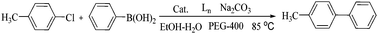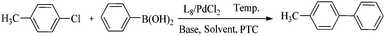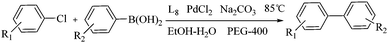Efficient symmetrical bidentate dioxime ligand-accelerated homogeneous palladium-catalyzed Suzuki–Miyaura coupling reactions of aryl chlorides†
Jinyi
Song
ab,
Hongyan
Zhao
ab,
Yang
Liu
ab,
Huatao
Han
ab,
Zhuofei
Li
ab,
Wenyi
Chu
*ab and
Zhizhong
Sun
*ab
aSchool of Chemistry and Materials Science, Heilongjiang University, Harbin 150080, P. R. China. E-mail: wenyichu@hlju.edu.cn; Fax: +86-451-86609135; Tel: +86-451-86609135
bKey Laboratory of Chemical Engineering Process & Technology for High-efficiency Conversion, College of Heilongjiang Province, Harbin 150080, P. R. China
First published on 12th December 2016
Abstract
A series of N,O-bidentate ligands were synthesized using the Vilsmeier–Haack reaction and oximation. 2,5-Dihydroxyterephthalaldehyde dioxime (L8) as an efficient N,O-symmetrical bidentate ligand was prepared from hydroquinone. It was studied as a high activity ligand for palladium-catalyzed Suzuki–Miyaura cross-coupling reactions of aryl chlorides with arylboronic acids under mild conditions. The coupling reactions were performed in the presence of PdCl2 as the catalyst, L8 as the ligand, Na2CO3 as the base, PEG-400 as the PTC and in ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an environmentally benign solvent at 85 °C. Plentiful biaryls were obtained by the optimized reaction with good yields at a low palladium loading of 0.20 mol%.
1) as an environmentally benign solvent at 85 °C. Plentiful biaryls were obtained by the optimized reaction with good yields at a low palladium loading of 0.20 mol%.
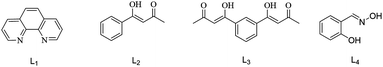
The palladium-catalyzed Suzuki–Miyaura reaction is one of the most widely used ways to synthesize biaryls,1–3 which are structural components of many natural products, materials and pharmaceutical chemicals.4–6 Aryl bromides and iodides are constantly used in Suzuki reactions, while aryl chlorides are rarely used despite their low cost and greater usability, because the high energy of the Caryl–Cl bond leads to reduced reaction activity.7,8 Therefore, it is necessary to develop an efficient catalytic system to get aryl chlorides involved in the reaction. Generally, the way to improve the efficiency of coupling reactions is by the employment of ancillary ligands.9–11 Phosphine ligands12–14 are commonly used in catalytic processes because of their high activity in cyclometalated transformations. However, the clear drawbacks of phosphines are the preparative difficulties, potential toxicity, instability etc. From early literature reports, some bidentate ligands could assist coupling reactions to give excellent yields. Therefore, our focus will be on bidentate ligands instead of phosphines. N,N-Bidentate ligands such as 1,10-phenanthroline,15,16 ethylenediamine,17,18 2,2′-bipyridine19,20etc. and O,O-bidentate ligands like dicarboxylic acids,21,22 β-diketones,23,24 pyrocatechol25etc. have attracted considerable attention as competent ligands for coupling reactions (see Scheme 1). Furthermore, N,O-bidentate ligands like salicylaldoximes26,27 are also frequently used to enhance catalytic activity. In our research, by comparing N,N-bidentate ligands with O,O-bidentate ligands, we found that the latter showed higher activity in our catalytic reaction. Notably, it was found that the catalytic activity of O,O-symmetrical bidentate ligands was significantly higher than for asymmetric ligands.28 Therefore, N,O-symmetrical bidentate ligands should be effective ligands in the Suzuki–Miyaura reaction.
