Self-assembly of [PNbxW12−xO40]n− Keggin anions – a simple way to mixed Nb–W polyoxometalates†
Pavel A.
Abramov
*ab,
Alexandra A.
Shmakova
ab,
Mohamed
Haouas
*c,
Gerhard
Fink
c,
Emmanuel
Cadot
c and
Maxim N.
Sokolov
ab
aNikolaev Institute of Inorganic Chemistry SB RAS, Novosibirsk, 630090, Russia. E-mail: abramov@niic.nsc.ru
bNovosibirsk State University, Novosibirsk, 630090, Russia
cLavoisier Institute of Versailles, UMR CNRS 8180, University of Versailles Saint-Quentin en Yvelines, 45 Avenue des Etats-Unis, 78035 Versailles, France. E-mail: mohamed.haouas@uvsq.fr
First published on 10th November 2016
Abstract
This paper summarizes preparations of mixed phosphoniobotungstates [PNbxW12−xO40](3+x)− (x = 1–3) in two different ways: (i) incorporation of Nb into pre-existing lacunary phosphotungstates and (ii) self-assembly reactions. A niobium oxalate complex as the source of niobium is used. The reaction products were monitored by 31P and 183W NMR in solution as well as by 31P MAS NMR techniques for precipitated samples. Single-substituted species [PNbW11O40]4− forms rapidly and selectively from lacunary [PW11O39]7− with one equivalent of [NbO(C2O4)2(H2O)2]−. With the increasing amount of the niobium oxalate the highest number of Nb atoms was found to be three per Keggin anion, but the disubstituted [PNb2W10O40]5− was always the preferable product whatever the tungsten reagent was. The reaction products with x = 2 and 3 are present as mixtures of all possible isomers, but the ratio between the individual isomers depends on the synthetic pathway.
Introduction
Of the polyoxoniobate family mixed-addendum Nb/W POMs, explored by Klemperer,1 Hill,2–6 and Finke7–9 groups, Lindqvist-type [NbxW6−xO19](2+x)−,10–12 Keggin-type [XW9Nb3O40]n− (X = SiIV, GeIV, n = 7; X = PV, AsV, n = 6)2,3,13–15 and Dawson-type [P2W12(NbO2)6O56]12−,16 [P2W15Nb3O62]9−,7,17 and [P2W17(NbO2)O61]7−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 merit particular attention because of their unique characteristics. Due to the presence of Nb, these POMs possess higher nucleophilicity than the parent tungstates.15,18,19 Such complexes demonstrate photocatalytic activity in oxidation5 and reduction20 processes, and can be used as precursors for hydrodesulfurization/isomerization catalysts12 or as active catalysts in the preparation of linearly-extended polyalkylenepolyamines.21
6 merit particular attention because of their unique characteristics. Due to the presence of Nb, these POMs possess higher nucleophilicity than the parent tungstates.15,18,19 Such complexes demonstrate photocatalytic activity in oxidation5 and reduction20 processes, and can be used as precursors for hydrodesulfurization/isomerization catalysts12 or as active catalysts in the preparation of linearly-extended polyalkylenepolyamines.21
The intrinsic high negative charge density of [Nb6O19]8− and other polyniobates can be reduced by the substitution of one or more NbV addenda sites with WVI, rendering these POMs less basic and more stable in neutral or acidic media. This goal can be achieved in various ways. Dabbabi and Boyer reported that Na2WO4 and K8[Nb6O19] in aqueous H2O2 yield the mixed metal complex [NbW5O19]3−.22 This compound is stable at pH 1.5–5 and gradually transforms into [cis-Nb2W4O19]4−, [fac-Nb3W3O19]5−, [cis-Nb4W2O19]6−, [Nb5WO19]7−, and [Nb6O19]8− with increasing alkalinity. The isomeric purity of [cis-Nb2W4O19]4−, [fac-Nb3W3O19]5−, and [cis-Nb4W2O19]6− was claimed from infrared studies23 and further confirmed, for [cis-Nb2W4O19]4−, by electrochemical measurements24 as well as by 17O and 183W NMR spectroscopy.10,25 The other traditional way of making the Nb/W mixed POMs consists of reacting peroxoniobates prepared in situ by solubilizing [Nb6O19]8− in H2O2 with lacunary polyoxotungstates or sodium tungstate. This methodology was extended to produce first Ta/W POMs.18 The peroxoniobates have good stability in neutral to acidic media owing to the presence of the strongly coordinating η2-peroxide ligands, which makes them compatible with lacunary POMs, unlike simple oxoniobates, which can be handled only in alkaline solutions where polytungstates are unlikely to survive.26 However, the use of peroxoniobates still presents several drawbacks, such as: (i) the excess of H2O2 destroys polytungstate precursors, and careful titration of peroxide with sodium sulfite is needed to avoid this;27,28 (ii) to destroy the niobium peroxo complexes prolonged heating is needed which is not always desirable; (iii) the control of the stoichiometric ratio Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W is complicated by some Nb ending up as insoluble Nb2O5. To avoid these difficulties, we have set out to develop an alternative approach based on the use of a niobium oxalate complex, (NH4)[NbO(C2O4)2(H2O)2]·3H2O, as a Nb precursor. The oxalate complex offers the advantages of being a well-defined stoichiometric solid, easily soluble in water and stable enough in solution even at a pH range between 1 and 3.29 We have found the only example of the use of niobium oxalate, namely, for the preparation of mixed Nb–W catalysts for decomposition of aromatics.30 In this work we used niobium oxalate for incorporation of Nb into lacunary Keggin phosphotungstates, and for self-assembly reactions with phosphate and tungstate, leading to mixed [PNbxW12−xO40]n− anions (x = 1–3).
W is complicated by some Nb ending up as insoluble Nb2O5. To avoid these difficulties, we have set out to develop an alternative approach based on the use of a niobium oxalate complex, (NH4)[NbO(C2O4)2(H2O)2]·3H2O, as a Nb precursor. The oxalate complex offers the advantages of being a well-defined stoichiometric solid, easily soluble in water and stable enough in solution even at a pH range between 1 and 3.29 We have found the only example of the use of niobium oxalate, namely, for the preparation of mixed Nb–W catalysts for decomposition of aromatics.30 In this work we used niobium oxalate for incorporation of Nb into lacunary Keggin phosphotungstates, and for self-assembly reactions with phosphate and tungstate, leading to mixed [PNbxW12−xO40]n− anions (x = 1–3).
Results and discussion
V and Nb-substituted heteropolytungstates
Vanadium substituted Keggin-type heteropolyanions [XVxM12−xO40]n− have been investigated as catalysts for selective oxidation of acids, aldehydes and hydrocarbons, oxidative dehydrogenation of alkanes etc.31–35 Vanadium for tungsten substitution in a Keggin anion does not affect its overall structure. Since X-ray diffraction analysis does not show any preferential position of vanadium in the Keggin structure, one can only obtain information about the cation site occupancy by analytical determination of the M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V ratio.34,36,37 Extra vanadium can also enter the Keggin-type anions by capping one or two rectangular faces of the Keggin polyhedra with {VO} caps.37,38
V ratio.34,36,37 Extra vanadium can also enter the Keggin-type anions by capping one or two rectangular faces of the Keggin polyhedra with {VO} caps.37,38
The niobium substituted Keggins have been studied on a more modest scale. A few of them, [XW9Nb3O40]n− (X = SiIV, GeIV, n = 7; X = PV, AsV, n = 6),9,39,40 [XW9O37(NbO2)3]n−,4,5 [γ-SiW10O38(NbO2)2]6−,41 and [XNbW11O40],5–42 have been obtained tested in alkene epoxidation, but their catalytic activity was found to be much lower than that of the vanadium analogues. However, the terminal oxide ligand at Nb seems to be more labile than that at W in substitution reactions, which offers some unexpected possibilities of making POMs more “soft”, in the HSAB theory sense. Thus Sécheresse et al. have shown that [PW11NbO40]4−, obtained by addition of a NbO3+ group from the controlled hydrolysis of NbCl5 into the monovacant α-[PW11O39]7−, selectively exchanges its terminal oxo group in the reaction with p-methoxyphenylthionophosphine sulfide (R2P2S4), with the formation of α-[PW11NbSO39]4−, with a sulfide replacing oxide.43 There remain also some general problems to be answered, such as the maximum number of Nb atoms entering a given Keggin structure, isomer formation and distribution etc.
Synthesis of P-NbxW12−x Keggin anions from lacunary type POM
Reactions between (NH4)[NbO(C2O4)2(H2O)2]·3H2O (Nb-Ox) and the trilacunary POMs, [A-PW9O34]9−, γ-[PW10O36]7−, and [PW11O39]7−, were carried out in water by addition of a calculated amount of Nb-Ox to a solution of the POM, and heating the final solution for 30 min at 80 °C at pH 3. Cs salts for 31P MAS NMR were precipitated with CsCl. The white precipitates were collected by filtration, extensively rinsed with water and dried in vacuo before the NMR experiments.The 31P MAS NMR spectra of the three products, together with the reference compound H3PW12O40·nH2O, are shown in Fig. 1. The multiple peaks in the range −14.9 to −15.1 ppm for H3PW12O40·nH2O result from different local environments of [PW12O40]3− due to the non-uniform degree of hydration in various domains of the bulk sample. These chemical shifts agree with the published values for the solid state (−15 ppm)44,45 and in solution (−14.4 ppm).46 The spectra of the solids from the reactions of Nb-Ox with [PW11O39]7− and [PW10O36]7− (Fig. 1b and c) are almost the same and exhibit three resonances at −15.0 (3%), −13.6 (85%), and −12.7 ppm (12%). In the spectrum of the product from the reaction with [PW9O34]9− (Fig. 1d) only two resonances are observed at −13.6 (5%) and −12.4 ppm (95%). Consequently, these two resonances at −13.6 and −12.4 ppm correspond to (Cs,K)4[PNbW11O40] (−13.6 ppm) and (Cs,Na)5[PNb2W10O40] (−12.4 ppm). Note the systematic shift in the 31P resonance (Δδ ∼ 1.3 ppm) for [PNbxW12−xO40](3+x)− with increasing x from 0 to 2. According to these results, with Nb-Ox stoichiometric (or quantitative) introduction of Nb into the Keggin phosphotungstate structure can be achieved only with [PW11O39]7−, while with [PW10O36]7− and [PW9O34]9− simultaneous incorporation of one and two Nb instead of strictly two and strictly three Nb atoms, respectively, expected, takes place. This means that partial decomposition and rearrangement of these bi- and trilacunary POMs occurs during the reaction. To gain further insight into these reactions, we followed them with conventional 31P NMR techniques in solution.
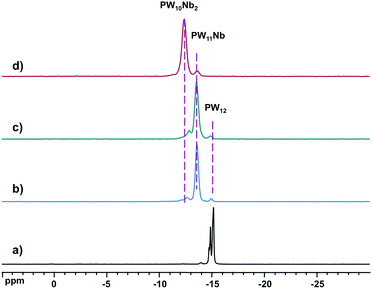 | ||
| Fig. 1 31P MAS NMR spectra of (a) H3PW12O40·nH2O, Cs salt of reaction products of Nb-Ox and (b) [PW11O39]7−, (c) [PW10O36]7−, and (d) [A-PW9O34]9−. | ||
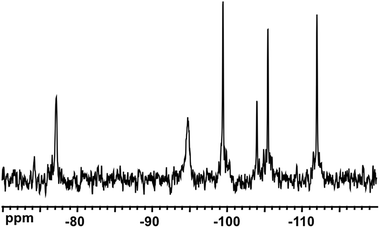
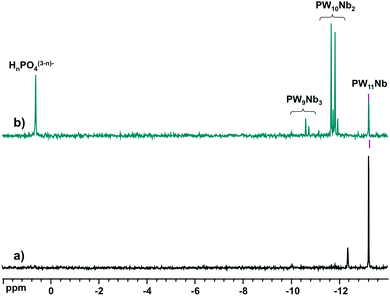
Fig. 2 shows the 31P NMR spectra of the reaction mixtures from Nb-Ox and K7[PW11O39] (Fig. 2a) and Na9[A-PW9O34] (Fig. 2b). It was difficult to perform the same experiment with Cs7[PW10O36] due to its low solubility leading to a suspension rather than to a clear solution. The spectrum of the reaction mixture with K7[PW11O39] (Fig. 2a) shows the dominant resonance line at −13.2 ppm assigned to [PNbW11O40]4− and an unidentified minor signal at −12.4 ppm. The latter was already present in the spectrum of the K7[PW11O39] precursor as an impurity and could correspond to [PW10O36]7−.47183W NMR of the reaction solution with K7[PW11O39] (Fig. 3) confirms the formation of [PNbW11O40]4− as the main product, since the six resonances at −77.1, −94.7, −99.5, −104.0, −105.5, and −112.0 ppm with relative intensities 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 are compatible with the Cs symmetry group of the monosubstituted Keggin structure. These NMR data agree with the published data for [PNbW11O40]4− POM.43,48,49
2 are compatible with the Cs symmetry group of the monosubstituted Keggin structure. These NMR data agree with the published data for [PNbW11O40]4− POM.43,48,49
 | ||
| Fig. 2 31P NMR spectra of the reaction mixture of (a) Nb-Ox and [PW11O39]7−, and (b) Nb-Ox and [A-PW9O34]9−. | ||
However, reaction with Na9[A-PW9O34] produces a complicated spectrum (Fig. 2b). The signal of [PNbW11O40]4− and two sets of resonances for the isomers of [PNb2W10O40]5− (−11.7 to −12.0 ppm) and [PNb3W9O40]6− (−10.1 to −11.4 ppm) are clearly visible, together with the signal from free phosphate (0.6 ppm). The signal distribution corresponds to a mixture consisting of 10% of [PNbW11O40]4−, 58% of [PNb2W10O40]5−, 12% of [PNb3W9O40]6−, and 20% of free HnPO4(3−n)−. These observations match the 31P MAS NMR data for the Cs salts, indicating that: (i) [PNb3W9O40]6− is less favored than [PNb2W10O40]5−, and (ii) structural decomposition/rearrangement takes place in the presence of excess of the Nb-Ox reagent. The starting [A-PW9O34]9− is entirely consumed, judging from the absence of its 31P NMR signature at −5 ppm.50
Self-assembly of P-NbxW12−x Keggin anions
Self-assembly of P-NbxW12−x Keggin anions in the range of chemical composition H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox
Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 = 1
Na2WO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12−n, n = 1–6, was monitored by 31P NMR (Fig. 4). The spectra revealed only the formation of [PW11O39]7− (−10.2 ppm), [PNbW11O40]4− (−13.2 ppm), and the isomers of [PNb2W10O40]5− (−11.6 to −12.0 ppm) and [PNb3W9O40]6− (−10.3 to −11.3 ppm), as well as free phosphate (0.7 ppm). The species distribution as a function of number of Nb equivalents is shown in Fig. 5. With addition of one equivalent of Nb all phosphate is consumed, and the prominent species is the monosubstituted Keggin [PNbW11O40]4− together with some [PNb2W10O40]5− and the lacunary POM [PW11O39]7− as minor products. Starting from two equivalents of Nb, the disubstituted [PNb2W10O40]5− becomes the dominant product. Simultaneously the share of unreacted phosphate increases with the increase in the niobium amount. The self-assembly effectively stops at [PNb2W10O40]5− even if some [PNb3W9O40]6− is also detectable. These results support previous observations and explain why di- and trilacunary phosphotungstates produced only mixtures of mono- and disubstituted (Cs,K)4[PNbW11O40] and (Cs,Na)5[PNb2W10O40].
12−n, n = 1–6, was monitored by 31P NMR (Fig. 4). The spectra revealed only the formation of [PW11O39]7− (−10.2 ppm), [PNbW11O40]4− (−13.2 ppm), and the isomers of [PNb2W10O40]5− (−11.6 to −12.0 ppm) and [PNb3W9O40]6− (−10.3 to −11.3 ppm), as well as free phosphate (0.7 ppm). The species distribution as a function of number of Nb equivalents is shown in Fig. 5. With addition of one equivalent of Nb all phosphate is consumed, and the prominent species is the monosubstituted Keggin [PNbW11O40]4− together with some [PNb2W10O40]5− and the lacunary POM [PW11O39]7− as minor products. Starting from two equivalents of Nb, the disubstituted [PNb2W10O40]5− becomes the dominant product. Simultaneously the share of unreacted phosphate increases with the increase in the niobium amount. The self-assembly effectively stops at [PNb2W10O40]5− even if some [PNb3W9O40]6− is also detectable. These results support previous observations and explain why di- and trilacunary phosphotungstates produced only mixtures of mono- and disubstituted (Cs,K)4[PNbW11O40] and (Cs,Na)5[PNb2W10O40].
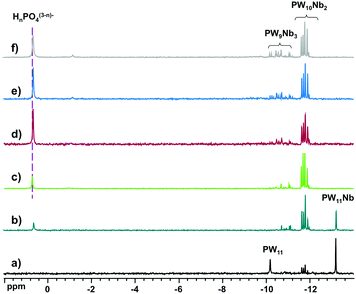 | ||
Fig. 4
31P NMR spectra of reaction mixtures of H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 at the molar ratios (a) 1 Na2WO4 at the molar ratios (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, (b) 1 11, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, (c) 1 10, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, (b) 1 9, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, (c) 1 8, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, (b) 1 7, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. 6. | ||
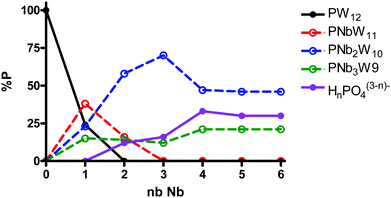 | ||
Fig. 5
31P NMR species distribution as a function of the number of Nb eq. in the reaction mixtures of H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 at the molar ratios 1 Na2WO4 at the molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12−n, where n = 0–6. 12−n, where n = 0–6. | ||
The selectivity of the formation of the monosubstituted compound from the lacunary K7[PW11O39] is superior to that in the one-pot self-assembly. The yield of [PNbW11O40]4− is 85% (based on P) with [PW11O39]7−, and drops to modest 38% in the one-pot synthesis. In order to check whether the precipitation with Cs+ alters the nature and distribution of the products, we precipitated the product of the one-pot reaction for the molar ratio H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox
Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 = 1
Na2WO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 with Cs+ and redissolved it in water. Fig. 6 shows the 31P NMR spectra before and after the precipitation–dissolution procedure. They show no significant changes after precipitation of the products as Cs+ salts. Even the isomer distribution is retained, indicating that the thermodynamic equilibrium is rapidly reached during the synthesis.
8 with Cs+ and redissolved it in water. Fig. 6 shows the 31P NMR spectra before and after the precipitation–dissolution procedure. They show no significant changes after precipitation of the products as Cs+ salts. Even the isomer distribution is retained, indicating that the thermodynamic equilibrium is rapidly reached during the synthesis.
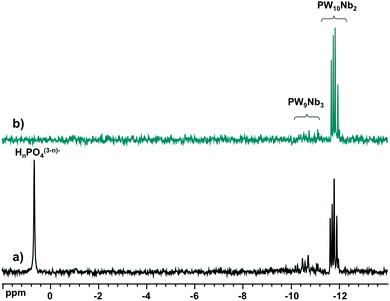 | ||
Fig. 6
31P NMR spectra of (a) aqueous reaction mixtures of H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 at a molar ratio of 1 Na2WO4 at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and (b) after precipitation with Cs+ and redissolution in H2O/D2O. 8 and (b) after precipitation with Cs+ and redissolution in H2O/D2O. | ||
Isomer distribution analysis
As already mentioned, two sets of resonances were observed, corresponding to the isomers of [PNb2W10O40]5− and [PNb3W9O40]6−. The numbers of isomers and their degeneracy for the series [PNbxW12−xO40](3+x)− have been computed with a homemade routine running in the Matlab software package (ESI†) and the results are summarized in Table 1. These calculations are based on statistical distribution resulting from random W/Nb site occupancies. There are five different isomers with a statistical distribution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for [PNb2W10O40]5−, and 13 isomers with multiplicity of 1
4 for [PNb2W10O40]5−, and 13 isomers with multiplicity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
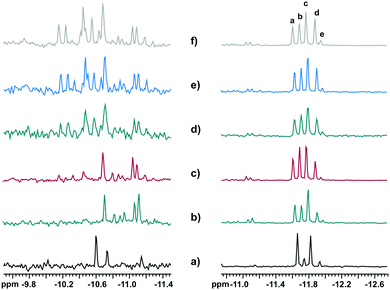
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 are possible for [PNb3W9O40]6−. We indeed observed five 31P resonances within the range −11.5 to −12.0 ppm, assigned to [PNb2W10O40]5− species, and ca. 15 signals in the range −10.0 to −11.5 ppm for [PNb3W9O40]6− (Fig. 4). Fig. 7 presents a zoom on the 31P NMR spectra in these ranges, as observed in the mixtures of H3PO4
6 are possible for [PNb3W9O40]6−. We indeed observed five 31P resonances within the range −11.5 to −12.0 ppm, assigned to [PNb2W10O40]5− species, and ca. 15 signals in the range −10.0 to −11.5 ppm for [PNb3W9O40]6− (Fig. 4). Fig. 7 presents a zoom on the 31P NMR spectra in these ranges, as observed in the mixtures of H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox
Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 at molar ratios 1
Na2WO4 at molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12−x, x = 2–6, as well as when Nb-Ox reacted with [A-PW9O34]9−, for comparison. It is possible to observe evolution in the relative signal intensity of the resonances of [PNb2W10O40]5−, labeled “a” through “e”, when the number of Nb equivalents increases (Fig. 8). There are three groups of signals: (i) the most intense, 30–40% (signal c), (ii) the less intense, 2–5% (signal e), and (iii) the three remaining evolving around the value of 20% of the total intensity. This may indicate that the statistical distribution is nearly reached in all self-assembly processes regardless of the W/Nb stoichiometry used (theoretical ratio is 9%
12−x, x = 2–6, as well as when Nb-Ox reacted with [A-PW9O34]9−, for comparison. It is possible to observe evolution in the relative signal intensity of the resonances of [PNb2W10O40]5−, labeled “a” through “e”, when the number of Nb equivalents increases (Fig. 8). There are three groups of signals: (i) the most intense, 30–40% (signal c), (ii) the less intense, 2–5% (signal e), and (iii) the three remaining evolving around the value of 20% of the total intensity. This may indicate that the statistical distribution is nearly reached in all self-assembly processes regardless of the W/Nb stoichiometry used (theoretical ratio is 9%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 × 18%
3 × 18%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36%, represented by dotted lines in Fig. 8). Interestingly enough, the distribution of the isomers in the product from Nb-Ox and the lacunary POM (Fig. 7a) is significantly different: a: 43%; b: 9%; c: 41%; d: 6%; e: 1%. This clearly indicates regioselective substitution occurring in this case.
36%, represented by dotted lines in Fig. 8). Interestingly enough, the distribution of the isomers in the product from Nb-Ox and the lacunary POM (Fig. 7a) is significantly different: a: 43%; b: 9%; c: 41%; d: 6%; e: 1%. This clearly indicates regioselective substitution occurring in this case.
| x | Number of isomers | Isomers distribution (degeneracy) |
|---|---|---|
| 0 | 1 | 1(1) |
| 1 | 1 | 1(1) |
| 2 | 5 | 1(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 × 1(2) 3 × 1(2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(4) 1(4) |
| 3 | 13 | 2 × 1(1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(2) 1(2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 × 1(3) 3 × 1(3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 × 1(6) 7 × 1(6) |
| 4 | 26 | 1(2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(3) 1(3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 × 1(4) 8 × 1(4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 × 1(8) 16 × 1(8) |
| 5 | 38 | 10 × 1(5)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 × 1(10) 28 × 1(10) |
| 6 | 47 | 2 × 1(2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 × 1(3) 2 × 1(3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 × 1(4) 2 × 1(4)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 × 1(6) 8 × 1(6)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 × 1(12) 33 × 1(12) |
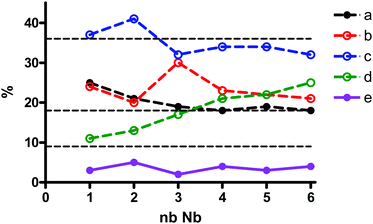 | ||
Fig. 8
31P NMR species distribution as a function of Nb equivalents introduced in the mixtures of H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb-Ox Nb-Ox![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na2WO4 at the molar ratios 1 Na2WO4 at the molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12−n, where n = 0–6. 12−n, where n = 0–6. | ||
From these distributions we can propose an assignment of the signals (Fig. 9). The most intense signal (“c”) should correspond to the most statistically abundant isomer, while the less intense signal (“e”) – to the least abundant isomer. The three remaining isomers occur at the same frequency, and one of them (signal “a”) was particularly prominent in the regioselective reaction with [PW9O34]9−. Such a degree of selectivity implies that a significant part of the starting lacunary POMs does not undergo any decomposition during the reaction. In this case the probability to find two Nb atoms in the same trimeric unit filling the vacancy of the PW9 unit becomes high, and this species should therefore correspond to signal “a”.
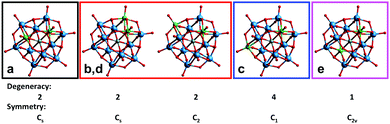 | ||
| Fig. 9 The five isomers in the mixed Keggin [PNb2W10O40]5− and possible 31P NMR assignments: signals a–e in Fig. 7. | ||
Conclusions
In this research we have developed an alternative synthetic way to niobium-substituted Keggin phosphotungstate with (NH4)[NbO(C2O4)2(H2O)2]·3H2O. In the reaction of [PW11O39]7− with this niobium oxalate selective formation of [PNbW11O40]4− is achieved. Reactions with bi- and tri-lacunary phosphotungstate or self-assembly reactions of the niobium oxalate complex, sodium tungstate and phosphoric acid give mixtures of niobium substituted Keggins, further complicated with the presence of all possible niobium positional isomers in the di- and tri-substituted products, even though the formation of [PNb2W10O40]5− is always preferable. Increasing the niobium-to-tungsten ratio above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 does not lead to the uptake of extra Nb into the Keggin backbone.
3 does not lead to the uptake of extra Nb into the Keggin backbone.
Application of this strategy to the synthesis of other mixed Nb–W polyoxometalates is in progress.
Experimental section
General information
(NH4)[NbO(C2O4)2(H2O)2]·3H2O (Nb-Ox) was prepared starting from K7H[Nb6O19]·13H2O.51 The purity of the compound was confirmed with powder X-ray diffraction by comparison of the XRD patterns with CCDC AMOXNB. The lacunary POMs Na9[A-PW9O34]·7H2O, Cs7[PW10O36]·H2O and K7[PW11O39]·17H2O were synthesized following the published protocols.52 Fourier transformed infrared (FT-IR) spectra were recorded on a 6700 FT-IR Nicolet spectrophotometer. The spectra were recorded on undiluted samples and ATR correction was applied. EDX analysis was carried out on a HITACHI TM 3000 Tabletop Microscope. TGA experiments were performed using a Seiko TG/DTA 320 thermogravimetric balance (5 °C min−1, under O2).Reactions of lacunary POMs with Nb-Ox
(a) 0.46 g (1.1 mmol) of solid Nb-Ox was added to a clear solution of 1.00 g (0.4 mmol) of Na9[A-PW9O34]·7H2O in 20 mL of distilled water. The pH value dropped down to pH 3.1 upon mixing. After complete dissolution of Nb-Ox, the mixture was kept at 80 °C for 30 min. Reaction products were then studied with 31P NMR in solution. For the solid-state NMR study, the sample was prepared by addition of 1.00 g (6 mmol) of CsCl. The crude product was collected by filtration, washed with water and dried in vacuo.(b) 0.47 g (1.2 mmol) of solid Nb-Ox was added to a suspension of 2.00 g (0.6 mmol) of Cs7[PW10O36]·H2O in 20 mL of distilled water. The pH value was adjusted to pH 3 with conc. HCl. After complete dissolution of Nb-Ox, the mixture was kept at 80 °C for 30 min. The crude product (1.80 g) was collected by filtration, washed with water and dried in vacuo.
(c) 0.12 g (0.3 mmol) of solid Nb-Ox was added to a clear solution of 2.00 g (0.6 mmol) of K7[PW11O39]·17H2O in 20 mL of distilled water. The pH value was adjusted to pH 3 with conc. HCl. After complete dissolution of Nb-Ox, the mixture was kept at 80 °C for 30 min. The reaction products were then studied with 31P and 183W NMR in solution. For the solid-state NMR study, the sample was prepared by precipitation with 2.00 g (0.012 mol) of CsCl. The crude product (1.90 g) was collected by filtration, washed with water and dried in vacuo. Cesium salt IR (diamond, cm−1): 1088 (sh), 1073 (s), 983 (vs), 882 (s), 781 (vs), 596 (m), 518 (m), 377 (m), 333 (m). TGA loss 6.2%. EDX calc. for Cs4[PNbW11O40]·12H2O Cs, P, Nb, W (%): 15.04, 0.88, 2.63, 57.23; found Cs, P, Nb, W (%) 14.82, 0.81, 2.45, 57.02. Potassium salt is more soluble and can be isolated by addition of 3 g of KCl to the final solution, it gives 0.8 g of the crude product. IR (diamond, cm−1): 1088 (sh), 1074 (s), 977 (vs), 882 (s), 781 (vs), 596 (m), 518 (m), 372 (m), 330 (m). TGA loss 4.4%. EDX calc. for K4[PNbW11O40]·7H2O K, P, Nb, W (%): 5.10, 1.01, 3.03, 65.90; found K, P, Nb, W (%) 5.09, 0.87, 3.12, 65.66.
Self-assembly reactions
All syntheses were done by dissolving a fixed amount of Na2WO42H2O in 20 mL of distilled water, followed by addition of the appropriate amounts of Nb-Ox and H3PO4 (85%). When the amount of niobium oxalate was small the pH was adjusted to 3 with 1M HCl. The reaction mixtures were thermostated for 30 min at 80 °C and then analyzed with 31P NMR. Depending on the required W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P molar ratio, the quantities are as follows:
P molar ratio, the quantities are as follows:
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :P 11
:P 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2.00 g (6 mmol) Na2WO4·2H2O, 0.22 g of Nb-Ox and 44 μL of H3PO4;
1; 2.00 g (6 mmol) Na2WO4·2H2O, 0.22 g of Nb-Ox and 44 μL of H3PO4;
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 10
P 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2.00 g (6 mmol) Na2WO4·2H2O, 0.48 g of Nb-Ox (10
1; 2.00 g (6 mmol) Na2WO4·2H2O, 0.48 g of Nb-Ox (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 44 μL of H3PO4;
2) and 44 μL of H3PO4;
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 9
P 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2.00 g (6 mmol) Na2WO4·2H2O, 0.79 g of Nb-Ox (9
1; 2.00 g (6 mmol) Na2WO4·2H2O, 0.79 g of Nb-Ox (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and 44 μL of H3PO4;
3) and 44 μL of H3PO4;
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 8
P 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2.00 g (6 mmol) Na2WO4·2H2O, 1.20 g of Nb-Ox (8
1; 2.00 g (6 mmol) Na2WO4·2H2O, 1.20 g of Nb-Ox (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and 44 μL of H3PO4;
4) and 44 μL of H3PO4;
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 7
P 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2.00g (6 mmol) Na2WO4·2H2O, 1.70 g of Nb-Ox (7
1; 2.00g (6 mmol) Na2WO4·2H2O, 1.70 g of Nb-Ox (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and 44 μL of H3PO4;
5) and 44 μL of H3PO4;
W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nb
Nb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 6
P 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 2.00 g (6 mmol) Na2WO4·2H2O, 2.40 g of Nb-Ox (6
1; 2.00 g (6 mmol) Na2WO4·2H2O, 2.40 g of Nb-Ox (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and 44 μL of H3PO4.
6) and 44 μL of H3PO4.
NMR experiments
The 31P MAS NMR experiments were performed on a Bruker Avance 500 spectrometer equipped with a 11.7 T wide-bore magnet. The 31P resonance frequency was 202.5 MHz and the experiments were performed on a 3.2 mm MAS probe with a spinning rate of 10 kHz. 31P CP/MAS NMR spectra were recorded with a contact time of 10 ms and a recycle delay of 15 s. Quantitative 31P MAS NMR spectra were also recorded using high-power proton decoupling. A total of 16 scans were accumulated with a π/2 pulse width of 1.96 μs and a 475 s recycle delay. Chemical shifts were referenced to 85% H3PO4 at 0 ppm.The solution 31P NMR (162 MHz) spectra in D2O were recorded in 5 mm outer diameter tubes on a Bruker 400 MHz spectrometer. Quantitative measurements were performed by accumulating 256 scans of π/2 pulses with a relaxation delay of 200 s. The NMR spectra were referenced to an external standard of 85% H3PO4 in H2O. The 183W NMR (20.8 MHz) spectra were recorded in 10 mm outer diameter tubes, on a Bruker Avance 500 spectrometer equipped with a Bruker low-frequency tunable probe. These spectra were referenced to an external standard (saturated Na2WO4–D2O solution) by the substitution method. Chemical shifts were reported on the δ scale with negative scale up-field from Na2WO4 (δ = 0). The 183W NMR scale was calibrated relative to the signal of D2O/H2O solution of H4SiW12O40 at −103.8 ppm with respect to the saturated Na2WO4 solution.
Acknowledgements
This work was supported by RFBR (grant number 15-33-20651), LIA FRANCE-RUSSIA No. 1144 CLUSPOM and COST STSM program (COST-STSM-ECOST-STSM-CM1203-101015-062506).Notes and references
- V. W. Day, W. G. Klemperer and C. Schwartz, J. Am. Chem. Soc., 1987, 109, 6030 CrossRef CAS.
- G. S. Kim, H. D. Zeng, D. Van Derveer and C. L. Hill, Angew. Chem., Int. Ed., 1999, 38, 3205 CrossRef CAS PubMed.
- M. K. Harrup, G. S. Kim, H. D. Zeng, R. P. Johnson, D. Van Derveer and C. L. Hill, Inorg. Chem., 1998, 37, 5550 CrossRef CAS PubMed.
- G. S. Kim, D. A. Judd, C. L. Hill and R. F. Schinazi, J. Med. Chem., 1994, 37, 816 CrossRef CAS PubMed.
- M. K. Harrup and C. L. Hill, J. Mol. Catal. A: Chem., 1996, 106, 57 CrossRef CAS.
- D. A. Judd, J. H. Nettles, N. Nevins, J. P. Snyder, D. C. Liotta, J. Tang, J. Ermolieff, R. F. Schinazi and C. L. Hill, J. Am. Chem. Soc., 2001, 123, 886 CrossRef CAS PubMed.
- A. Trovarelli and R. G. Finke, Inorg. Chem., 1993, 32, 6034 CrossRef CAS.
- R. G. Finke, K. Nomiya, C. A. Green and M. W. Droege, Inorg. Synth., 1992, 29, 239 CrossRef CAS.
- R. G. Finke and M. W. Droege, J. Am. Chem. Soc., 1984, 106, 7274 CrossRef CAS.
- F. Bannani, H. Driss, R. Thouvenot and M. Debbabi, J. Chem. Crystallogr., 2007, 37, 37 CrossRef CAS.
- T. M. Anderson, M. A. Rodriguez, T. A. Stewart, J. N. Bixler, W. Q. Xu, J. B. Parise and M. Nyman, Eur. J. Inorg. Chem., 2008, 3286 CrossRef CAS.
- K. Bouadjadja-Rohan, C. Lancelot, M. Fournier, A. Bonduelle-Skrzypczak, A. Hugon, O. Mentre and C. Lamonier, Eur. J. Inorg. Chem., 2015, 2067 CrossRef CAS.
- B. Yue, L. B. Tang, C. G. Rui, H. Z. Liu, S. L. Jin, S. S. Zhu, J. Liu and M. Q. Chen, Chem. Res. Chin. Univ., 1997, 13, 195 CAS.
- A. Stein, M. Fendorf, T. P. Jarvie, K. T. Mueller, A. J. Benesi and T. E. Mallouk, Chem. Mater., 1995, 7, 304 CrossRef CAS.
- E. V. Radkov and R. H. Beer, Inorg. Chim. Acta, 2000, 297, 191 CrossRef CAS.
- Y. H. Ren, Y. C. Hu, Y. C. Shan, Z. P. Kong, M. Gu, B. Yue and H. Y. He, Inorg. Chem. Commun., 2014, 40, 108 CrossRef CAS.
- C. C. Li, S. X. Liu, S. J. Li, Y. Yang, H. Y. Jin and F. J. Ma, Eur. J. Inorg. Chem., 2012, 3229–3234 CrossRef CAS.
- P. I. Molina, D. J. Sures, P. Miro, L. N. Zakharov and M. Nyman, Dalton Trans., 2015, 44, 15813 RSC.
- J. M. Maestre, J. P. Sarasa, C. Bo and J. M. Poblet, Inorg. Chem., 1998, 37, 3071 CrossRef CAS.
- D. D. Zhang, C. Zhang, P. T. Ma, B. S. Bassil, R. Al-Oweini, U. Kortz, J. P. Wang and J. Y. Niu, Inorg. Chem. Front., 2015, 2, 254 RSC.
- R. G. Bowmann, D. C. Molzahn and G. E. Hartwell, US Pat., US5030740 A, 1991.
- M. Dabbabi and M. Boyer, J. Inorg. Nucl. Chem., 1976, 38, 1011 CrossRef CAS.
- D. J. Sures, P. I. Molina, P. Miro, L. N. Zakharov and M. Nyman, New J. Chem., 2016, 40, 928 RSC.
- M. Dabbabi, M. Boyer, J. Pierrelaunay and Y. J. Jeannin, J. Electroanal. Chem., 1977, 76, 153 CrossRef CAS.
- W. Clegg, M. R. J. Elsegood, R. J. Errington and J. Havelock, J. Chem. Soc., Dalton Trans., 1996, 681 RSC.
- M. Nyman, Dalton Trans., 2011, 40, 8049 RSC.
- M. W. Droege and R. G. Finke, J. Mol. Catal., 1991, 69, 323 CrossRef CAS.
- N. Mizuno, H. Weiner and R. G. Finke, J. Mol. Catal. A: Chem., 1996, 114, 15 CrossRef CAS.
- J. M. Jehng and I. E. Wachs, J. Raman Spectrosc., 1991, 22, 83 CrossRef CAS.
- M. Hino, M. Kurashige, H. Matsuhashi and K. Arata, Appl. Catal., A, 2006, 310, 190 CrossRef CAS.
- S. M. Wang, L. Liu, Z. Y. Huang and Z. B. Han, RSC Adv., 2016, 6, 38782 RSC.
- X. B. Dong, D. J. Wang, K. B. Li, Y. Z. Zhen, H. M. Hu and G. L. Xue, Mater. Res. Bull., 2014, 57, 210 CrossRef CAS.
- J. Arichi, M. Eternot and B. Louis, Catal. Today, 2008, 138, 117 CrossRef CAS.
- J. E. Molinari, L. Nakka, T. Kim and I. E. Wachs, ACS Catal., 2011, 1, 1536 CrossRef CAS.
- B. C. Ma, Z. X. Zhang, W. F. Song, X. L. Xue, Y. Z. Yu, Z. S. Zhao and Y. Ding, J. Mol. Catal. A: Chem., 2013, 368, 152 CrossRef.
- O. W. Howarth, L. Pettersson and I. Andersson, J. Chem. Soc., Dalton Trans., 1991, 1799 RSC.
- Y. Xu, J. Q. Xu, C. X. Guo, G. Y. Yang, R. Z. Wang and H. R. Sun, Chem. J. Chin. Univ., 1999, 20, 14 CAS.
- Q. Lan, Z. M. Zhang, Y. G. Li, Y. Lu and E. B. Wang, Dalton Trans., 2014, 43, 16265 RSC.
- X. Lopez, C. Bo and J. M. Poblet, J. Am. Chem. Soc., 2002, 124, 12574 CrossRef CAS PubMed.
- K. Nomiya, C. Nozaki, A. Kano, T. Taguchi and K. Ohsawa, J. Organomet. Chem., 1997, 533, 153 CrossRef CAS.
- N. Satake, T. Hirano, K. Kamata, K. Suzuki, K. Yamaguchi and N. Mizuno, Chem. Lett., 2015, 44, 899 CrossRef CAS.
- D. R. Park, S. Park, Y. Bang and I. K. Song, Appl. Catal., A, 2010, 373, 201 CrossRef CAS.
- E. Cadot, V. Béreau and F. Sécheresse, Inorg. Chim. Acta, 1995, 239, 39 CrossRef CAS.
- E. V. Ramos-Fernandez, C. Pieters, B. Van der Linden, J. Juan-Alcaniz, P. Serra-Crespo, M. Verhoeven, H. Niemantsverdriet, J. Gascon and F. Kapteijn, J. Catal., 2012, 289, 42 CrossRef CAS.
- W. Salomon, C. Roch-Marchal, P. Mialane, P. Rouschmeyer, C. Serre, M. Haouas, F. Taulelle, S. Yang, L. Ruhlmann and A. Dolbecq, Chem. Commun., 2015, 51, 2972 RSC.
- K. Uehara, T. Oishi, T. Hirose and N. Mizuno, Inorg. Chem., 2013, 52, 11200 CrossRef CAS PubMed.
- W. H. Knoth and R. L. Harlow, J. Am. Chem. Soc., 1981, 103, 1865 CrossRef CAS.
- E. Radkov and R. H. Beer, Polyhedron, 1995, 14, 2139 CrossRef CAS.
- E. Radkov, Y. J. Lu and R. H. Beer, Inorg. Chem., 1996, 35, 551 CrossRef CAS.
- Z. G. Sun, Q. Liu and J. F. Liu, Transition Met. Chem., 2000, 25, 374 CrossRef CAS.
- M. Jurić, J. Popović, A. Šantić, K. Molčanov, N. Brničević and P. Planinić, Inorg. Chem., 2013, 52, 1832 CrossRef PubMed.
- P. J. Domaille, Inorg. Synth., 1992, 27, 96 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Matlab code (Topo_MH) to compute isomers in the series [PNbxW12−xO40](3+x)−. See DOI: 10.1039/c6nj02637k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |