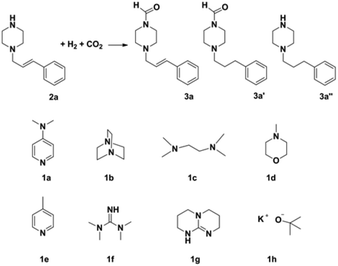
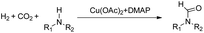
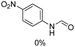
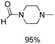
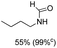
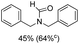
Synthesis of formamides containing unsaturated groups by N-formylation of amines using CO2 with H2†
Hangyu
Liu
ab,
Qingqing
Mei
ab,
Qingling
Xu
*b,
Jinliang
Song
a,
Huizhen
Liu
*ab and
Buxing
Han
*ab
aBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid and Interface and Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: liuhz@iccas.ac.cn; hanbx@iccas.ac.cn
bSchool of Chemistry and Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, P. R. China. E-mail: xuqingling@ucas.ac.cn
First published on 30th November 2016
Abstract
Formamides have wide applications in the industry and have been synthesized using CO2 as a carbon source and H2 as a reducing agent. However, previous systems required a noble catalyst and high temperature to achieve high efficiency, and the substrate scope was mostly limited to saturated amines. The selective N-formylation of amines containing unsaturated groups using CO2 and H2 is challenging because the efficient catalysts for the N-formylation are usually very active for hydrogenation of the unsaturated groups. Herein, we achieved for the first time a selective and efficient N-formylation of amines containing unsaturated groups using CO2 and H2 with a Cu(OAc)2–4-dimethylaminopyridine (DMAP) catalytic system. The substrates were converted to the desired formamides, while the unsaturated groups, such as the carbonyl group, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, C
C bond, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and the ester group remained. The main reason for the excellent selectivity of the Cu(OAc)2–DMAP catalytic system was that it was very active for the N-formylation reaction, but was not active for the hydrogenation of the unsaturated groups.
N bond and the ester group remained. The main reason for the excellent selectivity of the Cu(OAc)2–DMAP catalytic system was that it was very active for the N-formylation reaction, but was not active for the hydrogenation of the unsaturated groups.
Introduction
CO2 is an abundant and cheap C1 resource. Conversion of CO2 into value-added chemicals is an important approach for human beings to participate in the global carbon cycle.1–7 Catalytic chemical reduction is one of the most efficient routes. Some value-added chemicals have been synthesized by the chemical reduction of CO2.8–18Formamides are a class of chemicals with widespread applications in the industry as solvents and raw materials for the synthesis of other chemicals.19,20 Various routes and feedstocks have been used to synthesize formamides,21–36 and using CO2 as a carbon resource and H2 as the reducing reagent is an ideal route.22 Various saturated amines have been prepared using CO2 and H2 as feedstocks.37,38 Ding and his co-workers reported the N-formylation of a series of saturated amines with H2 and CO2 catalyzed by a ruthenium based homogeneous catalyst at 120 °C.39 Shi et al. found that the heterogeneous catalyst palladium was also active for this kind of reactions at 130 °C.40 Various saturated formamides have also been synthesized using organosilanes as the reducing reagent and CO2 as the carbon resource.25–36
The formamides containing unsaturated groups are more desirable in many cases because the unsaturated group can be easily further functionalized to produce useful compounds.41,42 So far, the formamides with unsaturated functional groups are generally synthesized by using CO, formic acid or methylformate as the carbon resources.44,45 It is interesting to produce this kind of formamides using CO2 to replace these carbon resources. Recently, Kobayashi and his co-workers reported the N-formylation of amines containing unsaturated groups using CO2 as the carbon resource and Ph2SiH2 as the reducing agent, and a chelating bis(NHC) rhodium complex (NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-heterocyclic carbene) was used as the catalyst.46
N-heterocyclic carbene) was used as the catalyst.46
It is well known that hydrogen is a commonly used reducing agent because it is abundant, economic, non-toxic, and the only byproduct is H2O. N-Formylation of amine derivatives containing unsaturated groups using CO2 and H2, in which the unsaturated group remains unreacted, is highly desirable, but is challenging. One of the main reasons is that CO2 is thermodynamically very stable and kinetically inert in typical organic syntheses. So the direct reaction between H2 and CO2 usually requires harsh reaction conditions, under which the unsaturated group, such as the carbonyl group, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, C
C bond, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and ester group, is easily hydrogenated.
N bond and ester group, is easily hydrogenated.
In this work, we discovered that the Cu–DMAP (1a) catalytic system could catalyze this kind of reactions very effectively, and the unsaturated groups (e.g. carbonyl group, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, C
C bond, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and ester group) could be retained. As far as we know, this is the first work for the synthesis of formamides containing unsaturated groups by N-formylation of amines using CO2 with H2.
N bond and ester group) could be retained. As far as we know, this is the first work for the synthesis of formamides containing unsaturated groups by N-formylation of amines using CO2 with H2.
Results
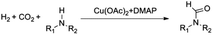
Compared to precious metal catalysts, abundant and inexpensive first-row transition metals, especially Cu, Ni, and Co, are advantageous for large-scale chemical processes. DMAP is a useful nucleophilic catalyst for a variety of reactions.43 We studied the performances of copper salts for the N-formylation reaction of 1-cinnamylpiperazine (2a) with or without additives. Cu(OAc)2 only afforded 20% yield of 3a in the absence of an additive (Table 1, entry 1). DMAP (1a) was the best additive among those tested and the Cu(OAC)2–DMAP catalytic system could afford 91% yield of 3a in 6 h (Table 1, entry 2). DABCO (1b, 1,4-diazabicyclo[2.2.2]octane) and N,N,N′,N′-tetramethyl-1,2-diaminopropane (1c) were also effective for promoting the reaction and the yields of 3a were 46% and 38%, respectively (Table 1, entries 3 and 4). The promoting effect of 4-methylmorpholine (1d) was poor, and the yield of 3a was only 28% (Table 1, entry 5). 4-Methylpyridine (1e), tetramethylguanidine (1f) and TBD (1g, 1,5,7-triazabicyclo[4.4.0]dec-5-ene) suppressed the reaction and afforded yields of 3a below 20% (Table 1, entries 6–8). An inorganic base potassium isopropoxide (1h) completely suppressed the reaction (Table 1, entry 9). The other metal complexes also exhibited high selectivity for the N-formylation reaction, while the activity was very low under the reaction conditions (Table 1, entries 10–14). Furthermore, the counterions of the copper salts containing oxygen led to higher activity (Table 1, entries 2 and 10–12). Cu(OAc)2–DMAP was the most active and selective catalytic system, and the yield of the desired product could be as high as 99% under the optimized conditions (Table 1, entry 15). The higher activity of Cu(OAc)2 is attributed to the basicity of OAC− and it may be of help in the activation of H2 by the interaction of oxygen of OAC− with H2. The reaction mechanism would be discussed below. In addition, the yield of the product was very low when only DMAP (1a) was used without a metal catalyst (Table 1, entry 16). It can be observed from entries 1, 15 and 16 that Cu(OAc)2 and DMAP had an excellent synergistic effect on the reaction. No formic acid was detected without amines which indicated that the N-formylation reaction catalyzed by Cu(OAc)2–DMAP didn't involve the hydrogenation of CO2 to formic acid (Table 1, entry 17) and this result is consistent with the result reported in the literature.47 The reaction can't occur without Cu(OAc)2 and DMAP (Table 1, entry 18). The composition of the gas after the reaction was also checked and only CO2 and H2 were detected. We also studied the effects of temperature and solvents on the reaction using the Cu(OAc)2–DMAP catalytic system for the N-formylation reaction of 1-cinnamylpiperazine (2a), and the results are listed in Table S1.† The yields of the desired product increased with increasing temperature in the range of 70 °C and 90 °C. Toluene/THF was the most effective among the solvents checked.| Entry | Metal precursor | Additive | Yieldb (%) | Selectivityc (%) |
|---|---|---|---|---|
| a Reaction conditions: 1-cinnamylpiperazine 1 mmol, PCO2 = PH2 = 40 atm, metal precursor 10 mol% based on the substrate, additive 2 mmol, THF 1.5 mL, 90 °C, 6 h. b Yield of 3a was determined by GC. c Selectivity of 3a was determined by GC. d 9 h. e Without amines. f 4 MPa CO2 were added firstly, and the solution was stirred for 1 h. Then discharge the CO2, while adding hydrogen (4 MPa) and stirred for 6 hours. | ||||
| 1 | Cu(OAc)2 | None | 20 | >99 |
| 2 | Cu(OAc)2 | 1a | 91 | >99 |
| 3 | Cu(OAc)2 | 1b | 46 | >99 |
| 4 | Cu(OAc)2 | 1c | 38 | >99 |
| 5 | Cu(OAc)2 | 1d | 28 | >99 |
| 6 | Cu(OAc)2 | 1e | 13 | >99 |
| 7 | Cu(OAc)2 | 1f | 19 | >99 |
| 8 | Cu(OAc)2 | 1g | 6 | >99 |
| 9 | Cu(OAc)2 | 1h | 0 | — |
| 10 | CuSO4 | 1a | 57 | >99 |
| 11 | Cu(NO3)2 | 1a | 17 | >99 |
| 12 | CuCl2 | 1a | 3 | >99 |
| 13 | Ni(OAc)2 | 1a | 19 | >99 |
| 14 | Co(OAc)2 | 1a | 21 | >99 |
| 15 | Cu(OAc)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
1a | 99 | >99 |
| 16 | None | 1a | 3 | >99 |
| 17 | Cu(OAc)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
1a | — | — |
| 18 | None | None | 0 | — |
| 19 | Cu(OAc)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
1a | 7 | >99 |
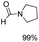
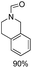
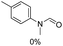
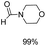
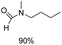
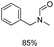
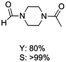
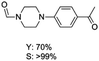
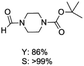
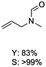
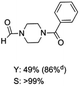
The results above indicate that Cu(OAc)2 and DMAP (1a) is an excellent combination for the N-formylation of 1-cinnamylpiperazine. We further explored the formylation of various amines with different unsaturated groups to examine the versatility of the catalytic system using H2 as a reductant in THF, and the results are listed in Table 2. The N-formylation reaction proceeded smoothly to selectively afford the corresponding formamides in good to excellent yields, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, carbonyl group and ester group could be retained. The Cu(OAc)2–DMAP catalytic system was effective for piperazine derivatives. The yield of 4-allylpiperazine-1-carbaldehyde could reach 95% in 12 h (Table 2, entry 1). The yields of 1-formyl-4-acetylpiperazine and 1-Boc-4-formylpiperazine were all above 80% (Table 2, entries 2 and 3). 86% yield of 4-benzoyl-1-piperazinecarboxaldehyde was obtained when the reaction time was prolonged to 20 h (Table 2, entry 4). The reactivity of chain secondary amines that contain the C
C bond, carbonyl group and ester group could be retained. The Cu(OAc)2–DMAP catalytic system was effective for piperazine derivatives. The yield of 4-allylpiperazine-1-carbaldehyde could reach 95% in 12 h (Table 2, entry 1). The yields of 1-formyl-4-acetylpiperazine and 1-Boc-4-formylpiperazine were all above 80% (Table 2, entries 2 and 3). 86% yield of 4-benzoyl-1-piperazinecarboxaldehyde was obtained when the reaction time was prolonged to 20 h (Table 2, entry 4). The reactivity of chain secondary amines that contain the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was lower than piperazine derivatives with the C
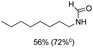
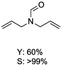
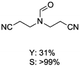
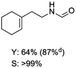
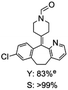
C bond was lower than piperazine derivatives with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (Table 2, entries 1 and 5–7). The yields of N,N-diallylformamide, N-(2-cyclohex-1-enyl-ethyl)-formamide and N-methyl-N-allyl-formamide were 60%, 64% and 83% in 12 h, respectively (Table 2, entries 5–7), while 87% yield of N-(2-cyclohex-1-enyl-ethyl)-formamide was obtained when the reaction time was prolonged to 20 h (Table 2, entry 6). The yield of N-formyl desloratadine was 83% (Table 2, entry 8). The yield of N,N-bis-(2-cyano-ethyl)-formamide was 31% in 12 h (Table 2, entry 9). The yield of 4-(4-acetylphenyl)piperazine-1-carbaldehyde was above 70% (Table 2, entries 10).
C bond (Table 2, entries 1 and 5–7). The yields of N,N-diallylformamide, N-(2-cyclohex-1-enyl-ethyl)-formamide and N-methyl-N-allyl-formamide were 60%, 64% and 83% in 12 h, respectively (Table 2, entries 5–7), while 87% yield of N-(2-cyclohex-1-enyl-ethyl)-formamide was obtained when the reaction time was prolonged to 20 h (Table 2, entry 6). The yield of N-formyl desloratadine was 83% (Table 2, entry 8). The yield of N,N-bis-(2-cyano-ethyl)-formamide was 31% in 12 h (Table 2, entry 9). The yield of 4-(4-acetylphenyl)piperazine-1-carbaldehyde was above 70% (Table 2, entries 10).
| Entry | Product | Entry | Product | Entry | Product |
|---|---|---|---|---|---|
| a Reaction conditions: amine 1 mmol, PCO2 = PH2 = 40 atm, Cu(OAc)2 10 mol% based on the substrate, DMAP 2 mmol, THF 1.5 mL, 90 °C, 12 h. b Yield was determined by GC. c Selectivity was determined by GC. d 20 h. e Yield was determined by 1H NMR. | |||||
| 1 |
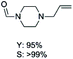
|
5 |
|
9 |
|
| 2 |
|
6 |
|
10 |
|
| 3 |
|
7 |
|
||
| 4 |
|
8 |
|
||
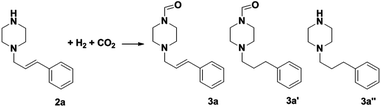
The above results indicate that the Cu(OAc)2–DMAP catalytic system was highly selective and efficient for the N-formylation of various amines with unsaturated groups using H2 and CO2. The efficiency and selectivity of the commonly used catalysts for the N-formylation reaction of saturated amines40,48–50 were also tested using the N-formylation reaction of 1-cinnamylpiperazine (2a) (Table 3). The conversion of 2a could reach 99% at 90 °C in 6 h, but almost no desired product 3a was detected over Pd/Al2O3 (Table 3, entry 1). A similar result was obtained over the Pd/C catalyst. When PdCl2 was used as the catalyst, the conversion of 2a was 94%, while the selectivity for 3a was only 3% (Table 3, entry 3). For all the Pd based catalysts checked, other byproducts such as n-propylbenzene and piperazine that resulted from the cleavage of the C–N bond were detected (Table 3, entries 1–3). The performance of the Ru complexes was also investigated (Table 3, entries 4–6). The conversion of 2a was 95%, and the selectivity of 3a, 3a′ and 3a′′ was only 11%, 13% and 11% respectively over [(C6H5)3P]3Ru(CO)(Cl)H and 65% selectivity of the byproducts from the cleavage of the C–N bond was detected (Table 3, entry 4). Under the same reaction conditions, the conversion of 2a was 75% over Ru3(CO)12 and the selectivity of 3a was 33% (Table 3, entry 5). Similarly, the conversion and selectivity of 3a over RhCl3 were low (Table 3, entry 7). All the results above indicate that the noble metal based catalysts, which are commonly used in the N-formylation reaction of saturated amines had very poor selectivity for the N-formylation of the amine containing the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond.
C bond.
| Entry | Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|---|
| 3a | 3a′ | 3a′′ | Othersa | |||
| Reaction conditions: 2a 1 mmol, PCO2 = PH2 = 40 atm, catalyst 10 mol%, THF 1.5 mL, 90 °C, 6 h. Conversion and selectivity were determined by GC.a The product from the cleavage of the C–N bond. | ||||||
| 1 | Pd/Al2O3 | >99 | Trace | 36 | 48 | 16 |
| 2 | Pd/C | >99 | 0 | 47 | 36 | 17 |
| 3 | PdCl2 | 94 | 3 | 22 | 50 | 25 |
| 4 | [(C6H5)3P]3Ru(CO)(Cl)H | 95 | 11 | 13 | 11 | 65 |
| 5 | Ru3(CO)12 | 75 | 33 | 7 | 7 | 53 |
| 6 | RhCl3 | 77 | 42 | Trace | Trace | 58 |
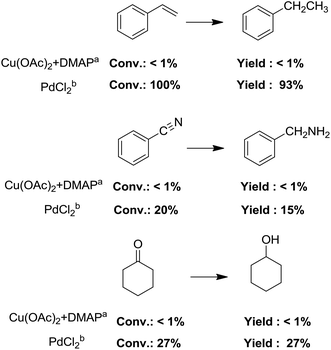
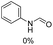
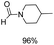
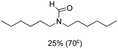
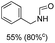
The most interesting part of this work is that the Cu(OAc)2–DMAP catalytic system was very selective and effective for the N-formylation reactions of various amines with unsaturated bonds. To investigate the reason for this interesting phenomenon, we studied its catalytic activity for the hydrogenation of a series of compounds containing different unsaturated bonds and the results are shown in Fig. 1. The catalytic system was not active for the hydrogenation of the unsaturated bonds (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), which can explain reasonably the high selectivity of the Cu(OAc)2–DMAP catalytic system for the N-formylation reactions. We also used PdCl2 as the catalyst for the hydrogenation of these substrates (Fig. 1). We found that these substrates converted to the corresponding saturated compounds, which can explain the low selectivity of the noble metal catalytic system for the N-formylation reactions.
O), which can explain reasonably the high selectivity of the Cu(OAc)2–DMAP catalytic system for the N-formylation reactions. We also used PdCl2 as the catalyst for the hydrogenation of these substrates (Fig. 1). We found that these substrates converted to the corresponding saturated compounds, which can explain the low selectivity of the noble metal catalytic system for the N-formylation reactions.
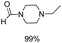
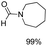
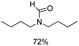
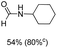
Noble metal based catalysts are commonly used in the N-formylation reaction of saturated amines.37–40,48–50 In this work, we also studied the catalytic performance of the Cu(OAc)2–DMAP catalytic system for the N-formylation reaction of typical saturated amines, and the results are provided in Table 4. It can be observed that the catalytic system was also very effective for the N-formylation of saturated amines. The excellent activity of the Cu(OAc)2–DMAP system results from the synergistic effect of Cu(OAc)2 and DMAP for N-formylation reactions, as discussed above (Table 1, entries 1, 15 and 16). However, Cu(OAC)2–DMAP catalytic system is not active for the N-formylation of aromatic amines (Table 1, Entries 17–20).
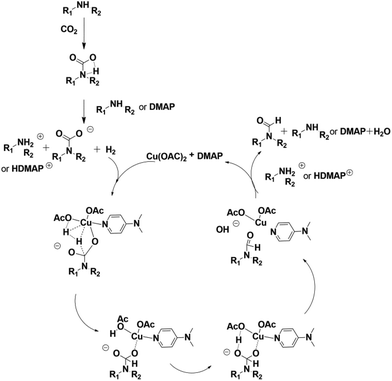
To study the reaction mechanism, a control experiment was performed (Table 1, entry 19). Firstly the reaction of 1-cinnamylpiperazine and 4 MPa CO2 was allowed to proceed for 1 h in the presence of Cu(OAc)2–DMAP. CO2 was removed and 4 MPa H2 was added and stirred for 6 h, and the product 3a was also produced, which indicated that CO2 and 1-cinnamylpiperazine or DMAP can form the salt51 and the salt further reacted with hydrogen to form the final product.
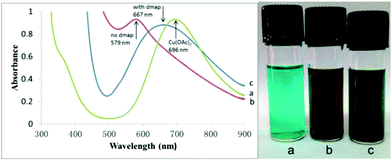
It has been reported that oxygen-containing ligands may help in the activation of hydrogen.53 Similarly, we obtained a transition state for OAc− assisted hydrogen cracking through calculations52 (Fig. S1 in the ESI†). On the basis of the experimental results, a possible reaction mechanism was proposed for the reaction over the Cu(OAc)2–DMAP catalytic system (Fig. 2). Amines can react with CO2 to form internal salts very easily.51 Next, the cleavage of the H–H bond was accompanied by the formation of a C–H bond and O–H bond. And finally H2O was lost and the final product was obtained. In order to further illustrate the role of DMAP, the reaction solutions with and without DMAP at 50 °C were examined using UV-Vis spectroscopy and the spectra are shown in Fig. 3. The absorption peak of copper acetate appears at 696 nm (a). Without DMAP, the absorption peak was observed at around 579 nm, which is characteristic of coordinate of copper and amine (b). For the reaction solution with DMAP, the peak shift to 667 nm indicated the coordination of copper and DMAP (c).
Conclusions
In summary, the Cu(OAc)2–DMAP catalytic system is very selective and active for the N-formylation reactions of amines containing unsaturated groups using H2 and CO2. The unsaturated groups in the substrates, including the carbonyl group, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, C
C bond, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond and ester group, are all stable under the reaction conditions. High yields of the desired formamides can be obtained with a selectivity of >99%. However, the selectivity of the desired product over Pd, Ru and Rh based catalysts, which are commonly used for the N-formylation reactions of saturated amines, is very low because the catalysts are also very active in the hydrogenation of the unsaturated bonds. We believe that the highly selective, active, and cheaper catalytic system has great potential of application for producing formamides with unsaturated groups.
N bond and ester group, are all stable under the reaction conditions. High yields of the desired formamides can be obtained with a selectivity of >99%. However, the selectivity of the desired product over Pd, Ru and Rh based catalysts, which are commonly used for the N-formylation reactions of saturated amines, is very low because the catalysts are also very active in the hydrogenation of the unsaturated bonds. We believe that the highly selective, active, and cheaper catalytic system has great potential of application for producing formamides with unsaturated groups.
Acknowledgements
This work was supported by the Recruitment Program of Global Youth Experts of China, National Natural Science Foundation of China (21603235, 21533011, and 21321063), and the Chinese Academy of Sciences (KJCX2.YW.H30).Notes and references
- M. Y. He, Y. H. Sun and B. X. Han, Angew. Chem., Int. Ed., 2013, 52, 9620–9633 CrossRef CAS PubMed.
- W. Wang, S. P. Wang, X. B. Ma and J. L. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462–1484 RSC.
- K. Iizuka, T. Wato, Y. Miseki, K. Saito and A. Kudo, J. Am. Chem. Soc., 2011, 133, 20863–20868 CrossRef CAS PubMed.
- Y. Xie, T. T. Wang, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960–1966 Search PubMed.
- W. Tu, Y. Zhou and Z. Zou, Adv. Mater., 2014, 26, 4607–4626 CrossRef CAS PubMed.
- S. Lin, C. S. Diercks, Y. B. Zhang, N. Kornienko, E. M. Nichols, Y. Zhao, A. R. Paris, D. Kim, P. Yang, O. M. Yaghi and C. J. Chang, Science, 2015, 349, 1208–1213 CrossRef CAS PubMed.
- Y. Y. Zhang, A. D. MacIntosh, J. L. Wong, E. A. Bielinski, P. G. Williard, B. Q. Mercado, N. Hazari and W. H. Bernskoetter, Chem. Sci., 2015, 6, 4291–4299 RSC.
- T. J. Schmeier, G. E. Dobereiner, R. H. Crabtree and N. Hazari, J. Am. Chem. Soc., 2011, 133, 9274–9277 CrossRef CAS PubMed.
- J. F. Hull, Y. Himeda, W. H. Wang, B. Hashiguchi, R. Periana, D. J. Szalda, J. T. Muckerman and E. Fujita, Nat. Chem., 2012, 4, 383–388 CrossRef CAS PubMed.
- C. Y. Wu, Z. F. Zhang, Q. G. Zhu, H. l. Han, Y. Y. Yang and B. X. Han, Green Chem., 2015, 17, 1467–1472 RSC.
- K. M. K. Yu, C. M. Y. Yeung and S. C. Tsang, J. Am. Chem. Soc., 2007, 129, 6360–6361 CrossRef CAS PubMed.
- F. Frusteri, G. Bonuraa, C. Cannillaa, G. Drago Ferrantea, A. Aloiseb, E. Catizzoneb, M. Migliorib and G. Giordanob, Appl. Catal., B, 2015, 176–177, 522–531 CrossRef CAS.
- X. F. Yang, S. Kattel, S. D. Senanayake, J. A. Boscoboinik, X. W. Nie, J. Graciani, J. A. Rodriguez, P. Liu, D. J. Stacchiola and J. G. G. Chen, J. Am. Chem. Soc., 2015, 137, 10104–10107 CrossRef CAS PubMed.
- Z. H. He, Q. L. Qian, J. Ma, Q. L. Meng, H. C. Zhou, J. L. Song, Z. M. Liu and B. X. Han, Angew. Chem., Int. Ed., 2016, 128, 747–751 CrossRef.
- H. Zhou, G. X. Wang, W. Z. Zhang and X. B. Lu, ACS Catal., 2015, 5, 6773–6779 CrossRef CAS.
- W. C. Chen, J. S. Shen, T. Jurca, C. J. Peng, Y. H. Lin, Y. P. Wang, W. C. Shih, G. P. A. Yap and T. G. Ong, Angew. Chem., Int. Ed., 2015, 54, 15207–15212 CrossRef CAS PubMed.
- E. Blondiaux, J. Pouessel and T. Cantat, Angew. Chem., Int. Ed., 2014, 53, 12186–12190 CrossRef CAS PubMed.
- K. Weissermel and H. J. Arpe, in Industrial Organic Chemistry, ed. C. R. Lindley, Wiley-VCH, Weinheim, 3rd edn, 1997 Search PubMed.
- S. T. Ding and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 9226–9237 CrossRef CAS PubMed.
- M. F. Ali, B. M. El Ali and J. G. Speight, Handbook of Industrial Chemistry-Organic Chemicals, McGraw-Hill, New York, 2005 Search PubMed.
- A. Tlili, E. Blondiaux, X. Frogneux and T. Cantat, Green Chem., 2015, 17, 157–168 RSC.
- Y. H. Wang, J. Zhang, H. J. Chen, Z. X. Zhang, C. F. Zhang, M. R. Li and F. Wang, Green Chem. 10.1039/c6gc02603f.
- Z. G. Ke, Y. Zhang, X. J. Cui and F. Shi, Green Chem., 2016, 18, 808–816 RSC.
- K. Motokura, N. Takahashi, D. Kashiwame, S. Yamaguchi, A. Miyaji and T. Baba, Catal. Sci. Technol., 2013, 3, 2392–2396 CAS.
- O. Jacquet, C. D. N. Gomes, M. Ephritikhine and T. Cantat, J. Am. Chem. Soc., 2012, 134, 2934–2937 CrossRef CAS PubMed.
- L. D. Hao, Y. F. Zhao, B. Yu, Z. Z. Yang, H. Y. Zhang, B. X. Han, X. Gao and Z. M. Liu, ACS Catal., 2015, 5, 4989–4993 CrossRef CAS.
- C. D. N. Gomes, O. Jacquet, C. Villiers, P. Thuéry, M. Ephritikhine and T. Cantat, Angew. Chem., Int. Ed., 2012, 51, 187–190 CrossRef PubMed.
- X. Frogneux, O. Jacquet and T. Cantat, Catal. Sci. Technol., 2014, 4, 1529–1533 CAS.
- C. C. Chong and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 12116–12120 CrossRef CAS PubMed.
- S. Das, F. D. Bobbink, S. Bulut, M. Soudania and P. J. Dyson, Chem. Commun., 2016, 52, 2497–2500 RSC.
- D. B. Nale, D. Rath, K. M. Parida, A. Gajengi and B. M. Bhanage, Catal. Sci. Technol., 2016, 6, 4872–4881 CAS.
- H. Lv, Q. Xing, C. T. Yue, Z. Q. Lei and F. W. Li, Chem. Commun., 2016, 52, 6545–6548 RSC.
- M. Hulla, F. D. Bobbink, S. Das and P. J. Dyson, ChemCatChem, 2016, 8, 3338–3342 CrossRef CAS.
- S. Q. Zhang, Q. Q. Mei, H. Y. Liu, H. Z. Liu, Z. P. Zhang and B. X. Han, RSC Adv., 2016, 6, 32370–32373 RSC.
- B. Dong, L. Y. Wang, S. Zhao, R. L. Ge, X. D. Song, Y. Wang and Y. A. Gao, Chem. Commun., 2016, 52, 7082–7085 RSC.
- C. Federsel, A. Boddien, R. Jackstell, R. Jennerjahn, P. J. Dyson, R. Scopelliti, G. Laurenczy and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9777–9780 CrossRef CAS PubMed.
- Q. Y. Bi, J. D. Lin, Y. M. Liu, S. H. Xie, H. Y. He and Y. Cao, Chem. Commun., 2014, 50, 9138–9140 RSC.
- L. Zhang, Z. B. Han, X. Y. Zhao, Z. Wang and K. L. Ding, Angew. Chem., Int. Ed., 2015, 54, 6186–6189 CrossRef CAS PubMed.
- X. J. Cui, Y. Zhang, Y. Q. Deng and F. Shi, Chem. Commun., 2014, 50, 189–191 RSC.
- E. Vardelle, D. G. Sanchez, A. M. Mingot, M. P. Jouannetaud, S. Thibaudeau and J. Marrot, Chem. Commun., 2008, 1473–1475 RSC.
- G. S. Nandra, P. S. Pang, M. J. Porter and J. M. Elliott, Org. Lett., 2005, 7, 3453–3455 CrossRef CAS PubMed.
- C. Zhang, B. Zhong, S. M. Yang, L. K. Pan, S. W. Yu, Z. J. Li, S. C. Li, B. Su and X. B. Meng, Bioorg. Med. Chem., 2015, 23, 3774–3780 CrossRef CAS PubMed.
- C. Borel, L. S. Hegedus, J. Krebs and Y. Satoh, J. Am. Chem. Soc., 1987, 109, 1101–1105 CrossRef CAS.
- J. Falbe and F. Korte, Eur. J. Org. Chem., 1965, 1928–1937 CAS.
- T. V. Q. Nguyen, W. J. Yoo and S. Kobayashi, Angew. Chem., Int. Ed., 2015, 54, 9209–9212 CrossRef CAS PubMed.
- R. Watari, Y. Kayaki, S. Hirano, N. Matsumoto and T. Ikariyab, Adv. Synth. Catal., 2015, 357, 1369–1373 CrossRef CAS.
- K. Kudo, H. Phala, N. Sugita and Y. Takezaki, Chem. Lett., 1977, 12, 1495–1496 CrossRef.
- Y. Morimoto, Y. Fujiwara, H. Taniguchi, Y. Hori and Y. Nagano, Tetrahedron Lett., 1986, 27, 1809–1810 CrossRef CAS.
- G. Süss-Fink, M. Langenbahn and T. Jenke, J. Organomet. Chem., 1989, 368, 103–109 CrossRef.
- C. Y. Wu, H. Y. Cheng, R. X. Liu, Q. Wang, Y. F. Hao, Y. C. Yu and F. Y. Zhao, Green Chem., 2010, 12, 1811–1816 RSC.
- See the ESI† for the complete references.
- I. Cano, M. A. Huertos, A. M. Chapman, G. Buntkowsky, T. Gutmann, P. B. Groszewicz and P. W. N. M. V. Leeuwen, J. Am. Chem. Soc., 2015, 137, 7718–7727 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6gc02243j |
| This journal is © The Royal Society of Chemistry 2017 |