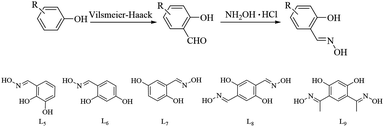
Herein, we designed and synthesized a variety of salicylaldoxime derivative ligands mainly via the Vilsmeier–Haack reaction29 and oximation (see Scheme 2). Among the synthesized ligands, L8, as a novel ligand, demonstrated the highest activity due to its electron-rich character and the existence of multiple bonding sites which are considered to increase the steric congestion around a metal centre. Moreover, the synthetic method for L8 was relatively more mild and economical than that for L9. Compared with phosphine ligands which are sensitive to water and air, L8 as an oxime derivative was described as being stable and showed high efficiency in reaction processes. It is noteworthy to mention that when the reaction was catalyzed by PdCl2 it was heterogeneous with low to moderate yields and reaction efficiency, while when using the novel ligand these issues could be avoided. We report the Suzuki–Miyaura coupling reaction of aryl chlorides with arylboronic acids over a neotype homogeneous catalytic system based upon L8 coordinated to PdCl2, making the method more attractive than previous methods.
The dioxime L8, as an efficient symmetrical bidentate ligand, was acquired in moderate yield by the formylation of hydroquinone using Vilsmeier–Haack reagent and oximation (Scheme 2).
The complex structure of L8 and PdCl2 was confirmed by elemental analysis, FT-IR, 1H and 13C NMR spectroscopy and mass spectral data. For the relevant data associated with the other synthesized salicylaldoxime derivative ligands and complexes, see the ESI†. The elemental analyses and the molecular ion peaks of the complexes were consistent with the proposed formulation. From the FT-IR data of the complex of L8, it can be seen that the νO–H band appears at 3451 cm−1, which is higher than that of the ligand (3337 cm−1) due to chemical changes. Compared with L8 (1651 cm−1), the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band appears at 1624 cm−1 owing to the donation of electrons from the nitrogen atom into the empty orbitals of the metal, resulting in a red shift. The Ar–C
N band appears at 1624 cm−1 owing to the donation of electrons from the nitrogen atom into the empty orbitals of the metal, resulting in a red shift. The Ar–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N signal in the 1H NMR spectrum appears at δ 8.41 ppm, and so compared to L8 (δ 8.24 ppm) a downfield shift of 0.17 ppm was observed. Meanwhile, an Ar–OH signal had disappeared, supporting the deprotonation of an OH group of the ligand, and at the same time the protonation of a nitrogen in an N–OH group. A similar downfield shift was also observed in the 13C NMR spectrum of the complex. It was confirmed that the N and O atoms mutually coordinated with PdCl2. The catalytic activities of the synthesized ligands and PdCl2 were verified using the Suzuki–Miyaura cross-coupling reaction. Initially, the reaction of 4-chlorotoluene with phenylboronic acid was selected as the model to evaluate the ligands and catalysts. The results of the experiments are summarized in Table 1.
N signal in the 1H NMR spectrum appears at δ 8.41 ppm, and so compared to L8 (δ 8.24 ppm) a downfield shift of 0.17 ppm was observed. Meanwhile, an Ar–OH signal had disappeared, supporting the deprotonation of an OH group of the ligand, and at the same time the protonation of a nitrogen in an N–OH group. A similar downfield shift was also observed in the 13C NMR spectrum of the complex. It was confirmed that the N and O atoms mutually coordinated with PdCl2. The catalytic activities of the synthesized ligands and PdCl2 were verified using the Suzuki–Miyaura cross-coupling reaction. Initially, the reaction of 4-chlorotoluene with phenylboronic acid was selected as the model to evaluate the ligands and catalysts. The results of the experiments are summarized in Table 1.
| Entry | Catalyst (quantity [mol%]) | Ligand (quantity [mol%]) | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 4-chlorotoluene (1.0 mmol, 1.0 equiv.), phenylboronic acid (1.2 equiv.), Na2CO3 (2.0 equiv.), ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 1 water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (8 mL), PEG-400 (0.01 equiv.), 85 °C, 5.0 h.
b Isolated yield. 1 (8 mL), PEG-400 (0.01 equiv.), 85 °C, 5.0 h.
b Isolated yield.
|
|||
| 1 | PdCl2 (0.20) | — | 20 |
| 2 | PdCl2 (0.20) | L1 (2.0) | 22 |
| 3 | PdCl2 (0.20) | L2 (2.0) | 30 |
| 4 | PdCl2 (0.20) | L3 (2.0) | 43 |
| 5 | PdCl2 (0.20) | L4 (2.0) | 45 |
| 6 | PdCl2 (0.20) | L5 (2.0) | 51 |
| 7 | PdCl2 (0.20) | L6 (2.0) | 46 |
| 8 | PdCl2 (0.20) | L7 (2.0) | 50 |
| 9 | PdCl2 (0.20) | L8 (2.0) | 80 |
| 10 | PdCl2 (0.20) | L9 (2.0) | 75 |
| 11 | PdCl2 (0.20) | L8 (1.0) | 55 |
| 12 | PdCl2 (0.20) | L8 (4.0) | 61 |
| 13 | Pd(OAc)2 (0.10) | — | 16 |
| 14 | Pd(OAc)2 (0.10) | L8 (2.0) | 43 |
| 15 | Pd(OAc)2 (0.20) | — | 33 |
| 16 | Pd(OAc)2 (0.20) | L8 (2.0) | 81 |
| 17 | Pd(OAc)2 (0.40) | — | 45 |
| 18 | Pd(OAc)2 (0.40) | L8 (2.0) | 80 |
| 19 | PdCl2 (0.05) | L8 (2.0) | 28 |
| 20 | PdCl2 (0.10) | L8 (2.0) | 48 |
| 21 | PdCl2 (0.40) | L8 (2.0) | 80 |
| 22 | PdCl2 (0.50) | L8 (2.0) | 79 |
| 23 | — | L8 (2.0) | 0 |
The experiments indicated that the yield of the reaction was very low without a ligand (Table 1, entry 1). Phenanthroline (L1) as an N,N-bidentate ligand showed some activity, resulting in a 22% yield (Table 1, entry 2). It could be seen that the catalytic effect of phenanthroline on the reaction was far less than that of the O,O-bidentate ligands L2 and L3, while when L3 was the O,O-symmetrical bidentate ligand it showed better activity than the O,O-asymmetrical bidentate ligand L2 (Table 1, entries 3 and 4). Salicylaldoxime (L4) as an N,O-bidentate ligand showed similar activity to L3 (Table 1, entries 4 and 5). When comparing similar N,O-asymmetrical bidentate ligands (L5, L6 and L7), the yields exhibited small fluctuations (Table 1, entries 6–8). The addition of N,O-symmetrical bidentate ligands L8 and L9 significantly increased the yield of the reaction to 80% and 75% (Table 1, entries 9 and 10). The results proved that the N,O-symmetrical bidentate dioxime L8 is a valid ligand for the coupling reaction. Then, the amount of L8 was selected; the data indicated that 2.0 mol% (compared with the molar quantity of 4-chlorotoluene) was the best (Table 1, entries 9, 11 and 12). Use of L8 in excessive amounts can cause an insufficient amount of the ligand to form the Pd(0) complex, as excess oxime could lead to the formation of oxime-ethers, as is seen in similar reaction systems.30 Moreover, Pd(II) is propitious to be reduced to Pd(0) in the presence of oximes.
