Tuning of synthesis conditions by thermal decomposition towards gadolinium-doped manganese carbonate nanoparticles with uniform size and high relaxivity†
Ting-jian
Wang‡
a,
Kang
Liu‡
b,
Xin
Shi
b,
Ling
Ye
b,
Wei
Gu
*b and
Chang-xiang
Yan
*a
aDepartment of Neurosurgery, Sanbo Brain Hospital, Capital Medical University, Beijing 100093, P. R. China. E-mail: yancx65828@sina.com
bSchool of Chemical Biology and Pharmaceutical Sciences, Capital Medical University, Beijing 100069, P. R. China
First published on 9th November 2016
Abstract
Gd-doped MnCO3 nanoparticles have been prepared by thermal decomposition of Mn-oleate with 20 mol% Gd-oleate at 310 °C. However, MnCO3 tends to transform into MnO at a temperature above 300 °C. Developing thermal decomposition below 300 °C for the synthesis of MnCO3 nanoparticles is therefore highly desirable. Herein, we tested the feasibility of the synthesis of Gd-doped MnCO3 nanoparticles by the thermal decomposition method at a low temperature of 280–300 °C and explored the effects of several parameters on the phase and uniformity of particles. It was revealed that the Gd-doped MnCO3 nanoparticles could be obtained only when the Gd doping concentration is within a range of 20–33 mol%, while the uniformity of the resulting Gd-doped MnCO3 nanoparticles could be improved either by elevating the reaction temperature or prolonging the aging time. In addition, the formation mechanism of MnCO3 nanoparticles with Gd doping was tentatively proposed based on the information acquired by thermogravimetric-gas chromatography-mass spectrometry. Moreover, the obtained uniform-sized Gd-doped MnCO3 nanoparticles present high relaxivity and notable positive contrast enhancement towards brain glioma in mice.
1. Introduction
Magnetic resonance imaging (MRI) is a powerful diagnostic technique in medical sciences, especially for neurological diseases.1 MRI contrast agents can shorten the proton relaxation time and are used commonly in clinics to intensify the contrast for an accurate diagnosis. The ability of contrast agents to shorten the relaxation time is evaluated quantitatively by relaxivity, which is correlated positively with the contrast enhancement effect.2 Currently available MRI contrast agents for neuroimaging are gadolinium (Gd)-chelates with high relaxivities.3,4 The contrast enhancement of Gd-chelates for brain tumors, however, could be realized only as a consequence of hypervascularization or a disrupted blood–brain barrier (BBB) within and around the brain tumor region.5,6Manganese (Mn) ions contain five unpaired electrons that make them paramagnetic with a shortened relaxation time.7 Manganese oxide (e.g. MnO) and manganese carbonate (MnCO3) nanoparticles (NPs) as convertible contrast agents that release Mn2+ in an acidic environment, resulting in high relaxivities and bright contrast in tumors, have been used for liver and brain tumor MRI.8–13 Mn ions can cross an uncompromised BBB,14 which makes Mn-based NPs an attractive alternative for neuroimaging, especially for early stage and nonenhancing brain tumors that lack a disrupted BBB. Nevertheless, most reported Mn-based NPs have a relatively low relaxivity.15,16
Gd ions possess seven unpaired electrons, which allow them to decrease the relaxation time of water protons in their proximity more efficiently.17 Gd-doped MnCO3 NPs with improved relaxivity were obtained by the thermal decomposition of Mn-oleate with 20 mol% Gd-oleate at the temperature of 310 °C.18 However, it was reported that decomposition of MnCO3 occurs above 300 °C.19,20 Developing thermal decomposition below 300 °C for the synthesis of MnCO3 NPs is highly desirable. In addition, the formation mechanism of Gd-doped MnCO3 NPs and the effect of Gd doping concentrations other than 20 mol% on the composition of NPs are unclear and require further investigation.
Herein, we tested the feasibility of preparing Gd-doped MnCO3 NPs through the thermal decomposition of Mn/Gd-oleate precursors at the temperature range of 280–300 °C. Meanwhile, the effects of several key experimental parameters (e.g., the Gd doping concentration, thermal decomposition temperature and aging time) on the phase and uniformity of particles were determined. In addition, a possible formation mechanism of Gd-doped MnCO3 NPs was proposed based on the information acquired from thermogravimetric-gas chromatography-mass spectrometry (TG-GC-MS).
2. Experimental section
2.1. Materials
Manganese chloride tetrahydrate (MnCl2·4H2O) and sodium oleate were purchased from Sinopharm Chemical Reagent Co. Ltd (Beijing, China). Gadolinium chloride hexahydrate (GdCl3·6H2O) was acquired from Sigma-Aldrich (St. Louis, MO, USA). N-(Trimethoxysilylpropyl)ethylenediamine triacetic acid (TETT) silane (45% in water) was supplied by Gelest, Inc. (Tokyo, Japan). All other chemicals and reagents were of analytical grade and were used as received without further purification.2.2. Synthesis
2.3. Characterization
Transmission electron microscopy (TEM) and elemental maps were acquired on a JEM-2100F (JEOL, Tokyo, Japan) at an operating voltage of 160 kV. The NP dispersion was dripped onto a copper grid and then mounted into the vacuum drying chamber for imaging. The average size of NPs was calculated based on 200 NPs. X-ray diffraction (XRD) patterns were obtained on a PANalytical X'pert Pro MPD diffractometer using Cu Kα irradiation at 40 kV and 40 mA (PANalytical, Holland). X-ray photoelectron spectroscopy (XPS) analysis was performed on an axis ultra-spectrometer (Kratos, UK) by using mono-Al Ka line (1486.71 eV) radiation at a power of 225 W. The elemental contents of Mn and Gd were measured on an inductively coupled plasma optical emission spectrometer (ICP-OES, Varian 710-ES, USA).2.4. Thermal decomposition behavior analysis
Thermal decomposition behavior analysis of Gd-doped MnCO3 NPs was investigated using a PerkinElmer simultaneous thermogravimetric (TG) analyzer (STA8000) and gas chromatography-mass spectrometry (SQ8 GC-MS). An equivalent amount of pure Mn-oleate precursors or mixed Mn/Gd-oleate precursors was heated up from 30 °C to 350 °C at a heating rate of 5 °C min−1 in the helium atmosphere for recording thermogravimetry (TG) and differential thermogravimetry (DTG) curves, and the composition of the evolved gas phase during thermal decomposition was monitored using GC-MS, which was connected to the TG instruments with an overall scan in the mass range m/z = 1–200.2.5. Relaxivity measurement
The samples of different (Mn + Gd) concentrations (0.54, 0.27, 0.135, 0.0675 and 0.03375 mM) were prepared in distilled water. The T1 relaxation times and the corresponding T1-weighted images of NPs were acquired on a 7.0 T MRI scanner (Bruker Pharmascan, Germany) with the RARE-T1-map MRI sequence: repetition times (TR) = 235, 400, 800, 1500, 3000, and 5000 ms, echo time (TE) = 11.00 ms, field of view (FOV) = 4.0 × 4.0 cm2, flip angle (FA) = 180.0°, and slice thickness = 1 mm. The r1 relaxivity value was calculated through the linear fitting of 1/T1 (s−1) versus the (Mn + Gd) concentration (mM).2.6. Brain glioma model
All animal experiments were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication no. 85-23, revised 1985) and the protocol approved by the Ethical Committee of Capital Medical University (Beijing, China). Mice were anesthetized with an intraperitoneal administration of 6% chloral hydrate at a dose of 0.10 mL/20 g of body weight. Approximately 5 × 105 C6 glioma cells in 5 μL of serum-free DMEM were injected into the left striatum. MR imaging was performed when the tumor size reached about 1.5–1.8 mm in diameter.2.7. In vivo MR imaging of brain glioma
The glioma-bearing mice were anesthetized by applying 1.5–2 vol% isoflurane via a nose cone at 1.5 L min−1 oxygen flow. The glioma bearing mice were then injected via the tail vein at a dosage of 15 mg Mn per kg of body weight. MR T1-weighted images of the mice were acquired before and 15 minutes after the NP injection on a 7.0 T MRI scanner (Bruker Pharmascan, Germany) with the following parameters: TR = 300 ms, TE = 8.6 ms, matrix = 256 × 256, FOV = 2.5 × 2.5 cm2, and slice thickness = 0.5 mm.3. Results and discussion
3.1. Preparation of Gd-doped MnCO3 NPs at low temperature
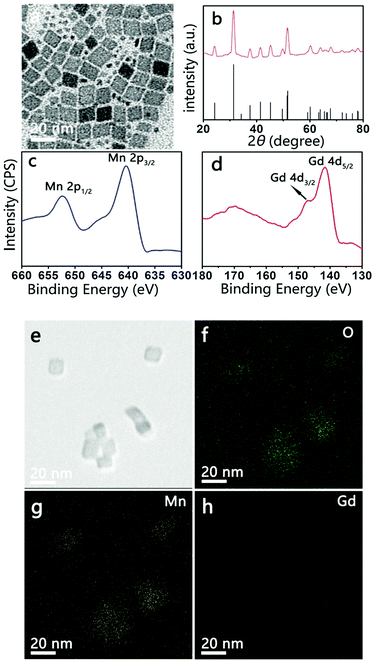
MnO NPs were synthesized by the thermal decomposition of Mn-oleate precursors in a high boiling point solvent (e.g., 1-octadecene) to ensure an accurate size control.20,21 To induce nucleation and particle growth, the Mn-oleate precursor is usually heated to above 300 °C, whereas MnCO3 tends to transform at this temperature. Therefore, to avoid this transformation, the temperature of Mn-oleate thermal decomposition with 20 mol% Gd doping was set at 280 °C for 30 min and 290 °C for 30 min. The morphologies and crystal structures of the resulting NPs were characterized by TEM and XRD. The TEM image (Fig. 1a) shows rhombohedral NPs with an average size of 12 nm accompanied by tiny irregular NPs. The corresponding XRD pattern (Fig. 1b) of these NPs matches the rhombohedral MnCO3 crystals as reported in the Joint Committee for Powder Diffraction Standards (JCPDS) standard card (44-1472), which suggests that both the large rhombohedral and tiny irregular NPs obtained are MnCO3 NPs. This consolidates the feasibility of a low-temperature thermal decomposition method for the preparation of MnCO3 NPs.The presence of Gd in the MnCO3 NPs was supported by XPS. The Mn 2p spectrum (Fig. 1c) displays two peaks at 653.4 eV and 641.1 eV, which are assigned to Mn 2p1/2 and Mn 2p3/2, respectively. These values are consistent with literature values of Mn2+ in MnCO3.22 The Gd 4d spectrum (Fig. 1d) presents two peaks at 147.2 eV and 141.4 eV, which are similar to the spectral patterns of Gd 4d3/2 and Gd 4d5/2 of Gd2O3 NPs and Gd-DTPA.23 The scanning TEM (STEM) images indicate that Gd is doped homogeneously over the entire MnCO3 NPs (Fig. 1e–h). XPS and STEM results demonstrate the successful doping of Gd into the MnCO3 NPs. The Gd doped in the MnCO3 NPs measured using ICP-OES is 17.0 mol%.
3.2. Effect of Gd doping concentration
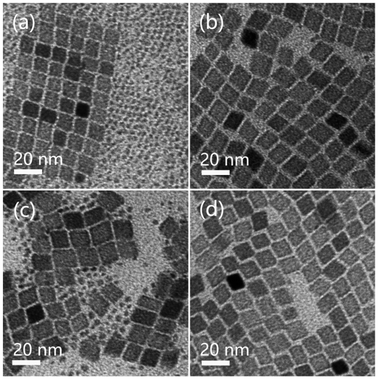
The thermal decomposition of pure Mn-oleate resulted in the formation of MnO NPs, whereas 20 mol% Gd doping resulted in the transformation of NPs into MnCO3 NPs. The relationship between the phase and Gd doping concentrations is therefore investigated. Fig. 2 presents the TEM and XRD patterns of the resulting NPs for 10, 33 and 50 mol% Gd doping, respectively. The TEM image of NPs with 10 mol% Gd doping results in cubic particles with an average size of 20 nm (Fig. 2a). When Gd doping increased to 33%, rhombohedral particles with an average size of 14 nm (Fig. 2b) and a few dots with a size of 3 nm are visible, which suggests that doping affects not only the nanocrystalline structure but also the size and shape. A TEM image of 50% Gd doping reveals rhombohedral and cubic NPs with different sizes and a considerable number of small irregular dots (Fig. 2c). Meanwhile, the crystallinity of as-synthesized NPs with different Gd doping was determined by XRD (Fig. 2d). Diffraction peaks for 10 mol% Gd doping can be indexed to cubic MnO by referring to the JCPDS standard card (01-1206). The XRD pattern for 33 mol% Gd doping matches that of the rhombohedral MnCO3 crystals (JCPDS No. 44-1472). Unlike the other two XRD patterns, which show either pure MnO or pure MnCO3, the XRD pattern for 50 mol% Gd doping shows mixed crystalline phases of MnO and MnCO3. Reflections other than MnO and MnCO3 are also visible.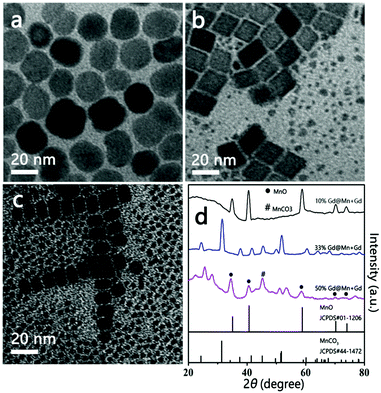 | ||
| Fig. 2 TEM images of the resulting NPs with 10 mol% (a), 33 mol% (b), and 50 mol% (c) Gd doping and their corresponding XRD patterns (d). | ||
Based on the TEM and XRD findings, it can be concluded that: (1) no MnCO3 NPs will form when the Gd doping is less than 10 mol%, (2) pure MnCO3 NPs are formed only when the Gd doping concentration is 20–33 mol% and (3) a higher Gd doping concentration (e.g. 50 mol%) results in the formation of a mixed product of MnO and MnCO3. These results indicate that Gd doping resulted in not only a complete conversion of the crystalline phase but also a gradual decrease in particle size.
3.3. Formation mechanism
The product obtained from the thermal decomposition of Mn-oleate depended critically on Gd doping. Thus, the thermal decomposition of Mn-oleate in the presence or absence of Gd-oleate was investigated using TG-GC-MS with 20 mol% Gd doping. Fig. 3a presents the TG curves of the pure Mn-oleate and mixed Mn/Gd-oleate precursors from 200 to 350 °C at 5 °C min−1. Below 250 °C, a lower mass derivative is observed in the DTG curves, with a corresponding minor mass loss of 3 wt% from the differential TG plot, which is likely because of the desorption of the bonded moisture and solvent on the NPs. A higher mass derivative appears on the DTG plots with increasing temperature, which indicates that the rapid mass loss is ascribed to the release of gaseous products formed during the thermal decomposition. TG of the pure Mn-oleate precursor reveals an increased mass loss of 4.1 wt% over that of mixed Mn/Gd-oleate precursors from 250 to 300 °C, which indicates that Gd could retain the generated gaseous products. These gaseous products during thermal decomposition were further identified using GC-MS. Fig. 3b confirms that the mass loss from 250 to 300 °C resulted primarily from CO2 (m/z = 44) and H2O (m/z = 18). Moreover, the ion current profile of mixed Mn/Gd-oleate exhibits weaker H2O and CO2 peaks compared with that of pure Mn-oleate, which validates the retention of H2O and CO2 by Gd. It is therefore proposed that these retained H2O and CO2 could convert to CO32−, react with Mn ions, and eventually lead to the formation of MnCO3. Obviously, a certain amount of Gd is required to ensure the occurrence of such a reaction.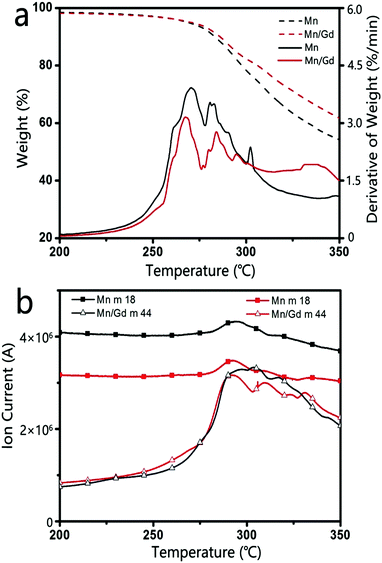 | ||
| Fig. 3 TG and DTG curves (a) of the thermal decomposition of Mn-oleate and Mn/Gd-oleate and selected MS scans of the decomposition products. Fragments: m/z = 18 (H2O), m/z = 44 (CO2) (b). | ||
3.4. Formation of uniform-sized Gd-doped MnCO3 NPs
As mentioned in Fig. 1, the Gd-doped MnCO3 NPs could be synthesized at 280 °C for 30 min and 290 °C for 30 min with 20 mol% Gd doping. However, the particles presented a distinct bimodal distribution. The size distribution of NPs influences their biological behaviors, such as stability, biodistribution, opsonization, metabolism, toxicity and clearance.24–29 Thus, the synthesis of uniform NPs is of importance. To clarify the particle growth process, we set two new thermal decomposition conditions of 280 °C for 60 min (S-1) and 280 °C for 90 min (S-2). Fig. 4a shows that S-1 has a bimodal distribution, whereas for S-2 in Fig. 4b, the smaller particles dissolve with time, accompanied by the appearance of larger particles, and the particles are rather uniform. Our results show that the growth of Gd-doped MnCO3 NPs follows an Ostwald ripening mechanism, that is, with prolonged aging time, the NPs with a smaller size shrink gradually, redissolve and in turn, help the larger particles grow. In addition to the influence of aging on particle uniformity, temperature was also investigated. We set two new reaction conditions of 290 °C for 60 min (S-3) and sequential heating at 280 °C for 30 min and 300 °C for 10 min (S-4). The XRD patterns of S-1–S-4 share the same features and match well with the rhombohedral MnCO3 crystals (JCPDS No. 44-1472). However, compared with as-synthesized MnCO3 NPs under sequential heating at 280 °C for 30 min and 290 °C for 30 min, more small irregular NPs are visible on the TEM image of S-3 (Fig. 4c), although we have increased the temperature while maintaining the total aging time. It is presumed that 280 °C plays a key role in the formation of uniform NPs because 280 °C is the temperature at which nucleation occurs. As shown in Fig. 4d, uniform Gd-doped MnCO3 NPs are obtained when the temperature is increased to 300 °C to accelerate the Ostwald ripening process in a relatively short aging time of 10 min. It is deduced that the preparation of uniform Gd-doped MnCO3 NPs can be achieved by increasing the temperature and/or prolonging the aging time. In conclusion, uniform Gd-doped MnCO3 NPs can be prepared by modulating the reaction temperature and aging time from 280 to 300 °C to help Ostwald ripening proceed to a point where smaller NPs are exhausted for the growth of large NPs.3.5. Relaxivity and in vivo MR imaging
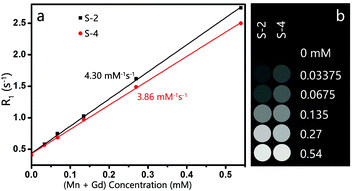
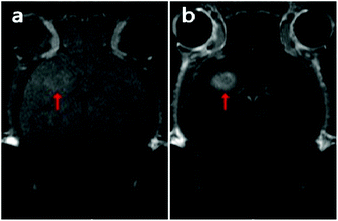
A higher relaxivity conforms to an improved enhancement effect, whereas the particle size, structure, composition, crystallinity, preparation and purification of NPs that are related closely to the reaction conditions affect the relaxivity strongly.30 After replacing the OA-ligand with TETT silane, the Gd-doped MnCO3 NPs could be readily dispersed in water. The T1 relaxation times of aqueous dispersions with different Gd + Mn concentrations were measured, and the relaxivity (r1) was calculated from the slope of the linear curve of inverse relaxation time as a function of (Mn + Gd) concentration. As plotted in Fig. 5a, S-2 presents a higher r1 value of 4.30 mM−1 s−1, compared with 3.86 mM−1 s−1 for S-4, considering that low temperature and prolonged aging time are favorable for improving the relaxivity. Nevertheless, the r1 relaxivities of both uniform-sized Gd-doped MnCO3 NPs are comparable to those of MnO NPs reported in the literature8–10,13 and Gd-chelates such as Magnevist.Furthermore, MR images of brain glioma-bearing mice were obtained pre- and post-injection of S-2 at a dosage of 15 mg Mn per kg of body weight. As shown in Fig. 6, S-2 led to a notable positive contrast enhancement in the brain glioma. This thus verifies the applicability of these uniform-sized Gd-doped MnCO3 NPs as an effective MR contrast agent for neuroimaging.
4. Conclusions
In summary, we reported the synthesis of Gd-doped MnCO3 nanoparticles by thermal decomposition at a low temperature of 280–300 °C. The introduced Gd ions could absorb CO2 and H2O that are produced during the thermal decomposition, which leads to the generation of CO32−. The CO32− formed will further react with Mn2+ to form MnCO3. A 20–33 mol% of Gd-oleate is required to ensure the occurrence of such a reaction. Uniform-sized Gd-doped MnCO3 nanoparticles could be produced by modulating the reaction temperature and aging time. The obtained uniform-sized Gd-doped MnCO3 nanoparticles present high relaxivity and notable positive contrast enhancement towards brain glioma in mice. Therefore, these uniform-sized Gd-doped MnCO3 nanoparticles hold great potential in serving as effective MR contrast agents.Acknowledgements
The authors gratefully acknowledge the financial support from the Natural Science Foundation of China (81271639), the Beijing Natural Science Foundation (7162023), and the Key Project from Beijing Commission of Education (KZ201610025022).References
- J. Wang, Y. Huang, A. E. David, B. Chertok, L. Zhang, F. Yu and V. C. Yang, Curr. Pharm. Biotechnol., 2012, 13, 2403–2416 CAS.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed.
- T. Roberts, N. Chuang and H. Roberts, Eur. J. Radiol., 2000, 34, 166–178 CrossRef CAS PubMed.
- R. Sethi, J. S. Ananta, C. Karmonik, M. Zhong, S. H. Fung, X. Liu, K. Li, M. Ferrari, L. J. Wilson and P. Decuzzi, Contrast Media Mol. Imaging, 2012, 7, 501–508 CrossRef CAS PubMed.
- G. Le Duc, S. Corde, A.-M. Charvet, H. Elleaume, R. Farion, J.-F. Le Bas and F. Estève, Invest. Radiol., 2004, 39, 385–393 CrossRef CAS PubMed.
- E. Terreno, D. D. Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019–3042 CrossRef CAS PubMed.
- M. Kueny-Stotz, A. Garofalo and D. Felder-Flesch, Eur. J. Inorg. Chem., 2012, 1987–2005 CrossRef CAS.
- C. C. Huang, N. H. Khu and C. S. Yeh, Biomaterials, 2010, 31, 4073–4078 CrossRef CAS PubMed.
- T. D. Schladt, M. I. Shukoor, K. Schneider, M. N. Tahir, F. Natalio, I. Ament, J. Becker, F. D. Jochum, S. Weber, O. Kohler, P. Theato, L. M. Schreiber, C. Sonnichsen, H. C. Schroder, W. E. Muller and W. Tremel, Angew. Chem., Int. Ed., 2010, 49, 3976–3980 CrossRef CAS PubMed.
- J. Shin, R. M. Anisur, M. K. Ko, G. H. Im, J. H. Lee and I. S. Lee, Angew. Chem., Int. Ed., 2009, 48, 321–324 CrossRef CAS PubMed.
- Y. Luo, J. Yang, J. Li, Z. Yu, G. Zhang, X. Shi and M. Shen, Colloids Surf., B, 2015, 136, 506–513 CrossRef CAS PubMed.
- R. Wang, Y. Luo, S. Yang, J. Lin, D. Gao, Y. Zhao, J. Liu, X. Shi and X. Wang, Sci. Rep., 2016, 6, 33844 CrossRef CAS PubMed.
- H. Yang, Y. Zhuang, H. Hu, X. Du, C. Zhang, X. Shi, H. Wu and S. Yang, Adv. Funct. Mater., 2010, 20, 1733–1741 CrossRef CAS.
- R. A. Yokel, NeuroMol. Med., 2009, 11, 297–310 CrossRef CAS PubMed.
- H. B. Na, J. H. Lee, K. An, Y. I. Park, M. Park, I. S. Lee, D. H. Nam, S. T. Kim, S. H. Kim and S. W. Kim, Angew. Chem., Int. Ed., 2007, 119, 5493–5497 CrossRef.
- J. Huang, J. Xie, K. Chen, L. Bu, S. Lee, Z. Cheng, X. Li and X. Chen, Chem. Commun., 2010, 46, 6684–6686 RSC.
- M. Tan and Z. R. Lu, Theranostics, 2010, 1, 83–101 Search PubMed.
- C. Shao, S. Li, W. Gu, N. Gong, J. Zhang, N. Chen, X. Shi and L. Ye, Anal. Chem., 2015, 87, 6251–6257 CrossRef CAS PubMed.
- W. Shaheen and M. Selim, J. Therm. Anal. Calorim., 2000, 59, 961–970 CrossRef CAS.
- T. D. Schladt, T. Graf and W. Tremel, Chem. Mater., 2009, 21, 3183–3190 CrossRef CAS.
- N. R. Jana, Y. Chen and X. Peng, Chem. Mater., 2004, 16, 3931–3935 CrossRef CAS.
- T. Cho, S. Park, M. Yoshio, T. Hirai and Y. Hideshima, J. Power Sources, 2005, 142, 306–312 CrossRef CAS.
- A. R. Chaudhuri, A. Fissel, V. Archakam and H. Osten, Appl. Phys. Lett., 2013, 102, 022904 CrossRef.
- H. Duan, M. Kuang, X. Wang, Y. A. Wang, H. Mao and S. Nie, J. Phys. Chem. C, 2008, 112, 8127–8131 CAS.
- B. Wang, X. He, Z. Zhang, Y. Zhao and W. Feng, Acc. Chem. Res., 2012, 46, 761–769 CrossRef PubMed.
- J.-H. Liu, S.-T. Yang, H. Wang, Y. Chang, A. Cao and Y. Liu, Nanomedicine, 2012, 7, 1801–1812 CrossRef CAS PubMed.
- T. K. Jain, M. K. Reddy, M. A. Morales, D. L. Leslie-Pelecky and V. Labhasetwar, Mol. Pharmaceutics, 2008, 5, 316–327 CrossRef CAS PubMed.
- A. Albanese, P. S. Tang and W. C. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed.
- R. Kumar, I. Roy, T. Y. Ohulchanskky, L. A. Vathy, E. J. Bergey, M. Sajjad and P. N. Prasad, ACS Nano, 2010, 4, 699–708 CrossRef CAS PubMed.
- S. C. Jackels, M. M. Durham, J. E. Newton and T. C. Henninger, Inorg. Chem., 1992, 31, 234–239 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nj02739c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |