1.5 | 203/64 | 70/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
This work |
| P2-Na2/3Ni1/3Mn2/3O2//Sb nanorods | P type layered TM oxide | 38 | 2.9 | 110/70 | 40/3000 | 97 |
| NaMnO2//NaTi2(PO4)3 | Tunnel-type TM oxides | 55 | ∼1.2 | 30/20 | 50/1000 | 105 |
| Na0.7CoO2//graphite | P type layered TM oxide | ∼100 | 2.2 | 60/30 | 503/5030a | 101 |
| NaxFe[Fe(CN)6]//FeOx–CNT | Prussian blue analogue | ∼120 | ∼2 | 136 | 68 | 100 |
| Na3V2(PO4)3@C//Na3V2(PO4)3@C | NASICON | 104.3 | ∼1.7 | 154.5 | 170a | 104 |
| Na3V2(PO4)3–rGO–CNT//Na3V2(PO4)3–rGO–CNT | NASICON | 115 | ∼1.7 | ∼150 | 425a | 102 |
| Na1.94Ni1.03Fe(CN)6//NaTi2(PO4)3 | Prussian blue analogue | 65 | ∼1.27 | 42.5/34 | 130/1200 | 106 |
| Na0.8Ni0.4Ti0.6O2//Na0.8Ni0.4Ti0.6O2 | O type layered TM oxide | ∼80 | 2.8 | 96 | 60 | 103 |
| Na3V2(PO4)3–C//Sb@TiO2−x | NASICON | 90 | 2.5 | 151/61 | 21/1830 | 99 |
| NaxNiyMn1−y[Fe(CN)6]//hard carbon | Prussian blue analogue | 62.2 | ∼3a | 81.42 | 90 | 98 |
Conclusions
For the first time, a sodium–selenium battery full cell is fabricated and electrochemically tested. The cell design is to pair selenium in a carbon nanosheet cathode with a pseudo-graphitic carbon anode that provides a good low voltage plateau, akin to graphite with Li. This novel next generation battery delivers remarkable Ragone chart characteristics. For example, it yields energy–power values of 203 and 50 W h kg−1 at 70 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W kg−1, as normalized by the active mass. The microstructure of the cathode consists of Se primarily impregnated into the micropores of cellulose-derived carbon nanosheets. The elastically compliant two-dimensional carbon supports 53 wt% Se mass loading, resulting in an electrode with a high electrical conductivity, rapid Na/Li ion transfer, and structural stability during extended cycling. Due to the stable immobilization of the selenium phase and greatly minimized chemical interaction between selenium and the organic carbonates in the electrolyte, the shuttle effect of the polyselenide intermediates is minimized.
000 W kg−1, as normalized by the active mass. The microstructure of the cathode consists of Se primarily impregnated into the micropores of cellulose-derived carbon nanosheets. The elastically compliant two-dimensional carbon supports 53 wt% Se mass loading, resulting in an electrode with a high electrical conductivity, rapid Na/Li ion transfer, and structural stability during extended cycling. Due to the stable immobilization of the selenium phase and greatly minimized chemical interaction between selenium and the organic carbonates in the electrolyte, the shuttle effect of the polyselenide intermediates is minimized.
Experimental section
Material preparation
Slurry of cellulose nanocrystals at a concentration of 11.5 wt% solids was purchased from Maine University. In a typical synthesis run, a slurry with a total of 1.5 g solids is used. Milli-Q water is added to increase the total volume to 50 mL. The slurry is further sonicated until a uniform suspension has formed. A volume of 3 mL of concentrated sulfuric acid is added into the suspension, which is further sonicated for an additional 10 min. Then the suspension is sealed in a 100 mL stainless steel autoclave and soaked at 180 °C for 24 h. The obtained solid is collected via filtration, washed with MQ-water and dried in an oven. The dark brown products are carbonized and activated at 800 °C for 1 h with the presence of an equal mass of potassium hydroxide under an argon flow. The remaining active reagent is washed away with 2 M HCl and MQ-water. The obtained carbons are thoroughly dried in a vacuum oven.To prepare the candidate material, selenium powder (Alfa Aesar, 99.999%) and the prepared carbons were thoroughly mixed using a planetary ball milling method for 0.5 h in a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under an argon atmosphere. Selenium impregnation includes two separate steps. The first step is a selenium diffusion process which is conducted at 260 °C for 12 h. Before the second step, the obtained powder is sealed in a small volume glass tube inside an argon filled glovebox. In the second step, the glass tube containing a black powder is further soaked at 600 °C for 3 h under an inert atmosphere. The obtained black product (named Se-CCN) is then employed as the active material without further processing. A baseline material is prepared via 30 min of manually mixing the selenium power with active carbon (NORIT A SUPRA) using a mortar and pestle (called the Se/C mixture). Pristine selenium powder is also used in some of the baseline analyses. A detailed synthesis method for the pseudo-graphitic carbon (PGC) anode is provided in our previously published study.65
1 under an argon atmosphere. Selenium impregnation includes two separate steps. The first step is a selenium diffusion process which is conducted at 260 °C for 12 h. Before the second step, the obtained powder is sealed in a small volume glass tube inside an argon filled glovebox. In the second step, the glass tube containing a black powder is further soaked at 600 °C for 3 h under an inert atmosphere. The obtained black product (named Se-CCN) is then employed as the active material without further processing. A baseline material is prepared via 30 min of manually mixing the selenium power with active carbon (NORIT A SUPRA) using a mortar and pestle (called the Se/C mixture). Pristine selenium powder is also used in some of the baseline analyses. A detailed synthesis method for the pseudo-graphitic carbon (PGC) anode is provided in our previously published study.65
Material characterization
For the morphology and element mapping characterization, a Hitachi S-3000 SEM with an Oxford INCA EDXS system was used. TEM and energy-dispersive X-ray (EDX) spectroscopy were performed using a JEOL 2200FS (200 kV) with an in-column Ω filter in the scanning mode (STEM) with a nominal analytical beam size of 0.5 nm. X-ray diffraction (XRD) analysis was performed using a Bruker AXS D8 Discover diffractometer with Cu Kα radiation. The Raman spectra were recorded with a confocal microprobe Raman system (Thermo Nicolet Almega XR Raman Microscope). The surface area and porous texture were characterized from the nitrogen adsorption at 77 K (Quantachrome Autosorb-1). The pore size distributions were evaluated using a nonlocal density functional theory (DFT) method using nitrogen adsorption data and assuming slit-pore geometry. X-ray photoelectron spectroscopy (XPS) was carried out on an Axis Ultra spectrometer. Thermogravimetric analysis (TGA, Perkin-Elmer TGA 4000) was undertaken with a heating rate of 5 °C min−1 under 200 mL min−1 of an argon flow.Electrochemical measurements
Electrochemical tests were carried out using CR2032 coin cells, either in a half-cell or a full cell configuration. A slurry of 80% active material, 10% carbon black (Super-P) and 10% PVDF binder, dissolved in N-methylpyrrolidone, was dropped on stainless steel disks and then dried at 80 °C overnight in a vacuum oven. The typical mass loading of the cathode is 2 mg cm−2. An electrolyte of 1 M NaClO4 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (volume ratio) ethylene carbonate (EC)
1 (volume ratio) ethylene carbonate (EC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dimethyl carbonate (DMC), and 25 μm thick polyethene-based separators were used. For half-cells, Na foils were used as the counter electrodes. Cycling voltammetry measurements (CV) were performed using a Solartron 1470 Multistat system. Galvanostatic charge/discharge profiles were obtained using a BT2000 Arbin electrochemical workstation. All the capacities and energy densities in the half cell configuration were calculated on the basis of the selenium mass in the composites. The energy density in the full cell was calculated on the basis of the total mass of all the electrode materials. All tests were conducted at room temperature.
dimethyl carbonate (DMC), and 25 μm thick polyethene-based separators were used. For half-cells, Na foils were used as the counter electrodes. Cycling voltammetry measurements (CV) were performed using a Solartron 1470 Multistat system. Galvanostatic charge/discharge profiles were obtained using a BT2000 Arbin electrochemical workstation. All the capacities and energy densities in the half cell configuration were calculated on the basis of the selenium mass in the composites. The energy density in the full cell was calculated on the basis of the total mass of all the electrode materials. All tests were conducted at room temperature.
For the sodium full cells, Se-CCN was used as the cathode and an in-house fabricated pseudo-graphitic carbon (PGC) (described in the Introduction) was used as the anode. The typical mass loading was 2 mg cm−2 for the Se-CCN cathode and 3 mg cm−2 for the PGC anode. Approximately 50 μL electrolyte was added for each cell preparation. Before being assembled together into a full cell, both the PGC anode and Se-CCN cathode were partially sodiated in the half-cells. After pre-sodiation, both the PGC and Se-CCN electrodes were removed from the disassembled half-cells and then reassembled into a full battery, all the operations were performed inside a glove box. The initial open circuit potential (OCP) of the as-assembled battery was around 0.4 V. The energy and power density calculations for the half-cell were based on the electrode active mass. Calculations for the full cell performance were based on the total mass of the cathode and anode materials, including the mass of the CCN in the cathode and the mass of the excess PGC. In a full cell the reported current density was based on the mass of the cathode.
References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-Gonzalez and T. Rojo, Energy Environ. Sci., 2012, 5, 5884–5901 CAS.
- S. Y. Hong, Y. Kim, Y. Park, A. Choi, N. S. Choi and K. T. Lee, Energy Environ. Sci., 2013, 6, 2067–2081 CAS.
- B. Ahmed, D. H. Anjum, M. N. Hedhili and H. N. Alshareef, Small, 2015, 11(34), 4341–4350 CrossRef CAS PubMed.
- J. Wan, F. Gu, W. Bao, J. Dai, F. Shen, W. Luo, X. Han, D. Urban and L. Hu, Nano Lett., 2015, 15, 3763–3769 CrossRef CAS PubMed.
- W. Luo, F. Shen, C. Bommier, H. Zhu, X. Ji and L. Hu, Acc. Chem. Res., 2016, 49, 231–240 CrossRef CAS PubMed.
- Z. Li, L. Ma, T. Surta, C. Bommier, Z. Jian, Z. Xing, W. Stickle, M. Dolgos, K. Amine, J. Lu, T. Wu and X. Ji, ACS Energy Lett., 2016, 1, 395–401 CrossRef CAS.
- B. L. Ellis, K. T. Lee and L. F. Nazar, Chem. Mater., 2010, 22, 691–714 CrossRef CAS.
- S. W. Kim, D. H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- X. Su, Q. Wu, J. Li, X. Xiao, A. Lott, W. Lu, B. W. Sheldon and J. Wu, Adv. Energy Mater., 2014, 4, 1300882 CrossRef.
- Z. Li, J. Ding and D. Mitlin, Acc. Chem. Res., 2015, 48, 1657–1665 CrossRef CAS PubMed.
- J. Ding, Z. Li, H. Wang, K. Cui, A. Kohandehghan, X. Tan, D. Karpuzov and D. Mitlin, J. Mater. Chem. A, 2015, 3, 7100–7111 CAS.
- T. Ohzukuk, A. Ueda, M. Nagayama, Y. Iwakoshi and H. Komori, Electrochim. Acta, 1993, 38, 1159–1167 CrossRef.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4301 CrossRef CAS PubMed.
- L. X. Yuan, Z. H. Wang, W. X. Zhang, X. L. Hu, J. T. Chen, Y. H. Huang and J. B. Goodenough, Energy Environ. Sci., 2011, 4, 269–284 CAS.
- Q. An, F. Xiong, Q. Wei, J. Sheng, L. He, D. Ma, Y. Yao and L. Mai, Adv. Energy Mater., 2015, 5, 1401963 CrossRef.
- H. Pan, Y. Hu and L. Chen, Energy Environ. Sci., 2013, 6, 2338–2360 CAS.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512–517 CrossRef CAS PubMed.
- A. Manthiram, Y. Fu, S. H. Chung, C. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- S. Evers and L. F. Nazar, Acc. Chem. Res., 2013, 46, 1135–1143 CrossRef CAS PubMed.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J. M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 5, 500–506 CrossRef PubMed.
- Y. Yin, S. Xin, Y. Guo and L. Wan, Angew. Chem., Int. Ed., 2013, 52, 13186–13200 CrossRef CAS PubMed.
- S. Xin, L. Gu, N. H. Zhao, Y. X. Yin, L. J. Zhou, Y. G. Guo and L. J. Wan, J. Am. Chem. Soc., 2012, 134, 18510–18513 CrossRef CAS PubMed.
- Y. Qu, Z. Zhang, X. Zhang, G. Ren, Y. Lai, Y. Liu and J. Li, Carbon, 2015, 84, 399–408 CrossRef CAS.
- J. Song, M. L. Gordin, T. Xu, S. Chen, Z. Yu, H. Sohn, J. Lu, Y. Ren, Y. Duan and D. Wang, Angew. Chem., 2015, 127, 4399–4403 CrossRef.
- X. Liang, C. Kwok, F. Lodi-Marzano, Q. Pang, M. Cuisinier, H. Huang, C. J. Hart, D. Houtarde, K. Kaup, H. Sommer, T. Brezesinski, J. Janek and L. F. Nazar, Adv. Energy Mater., 2016, 6, 1501636 CrossRef.
- R. Demir-Cakan, M. Morcette, F. Nouar, C. Davoisne, T. Devic, D. Gonbeau, R. Dominko, C. Serre, G. Ferey and J. M. Tarascon, J. Am. Chem. Soc., 2011, 133, 16154–16160 CrossRef CAS PubMed.
- Q. Pang, X. Liang, C. Y. Kwok and L. F. Nazar, J. Electrochem. Soc., 2015, 162, A2567–A2576 CrossRef CAS.
- Q. Pang, D. Kundu, M. Cuisinier and L. F. Nazar, Nat. Commun., 2014, 5, 4759 CrossRef CAS PubMed.
- J. Chen, D. Wu, E. Walter, M. Engelhard, P. Bhattacharya, H. Pan, Y. Shao, F. Gao, J. Xiao and J. Liu, Nano Energy, 2015, 13, 267–274 CrossRef CAS.
- J. Kim, K. Fu, J. Choi, S. Sun, J. Kim, L. Hu and U. Paik, Chem. Commun., 2015, 51, 13682–13685 RSC.
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 18, 928–935 CrossRef PubMed.
- K. B. Hueso, M. Armand and T. Rojo, Energy Environ. Sci., 2013, 6, 734–749 CAS.
- T. H. Hwang, D. S. Jung, J. S. Kim, B. G. Kim and J. W. Choi, Nano Lett., 2013, 13, 4532–4538 CrossRef CAS PubMed.
- X. Yu and A. Manthiram, Adv. Energy Mater., 2015, 5, 1500350 CrossRef.
- S. Xin, Y. X. Yin, Y. G. Guo and L. J. Wan, Adv. Mater., 2014, 26, 1261–1265 CrossRef CAS PubMed.
- X. Yu and A. Manthiram, Chem. Mater., 2016, 28, 896–905 CrossRef CAS.
- S. Wei, S. Xu, A. Agrawral, S. Choudhury, Y. Lu, Z. Tu, L. Ma and L. A. Archer, Nat. Commun., 2016, 7, 11722 CrossRef CAS PubMed.
- D. L. Perry, Handbook of Inorganic Compounds, CRC Press, Boca Raton, FL, 2011 Search PubMed.
- K. Han, Z. Liu, J. Shen, Y. Lin, F. Dai and H. Ye, Adv. Funct. Mater., 2015, 25, 455–463 CrossRef CAS.
- A. Abouimrane, D. Dambournet, K. W. Chapman, P. J. Chupas, W. Weng and K. Amine, J. Am. Chem. Soc., 2012, 134, 4505–4508 CrossRef CAS PubMed.
- C. Luo, J. Wang, L. Suo, J. Mao, X. Fan and C. Wang, J. Mater. Chem. A, 2015, 3, 555–561 CAS.
- C. Luo, Y. Xu, Y. Zhu, Y. Liu, S. Zheng, Y. Liu, A. Langrock and C. S. Wang, ACS Nano, 2013, 7, 8003–8010 CrossRef CAS PubMed.
- L. Zeng, W. Zeng, Y. Jiang, X. Wei, W. Li, C. Yang, Y. Zhu and Y. Yu, Adv. Energy Mater., 2015, 5, 1401377 CrossRef.
- L. Zeng, X. Wei, J. Wang, Y. Jiang, W. Li and Y. Yu, J. Power Sources, 2015, 281, 461–469 CrossRef CAS.
- H. Wang, S. Li, Z. Chen, H. Liu and Z. Guo, RSC Adv., 2014, 4, 61673–61678 RSC.
- X. Li, J. Liang, Z. Hou, W. Zhang, Y. Wang, Y. Zhu and Y. Qian, Adv. Funct. Mater., 2015, 25, 5229–5238 CrossRef CAS.
- K. Han, Z. Liu, H. Ye and F. Dai, J. Power Sources, 2014, 263, 85–89 CrossRef CAS.
- L. Liu, Y. Hou, Y. Yang, M. Li, X. Wang and Y. Wu, RSC Adv., 2014, 4, 9086–9091 RSC.
- J. Lee, H. Kim, M. Oschatz, D. C. Lee, F. Wu, H. T. Lin, B. Zdyrko, W. Cho, S. Kaskel and G. Yushin, Adv. Energy Mater., 2015, 5, 1400981 CrossRef.
- J. Zhang, Y. Xu, L. Fan, Y. Zhu, J. Liang and Y. Qian, Nano Energy, 2015, 13, 592–600 CrossRef CAS.
- C. Yang, S. Xin, Y. Yin, H. Ye, J. Zhang and Y. Guo, Angew. Chem., Int. Ed., 2013, 52, 8363–8367 CrossRef CAS PubMed.
- Z. Li and L. Yin, Nanoscale, 2015, 7, 9597–9606 RSC.
- J. He, Y. Chen, W. Lv, K. Wen, P. Li, Z. Wang, W. Zhang, W. Qin and W. He, ACS Energy Lett., 2016, 1, 16–20 CrossRef CAS.
- Z. Li, L. Yuan, Z. Yi, Y. Liu and Y. Huang, Nano Energy, 2014, 9, 229–236 CrossRef CAS.
- C. Yang, Y. Yin and Y. G. Guo, J. Phys. Chem. Lett., 2015, 6, 256–266 CrossRef CAS PubMed.
- C. Zu and A. Manthiram, J. Phys. Chem. Lett., 2014, 5, 2522–2527 CrossRef CAS PubMed.
- Z. Wang, Y. Dong, H. Li, Z. Zhao, H. Wu, C. Hao, S. Liu, J. Qiu and X. W. Lou, Nat. Commun., 2014, 5, 5002 CrossRef CAS PubMed.
- H. Wang, Z. Xu, Z. Li, K. Cui, J. Ding, A. Kohandehghan, X. Tan, B. Zahiri, B. C. Olsen, C. M. Holt and D. Mitlin, Nano Lett., 2014, 14, 1987–1994 CrossRef CAS PubMed.
- L. Sun, C. Tian, M. Li, X. Meng, L. Wang, R. Wang, J. Yin and H. Fu, J. Mater. Chem. A, 2013, 1, 6462–6470 CAS.
- H. Wang, Z. Xu, A. Kohandehghan, Z. Li, K. Cui, X. Tan, T. Stephenson, C. K. Kingondu, C. Holt, B. Olsen, J. Tak, D. Harfield, A. O. Anyia and D. Mitlin, ACS Nano, 2013, 7, 5131–5141 CrossRef CAS PubMed.
- J. Ding, H. Wang, Z. Li, K. Cui, D. Karpuzov, X. Tan, A. Kohandehghan and D. Mitlin, Energy Environ. Sci., 2015, 8, 941–955 CAS.
- J. Ding, H. Wang, Z. Li, A. Kohandehghan, K. Cui, Z. Xu, B. Zahiri, X. Tan, E. M. Lotfabad, B. Olsen and D. Mitlin, ACS Nano, 2013, 12, 11004–11015 CrossRef PubMed.
- E. M. Lotfabad, P. Kalisvaart, A. Kohandehghan, D. Karpuzov and D. Mitlin, J. Mater. Chem. A, 2014, 2, 19685–19695 Search PubMed.
- E. M. Lotfabad, J. Ding, K. Cui, A. Kohandehghan, W. P. Kalisvaart, M. Hazelton and D. Mitlin, ACS Nano, 2014, 8, 7115–7129 CrossRef CAS PubMed.
- V. V. Poborchii, A. V. Kolobov, J. Caro, V. V. Zhuravlev and K. Tanaka, Chem. Phys. Lett., 1997, 280, 17–23 CrossRef CAS.
- V. V. Poborchii, Chem. Phys. Lett., 1996, 251, 230–234 CrossRef CAS.
- P. K. Babu, A. Lewera, J. H. Chung, R. Hunger, W. Jaegermann, N. Alonso-Vante, A. Wieckowski and E. Oldfield, J. Am. Chem. Soc., 2007, 129, 15140–15141 CrossRef CAS PubMed.
- K. Grenader, M. Kind, L. Silies, A. Peters, J. W. Bats, M. Bolte and A. Terfort, J. Mol. Struct., 2013, 1039, 61–70 CrossRef CAS.
- E. E. Aynsley, N. N. Greenwood and M. J. Sprague, J. Chem. Soc., 1965, 2395–2402 RSC.
- K. Helios, A. Pietraszko, W. Zierkiewicz, H. Wojtowicz and D. Michalska, Polyhedron, 2011, 30, 2466–2472 CrossRef CAS.
- H. Ye, Y. Yin, S. F. Zhang and Y. G. Guo, J. Mater. Chem. A, 2014, 2, 13293–13298 CAS.
- J. Ding, Z. Li, K. Cui, S. Boyer, D. Karpuzov and D. Mitlin, Nano Energy, 2016, 23, 129–137 CrossRef CAS.
- Y. Cui, A. Abouimrane, J. Lu, T. Bolin, Y. Ren, W. Weng, C. Sun, V. A. Maroni, S. M. Heald and K. Amine, J. Am. Chem. Soc., 2013, 135, 8047–8056 CrossRef CAS PubMed.
- D. Kim, S. H. Kang, M. Slater, S. Rood, J. T. Vaughey, N. Karan, M. Balasubramanian and C. S. Johnson, Adv. Energy Mater., 2011, 1, 333–336 CrossRef CAS.
- S. Tepavcevic, H. Xiong, V. R. Stamenkovic, X. Zuo, M. Balasubramanian, V. B. Prakapenka, C. S. Johnson and T. Rajh, ACS Nano, 2012, 6, 530–538 CrossRef CAS PubMed.
- Y. Liu, Y. Xu, X. Han, C. Pellegrinelli, Y. Zhu, H. Zhu, J. Wan, A. C. Chung, O. Vaaland, C. Wang and L. Hu, Nano Lett., 2012, 12, 5664–5668 CrossRef CAS PubMed.
- S. Lim, H. Kim, R. A. Shakoor, Y. Jung and J. W. Choi, J. Electrochem. Soc., 2012, 159, A1393–A1397 CrossRef CAS.
- F. Sauvage, L. Laffont, J.-M. Tarascon and E. Baudrin, Inorg. Chem., 2007, 46, 3289–3294 CrossRef CAS PubMed.
- H. Kim, R. A. Shakoor, C. Park, S. Y. Lim, J. S. Kim, Y. N. Jo, W. Cho, K. Miyasaka, R. Kahraman, Y. Jung and J. W. Choi, Adv. Funct. Mater., 2013, 23, 1147–1155 CrossRef CAS.
- R. Gover, A. Bryan, P. Burns and J. Barker, Solid State Ionics, 2006, 177, 1495–1500 CrossRef CAS.
- E. M. Lotfabad, P. Kalisvaart, A. Kohandehghan, K. Cui, M. Kupsta, B. Farbod and D. Mitlin, J. Mater. Chem. A, 2014, 2, 2504–2516 CAS.
- A. Kohandehghan, P. Kalisvaart, K. Cui, M. Kupsta, E. M. Lotfabad and D. Mitlin, J. Mater. Chem. A, 2013, 1, 12850–12861 CAS.
- A. Kohandehghan, P. Kalisvaart, M. Kupsta, B. Zahiri, B. S. Amirkhiz, Z. Li, E. Memarzadeh, L. A. Bendersky and D. Mitlin, J. Mater. Chem. A, 2013, 1, 1600–1612 CAS.
- L. Ji, M. Gu, Y. Shao, X. Li, M. H. Engelhard, B. W. Arey, W. Wang, Z. Nie, J. Xiao, C. Wang, J. Zhang and J. Liu, Adv. Mater., 2014, 26, 2901–2908 CrossRef CAS PubMed.
- Z. Li, L. Yuan, Z. Yi, Y. Sun, Y. Liu, Y. Jiang, Y. Shen, Y. Xin, Z. Zhang and Y. Huang, Adv. Energy Mater., 2014, 4, 1301473 CrossRef.
- M. Moradi, Z. Li, J. Qi, W. Xing, K. Xiang, Y. M. Chiang and A. M. Belcher, Nano Lett., 2015, 15, 2917–2921 CrossRef CAS PubMed.
- J. Billaud, R. J. Clement, A. R. Armstrong, J. C. Vazquez, P. Rozier, C. P. Grey and P. G. Bruce, J. Am. Chem. Soc., 2014, 136, 17243–17248 CrossRef CAS PubMed.
- V. Raju, J. Rains, C. Gates, W. Luo, X. Wang, W. F. Stickle, G. D. Stucky and X. Ji, Nano Lett., 2014, 14, 419–4124 Search PubMed.
- D. W. Choi, D. H. Wang, V. V. Viswanathan, I. T. Bae, W. Wang, N. M. Nie, J. G. Zhang, G. L. Graff, J. Liu, Z. G. Yang and T. Duong, Electrochem. Commun., 2010, 12, 378–381 CrossRef CAS.
- C. Zhu, K. Song, P. A. Aken, J. Maier and Y. Yu, Nano Lett., 2014, 14, 2175–2180 CrossRef CAS PubMed.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271–1273 CrossRef CAS.
- H. Wang, D. Mitlin, J. Ding, Z. Li and K. Cui, J. Mater. Chem. A, 2016, 4, 5149–5158 CAS.
- L. Liang, Y. Xu, C. Wang, L. Wen, Y. Fang, Y. Mi, M. Zhou, H. Zhao and Y. Lei, Energy Environ. Sci., 2015, 8, 2954–2962 CAS.
- D. Yang, J. Xu, X. Liao, Y. He, H. Liu and Z. Ma, Chem. Commun., 2014, 50, 13377–13380 RSC.
- N. Wang, Z. Bai, Y. Qian and J. Yang, Adv. Mater., 2016, 28, 4126–4133 CrossRef CAS PubMed.
- H. Ye, Y. Wang, F. Zhao, W. Huang, N. Han, J. Zhou, M. Zeng and Y. Li, J. Mater. Chem. A, 2016, 4, 1754–1761 CAS.
- I. Hasa, X. Dou, D. Buchholz, Y. Horn, J. hassoun, S. Passerini and B. Scrosati, J. Power Sources, 2016, 310, 26–31 CrossRef CAS.
- C. Zhu, P. Kopold, P. A. Aken, J. Maier and Y. Yu, Adv. Mater., 2016, 28, 2409–2416 CrossRef CAS PubMed.
- S. Guo, H. Yu, P. Liu, Y. Ren, T. Zhang, M. Chen, M. Ishida and H. Zhou, Energy Environ. Sci., 2015, 8, 1237–1244 CAS.
- W. Duan, Z. Zhu, H. Li, Z. Hu, K. Zhang, F. Cheng and J. Chen, J. Mater. Chem. A, 2014, 2, 8668–8675 CAS.
- Z. Hou, X. Li, J. Liang, Y. Zhu and Y. Qian, J. Mater. Chem. A, 2015, 3, 1400–1404 CAS.
- X. Wu, Y. Cao, X. Ai, J. Qian and H. Yang, Electrochem. Commun., 2013, 31, 145–148 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ee02274j |
| This journal is © The Royal Society of Chemistry 2017 |