The catalysts of coupling reactions are principally Pd-based. Comparing Pd salts in the same quantities (Table 1, entries 1 and 15), PdCl2 catalyzed the reaction without a ligand to give only a 20% yield, a catalytic effect in arrears of Pd(OAc)2 in the absence of a ligand. However, the addition of L8 to PdCl2 catalyzed the reaction, and the yield could reach up to 80% (Table 1, entry 9) which far surpassed the result of Pd(OAc)2. In the presence of L8 and Pd(OAc)2, the highest yield that could be reached was 81%, which was close to that achieved by PdCl2 when combined with L8 (Table 1, entries 14, 16 and 18). We preferred to use PdCl2 as the catalyst as it is relatively inexpensive which makes the reaction more cost-effective. From the catalyst quantity data (Table 1, entries 9 and 19–22), the preliminary results showed that a 0.20 mol% palladium loading favored the reaction. Decreasing the amount of the catalyst was accompanied by a drop in the yield (Table 1, entries 9, 19 and 20), while continuing to increase the amount of the catalyst had no significant effect on the yield of the reaction (Table 1, entries 9, 21 and 22). The reaction without a catalyst did not occur at all (Table 1, entry 23).
The other reaction conditions including the base, solvent and temperature were selected using the data in Table 2. The solvent has previously been exhibited to be critical for the reaction. Aprotic and protic solvents were selected. When using protic solvents as the reaction medium (Table 2, entries 1–3), a 56% yield was obtained for the coupling reaction in EtOH. When comparing the use of ethanol and water in different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), yields of 80%, 72% and 75% were achieved, respectively (Table 2, entries 4–6). When using aprotic solvents as the reaction medium (Table 2, entries 7–11), DMF was useful for the reaction, giving an 82% yield, but the disadvantage was the high temperatures required. Above all, ethanol and water (1
1), yields of 80%, 72% and 75% were achieved, respectively (Table 2, entries 4–6). When using aprotic solvents as the reaction medium (Table 2, entries 7–11), DMF was useful for the reaction, giving an 82% yield, but the disadvantage was the high temperatures required. Above all, ethanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was the most suitable solvent system for the reaction. The base also plays a vital role in the reaction. When organic bases were selected including Et3N and pyridine (Table 2, entries 12 and 13), the results showed that the reaction progressed in poor yield.
1) was the most suitable solvent system for the reaction. The base also plays a vital role in the reaction. When organic bases were selected including Et3N and pyridine (Table 2, entries 12 and 13), the results showed that the reaction progressed in poor yield.
| Entry | Solvent (V/V) | Base | PTC | T/°C | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-chlorotoluene (1.0 mmol, 1.0 equiv.), phenylboronic acid (1.2 equiv.), PdCl2 (0.20 mol%), L8 (2.0 mol%), base (2.0 equiv.), solvent (8 mL), PTC (0.01 equiv.), 5.0 h. b Isolated yield. | |||||
| 1 | Glycerin | Na2CO3 | PEG-400 | 85 | 31 |
| 2 | EtOH | Na2CO3 | PEG-400 | 85 | 56 |
| 3 | H2O | Na2CO3 | PEG-400 | 85 | 20 |
| 4 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Na2CO3 | PEG-400 | 85 | 80 |
| 5 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | PEG-400 | 85 | 72 |
| 6 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 2 H2O = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Na2CO3 | PEG-400 | 85 | 75 |
| 7 | DMF | Na2CO3 | PEG-400 | 115 | 82 |
| 8 | DMSO | Na2CO3 | PEG-400 | 85 | 42 |
| 9 | THF | Na2CO3 | PEG-400 | 85 | 30 |
| 10 | Toluene | Na2CO3 | PEG-400 | 85 | 43 |
| 11 | Dioxane | Na2CO3 | PEG-400 | 85 | 31 |
| 12 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Et3N | PEG-400 | 85 | 25 |
| 13 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Pyridine | PEG-400 | 85 | 20 |
| 14 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K3PO4 | PEG-400 | 85 | 47 |
| 15 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NaHCO3 | PEG-400 | 85 | 38 |
| 16 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NaOH | PEG-400 | 85 | 59 |
| 17 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
K2CO3 | PEG-400 | 85 | 61 |
| 18 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Na2CO3 | — | 85 | 55 |
| 19 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1 H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Na2CO3 | PEG-400 | 75 | 68 |
Conversely, inorganic bases were found to be more helpful in the reaction. In particular, the use of Na2CO3 improved the reaction process to give the highest yield in ethanol/water solvent (80%, Table 2, entry 4). When experiments were carried out in the presence of PEG-400 as a phase transfer catalyst (PTC) the yield was significantly increased to 80%. When no phase transfer catalyst was present, the yield only reached 55% (Table 2, entries 4 and 18). PEG-400 likely acts as a stabilizer for some low ligated palladium species involved in the catalysis, thereby improving the yields of the coupling products. The temperatures selected were 75 °C and 85 °C (reflux temperature) and gave isolated yields of 68% and 80% (Table 2, entries 19 and 4). Therefore, the elevated temperature could accelerate the rate of the reaction, so 85 °C was a reasonable choice.
With the optimum reaction system conditions, we expanded the substrate scope to a variety of aryl chlorides and arylboronic acids varying their electronic and steric characteristic in the Suzuki–Miyaura reaction, these are compiled in Table 3. As shown in Table 3, the effects of the aryl chlorides were firstly investigated. Aryl chlorides with either electron-withdrawing or electron-donating groups could perform the reaction under the optimum conditions. Moreover, the yields correlated with the properties and positions of the substituents. Chlorobenzene was used as the substrate to generate biphenyl in 90% yield within 0.5 h (Table 3, entry 1). para-Substituted aryl chlorides with electron-withdrawing groups such as nitro, formyl, and cyano groups (Table 3, entries 2–4) brought about overall superior yields of 88%, 85% and 83% within 3 hours, respectively.
| Entry | R1 | R2 | Time (h) | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: aryl chloride (1.0 mmol, 1.0 equiv.), arylboronic acid (1.2 equiv.), PdCl2 (0.20 mol%), L8 (2.0 mol%), PEG-400 (0.01 equiv.), Na2CO3 (2.0 equiv.), ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 1 water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (8 mL). The progress of the reactions was monitored by TLC.
b Isolated yield.
c Reaction conditions: aryl chloride (1.0 mmol, 1.0 equiv.), arylboronic acid (2.4 equiv.), PdCl2 (0.20 mol%), L8 (2.0 mol%), PEG-400 (0.01 equiv.), Na2CO3 (4.0 equiv.), ethanol 1 (8 mL). The progress of the reactions was monitored by TLC.
b Isolated yield.
c Reaction conditions: aryl chloride (1.0 mmol, 1.0 equiv.), arylboronic acid (2.4 equiv.), PdCl2 (0.20 mol%), L8 (2.0 mol%), PEG-400 (0.01 equiv.), Na2CO3 (4.0 equiv.), ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 1 water = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (8 mL). 1 (8 mL).
|
||||
| 1 | H | H | 0.5 | 90 |
| 2 | 4-NO2 | H | 3.0 | 88 |
| 3 | 4-CHO | H | 3.0 | 85 |
| 4 | 4-CN | H | 3.0 | 83 |
| 5 | 4-Me | H | 5.0 | 80 |
| 6 | 4-OMe | H | 5.5 | 78 |
| 7 | 3-OMe | H | 7.0 | 65 |
| 8 | 2-OMe | H | 8.0 | 60 |
| 9 | 2-NO2 | H | 8.0 | 62 |
| 10 | 3,4-OMe | H | 8.0 | 70 |
| 11 | 3,4-OBn | H | 10.0 | 75 |
| 12 | 4-Me | 4-CF3 | 8.0 | 70 |
| 13 | 4-OMe | 4-CF3 | 8.0 | 72 |
| 14 | 4-NO2 | 4-CF3 | 5.0 | 78 |
| 15 | 4-CHO | 4-CF3 | 5.0 | 76 |
| 16 | 4-CN | 4-CF3 | 5.0 | 73 |
| 17 | 4-OMe | 4-Me | 5.0 | 74 |
| 18 | 4-CN | 4-OMe | 5.0 | 75 |
| 19 | 3,4-OBn | 4-OMe | 10.0 | 70 |
| 20 | 3,4-OBn | 4-Phenyl | 9.5 | 77 |
| 21 | 2-NO2 | 3,5-F | 7.0 | 65 |
| 22 | 4-NO2 | 2,5-Me | 5.0 | 71 |
| 23 | 4-Me | 3,4-OMe | 7.0 | 70 |
| 24 | H | 2-Me | 6.0 | 68 |
| 25 | 4-OMe | 2-Me | 6.5 | 65 |
| 26 | 4-CHO | 2-Me | 6.0 | 67 |
| 27 | 4-NO2 | 2-Me | 5.5 | 68 |
| 28 | 2-NO2 | 2-Me | 6.5 | 64 |
| 29 | H | 1-Naphthalene | 8.0 | 78 |
| 30 | 4-OMe | 1-Naphthalene | 8.5 | 77 |
| 31 | 4-CHO | 1-Naphthalene | 8.0 | 79 |
| 32 | H | 2-Naphthalene | 8.0 | 76 |
| 33 | 3,4-OBn | 2-Naphthalene | 10.0 | 75 |
| 34 | 2-OBn, 4-OMe | 2-Naphthalene | 9.0 | 74 |
| 35c | 2,6-Cl pyridine | H | 6.0 | 70 |
When the substituents were electron-donating groups like methyl and methoxy groups (Table 3, entries 5 and 6), the reaction also proceeded easily in approximately 80% yield though over 5 hours. The reason for this is that an electron-withdrawing group is conducive to an oxidative addition reaction, which is the rate-determining step in the Suzuki–Miyaura reaction. Apart from the electronic features, the impact of steric hindrance was also investigated (Table 3, entries 2 and 6–9). ortho-Substituted chlorides were compared with meta- and para-substituted chlorides, and the former gave a 60% yield (Table 3, entry 8). When the aryl chlorides had multiple substituents (Table 3, entries 10 and 11), the reaction yield exceeded 70% over 8 hours.
For arylboronic acids with electron-withdrawing or electron-donating groups, such as trifluoromethyl, methoxy and phenyl groups (Table 3, entries 12–20), the reactions could afford the corresponding biaryls in moderate to good yields. Phenylboronic acid could give better yields than substituted phenylboronic acids (Table 3, entries 2–6 and 12–16). Likewise, when the arylboronic acids had multiple substituents (Table 3, entries 21–23), the reactions also occurred. For instance, from the reaction with 2,5-dimethyl phenylboronic acid the product was isolated in 71% yield after 5 hours (Table 3, entry 22). Encouraged by the successful results of the reaction of the aryl chlorides with phenylboronic acid, further study was conducted with some sterically hindered arylboronic acids. Under the L8/PdCl2 catalytic system, a higher yield was obtained for p-tolylboronic acid relative to o-tolylboronic acid (65%, Table 3, entry 25), and aryl chlorides with both electron donating and electron withdrawing groups furnished the product in moderate to good yields (Table 3, entries 25–27). It is noteworthy to mention that 2-nitrochlorobenzene coupled with o-tolylboronic acid in 64% yield (Table 3, entry 28). The steric hindrance of the arylboronic acid had little effect on the reaction. 1-Naphthyl and 2-naphthyl boronic acids also participated with excellent reactivity (Table 3, entries 29–34). 4-Bromoanisole coupled with 1-naphthalene boronic acid in almost 77% yield. Similarly, an electron-poor aryl chloride also provided the intended product with 1-naphthalene boronic acid in high yields. 2-Naphthalene boronic acid was also investigated and gave the relative products in moderate yields (Table 3, entries 33 and 34). It should be emphasized that 2,6-dichloropyridine as an aryl chloride demonstrated compatibility as a heteroaromatic aryl chloride and a multiply chloro substituted aromatic in the coupling reaction process (Table 3, entry 35). To our great delight, five novel biaryl compounds were synthesized with good yields using the catalyst system (Table 3, entries 19–21, 33 and 34).
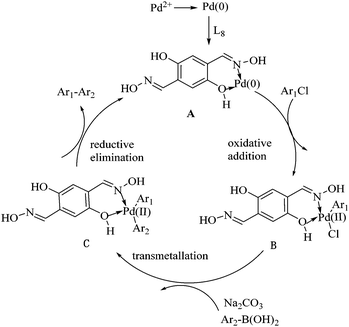
Based on literature reports,11,31,32 we propose that the mechanism of the Suzuki–Miyaura reaction could be shown as in Scheme 3. Initially, Pd(0) is formed by the reduction of Pd(II) in the Suzuki reaction system, Pd(0) is then stabilized by the ligand L8 and the complex A as the effective catalyst is formed. Then an oxidative addition occurs between the complex A and the aryl chloride to produce the intermediate B, which undergoes transmetallation with the arylboronic acid to afford the intermediate C in the presence of Na2CO3. Finally, a reductive elimination provides the corresponding biaryl products and the regeneration of the complex A, thereby resuming the catalytic cycle.
In conclusion, a series of salicylaldoxime derivative ligands were synthesized using a straightforward two-step procedure for the catalyzed Suzuki–Miyaura cross-coupling reaction of electron-rich, electron-poor, and sterically hindered aryl chlorides with arylboronic acids under alcohol–water conditions. L8 facilitated the coupling reaction, not only as an efficient ligand for palladium coordination, but also to provide a homogeneous system to promote the integrality of the reaction. Moreover, the reaction can be performed in good yields at a low palladium loading of 0.20 mol%. Furthermore, five novel biaryls were synthesized under the catalyst system. Extension of the application of the synthesized ligands to other reactions is still underway.
Experimental
In a 20 mL reaction flask, an aryl halide (1.0 mmol, 1.0 equiv.), an aryl boronic acid (1.2 equiv.), Na2CO3 (2.0 equiv.), PEG-400 (0.01 equiv.), the ligand (2.0 mol%) and PdCl2 (0.20 mol%) were charged and dissolved in 8 mL of ethanol aqueous solution (Vethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vwater = 1
Vwater = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reaction mixture was stirred at 85 °C and monitored by TLC. At the end of the reaction, the reaction mixture was poured into water and the aqueous layer was extracted with ethyl acetate 3 times (3 × 10 mL), and the organic extracts were then dried over anhydrous sodium sulfate. After filtration and removal of the solvent, the residue was purified by column chromatography to give the biaryl products. The purity of the products matched with authentic samples.
1). The reaction mixture was stirred at 85 °C and monitored by TLC. At the end of the reaction, the reaction mixture was poured into water and the aqueous layer was extracted with ethyl acetate 3 times (3 × 10 mL), and the organic extracts were then dried over anhydrous sodium sulfate. After filtration and removal of the solvent, the residue was purified by column chromatography to give the biaryl products. The purity of the products matched with authentic samples.
This work was supported by a fund from the Natural Science Foundation of Heilongjiang Province of China (No. B201208).
References
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- T. Y. Luh, M. K. Leung and K. T. Wong, Chem. Rev., 2000, 100, 3187–3204 CrossRef CAS PubMed.
- A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722 CrossRef CAS PubMed.
- S. R. Chemler, D. Trauner and S. J. Danishefsky, Angew. Chem., Int. Ed., 2001, 40, 4544 CrossRef CAS PubMed.
- S. Paul and J. H. Clark, Green Chem., 2003, 5, 635 RSC.
- A. F. Littke, C. Y. Dai and G. C. Fu, J. Am. Chem. Soc., 2000, 122, 4020–4028 CrossRef CAS.
- A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS.
- J. K. Wang, Y. X. Zong, X. C. Wang, Y. L. Hu, G. R. Yue and Y. Pan, Green Chem., 2016, 18, 967–973 RSC.
- J. J. Ning, J. F. Wang, Z. G. Ren, D. J. Young and J. P. Lang, Tetrahedron, 2015, 71, 4000–4006 CrossRef CAS.
- D. X. Liu, W. J. Gong, H. X. Li, J. Gao, F. L. Li and J. P. Lang, Tetrahedron, 2014, 70, 3385–3389 CrossRef CAS.
- Q. Li, L. M. Zhang, J. J. Bao, H. X. Li, J. B. Xie and J. P. Lang, Appl. Organomet. Chem., 2014, 28, 861–867 CrossRef CAS.
- D. W. Old, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 9722–9723 CrossRef CAS.
- M. G. Andreu, A. Zapf and M. Beller, Chem. Commun., 2000, 2475–2476 RSC.
- R. Ghost, N. N. Adarsh and A. Sarkar, Eur. J. Org. Chem., 2010, 5320–5322 Search PubMed.
- M. Rakibuddin, S. Gazi and R. Ananthakrishnan, Catal. Commun., 2015, 58, 53–58 CrossRef CAS.
- Y. Q. Lu, X. L. Wang, M. Wang, L. Q. Kong and J. S. Zhao, Electrochim. Acta, 2015, 180, 86–95 CrossRef CAS.
- J. C. Antilla, J. M. Baskin, T. E. Barder and S. L. Buchwald, J. Org. Chem., 2004, 69, 5578–5587 CrossRef CAS PubMed.
- M. R. Rivero and S. L. Buchwald, Org. Lett., 2007, 9, 973–976 CrossRef CAS PubMed.
- C. Nájera, J. G. Moltó, S. Karlstrëm and L. R. Falvello, Org. Lett., 2003, 5, 1451–1454 CrossRef PubMed.
- R. J. Sullivan, J. Kim, C. Hoyt, L. A. Silks and M. Schlaf, Polyhedron, 2016, 108, 104–114 CrossRef CAS.
- H. Y. Liu, H. L. Liu, R. X. Li and H. Chen, Tetrahedron Lett., 2014, 55, 415–418 CrossRef CAS.
- F. A. Cruz, Z. W. Chen, S. I. Kurtoic and V. M. Dong, Chem. Commun., 2016, 52, 5836–5839 RSC.
- V. R. Akhmetova, N. S. Akhmadiev, Z. A. Starikova, A. R. Tulyabaev, E. S. Mescheryakova and A. G. Ibragimov, Tetrahedron, 2015, 71, 7722–7728 CrossRef CAS.
- Z. Ma, M. Sutradhar, A. V. Gurbanov, A. M. Gurbanov, A. M. Maharramov, R. A. Aliyeva, F. S. Aliyeva, F. N. Bahmanova, V. I. Mardanova, F. M. Chyragov and K. T. Mahmnudov, Polyhedron, 2015, 101, 14–22 CrossRef CAS.
- M. Albrecht, M. Schneider and H. Röttele, Angew. Chem., Int. Ed., 1999, 38, 557–559 CrossRef CAS.
- Y. L. Wang, J. Luo and Z. L. Liu, Appl. Organomet. Chem., 2013, 27, 601–605 CAS.
- S. Akine, T. Taniguchi, W. K. Dong, S. Masubuchi and T. Nabeshima, J. Org. Chem., 2005, 70, 1704–1711 CrossRef CAS PubMed.
- M. Wang, X. B. Yuan, H. Y. Li, L. M. Ren, Z. Z. Sun, Y. J. Hou and W. Y. Chu, Catal. Commun., 2015, 58, 154–157 CrossRef CAS.
- I. M. Downie, M. J. Earle, H. Heaney and K. F. Shuhaibar, Tetrahedron, 1993, 49, 4015–4034 CrossRef CAS.
- P. De, K. Pandurangan, U. Maitra and S. Wailes, Org. Lett., 2007, 9, 2767–2770 CrossRef CAS PubMed.
- J. P. Simeone and J. R. Sowa, Tetrahedron, 2007, 63, 12646–12654 CrossRef CAS.
- W. B. Yang, C. Liu and J. S. Qiu, Chem. Commun., 2010, 46, 2661 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nj02815b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |