Rationally encapsulated gold nanorods improving both linear and nonlinear photoacoustic imaging contrast in vivo†
Fei
Gao‡
a,
Linyi
Bai‡
b,
Siyu
Liu
a,
Ruochong
Zhang
a,
Jingtao
Zhang
b,
Xiaohua
Feng
a,
Yuanjin
Zheng
*a and
Yanli
Zhao
*bc
aSchool of Electrical and Electronic Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798. E-mail: yjzheng@ntu.edu.sg
bDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371. E-mail: zhaoyanli@ntu.edu.sg
cSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798
First published on 18th November 2016
Abstract
Photoacoustic tomography has emerged as a promising non-invasive imaging technique that integrates the merits of high optical contrast with high ultrasound resolution in deep scattering medium. Unfortunately, the blood background in vivo seriously impedes the quality of imaging due to its comparable optical absorption with contrast agents, especially in conventional linear photoacoustic imaging modality. In this study, we demonstrated that two hybrids consisting of gold nanorods (Au NRs) and zinc tetra(4-pyridyl)porphyrin (ZnTPP) exhibited a synergetic effect in improving optical absorption, conversion efficiency from light to heat, and thermoelastic expansion, leading to a notable enhancement in both linear (four times greater) and nonlinear (more than six times) photoacoustic signals as compared with conventional Au NRs. Subsequently, we carefully investigated the interesting factors that may influence photoacoustic signal amplification, suggesting that the coating of ZnTPP on Au NRs could result in the reduction of gold interfacial thermal conductance with a solvent, so that the heat is more confined within the nanoparticle clusters for a significant enhancement of local temperature. Hence, both the linear and nonlinear photoacoustic signals are enhanced on account of better thermal confinement. The present work not only shows that ZnTPP coated Au NRs could serve as excellent photoacoustic nanoamplifiers, but also brings a perspective for photoacoustic image-guided therapy.
Photoacoustic (PA) tomography or microscopy, as a rapidly developing non-invasive imaging technology, well integrates molecular excitation by laser light with high resolution of ultrasound imaging in deep scattering medium, which has been widely used in biomedical imaging, image-guided drug delivery, and preclinical diagnostics.1–3 In conventional linear PA imaging, pulsed light irradiation is generally absorbed by contrast agents and then converted to heat with temperature rise.4,5 The transient temperature rise leads to thermoelastic expansion and acoustic emission that could be probed by using an ultrasound transducer to construct the PA image.6,7 Overall, the contrast in PA imaging mainly depends on light absorbance and optical to acoustic conversion efficiency, since the amplitude of the generated PA signal is linearly proportional to the optical absorption and the thermoelastic properties of the absorbing medium.8–11 On the other hand, the utilization of multiple laser pulse irradiation to realize nonlinear signal amplification has also attracted a lot of attention. Nonlinear PA imaging is mainly based on the increased Gruneisen coefficient (thermal expansion coefficient) on account of the heat accumulation and temperature increase of an object in the process of laser irradiation and heating. Thus, optical absorption and photothermal conversion efficiency of the contrast agents play an important role in both linear and nonlinear PA imaging.
Some metallic nanomaterials could serve as contrast enhancers to improve the PA effect, owing to their superior photothermal performance with a strong optical absorption coefficient, good biocompatibility, and ease to conjugate with biomarkers for deep tissue monitoring. Among them, gold nanoparticles (Au NPs) and nanorods (Au NRs) as nanosized contrast and therapeutic agents have attracted much attention on account of their facile preparation, efficient photothermal conversion and good biocompatibility.12–16 Particularly in the PA imaging field, they are usually employed to enhance the PA signal, improve the image contrast, and evaluate other PA agents as the reference.17–21 However, some drawbacks and limitations are still present in these metallic agents for practical PA applications. For example, their optical absorbance band is relatively narrow, restricting experimental operation conditions.22,23 Moreover, strong background PA signals from the blood could degrade the image contrast especially in conventional linear PA imaging due to similar optical absorption properties.17,24 Herein, ideal PA agents having broad optical absorbance and stable protection materials to ensure the maximum heat accumulation and temperature rise are required for both linear and nonlinear PA techniques to enhance the image contrast.25,26
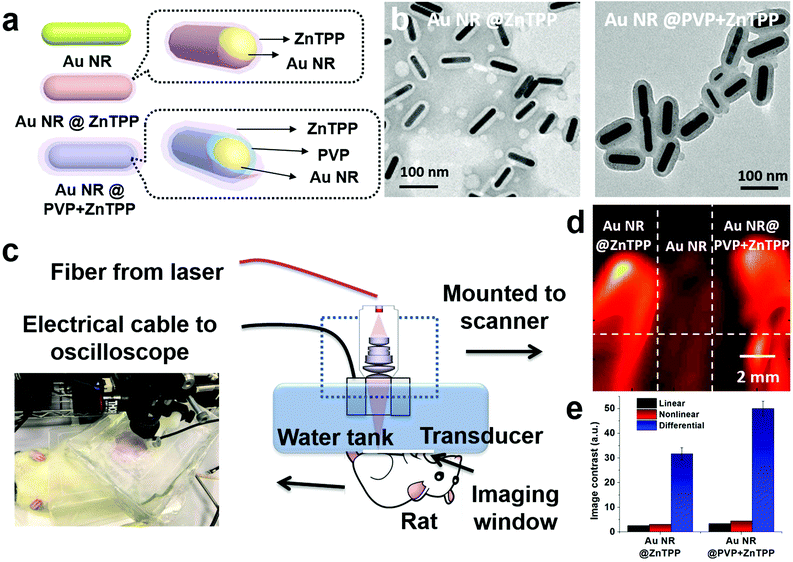
In this study, we developed two hybrid materials by encapsulating Au NRs within zinc tetra(4-pyridyl)porphyrin (ZnTPP) shells (Fig. 1a). The obtained hybrids (Fig. 1b) exhibited a synergetic effect in improving thermal conduction and thermoelastic expansion, leading to a notable contrast enhancement in both linear (four times greater) and nonlinear photoacoustic signals (more than six times) as compared with conventional Au NRs (Fig. 1c and d). Subsequently, we carefully investigated some interesting factors that could influence the signal amplification of these hybrid materials, including the thickness of ZnTPP shells, Au NR/ZnTPP interfaces, and linear/nonlinear PA imaging modalities. The introduction of a ZnTPP shell not only effectively improves their thermal confinement capability to maximize both linear and nonlinear PA signals, but also broadens their optical absorbance as compared with pure Au NRs. More importantly, the enhancement of both linear and nonlinear PA signals further improves the image contrast, which is much better than conventional linear PA imaging with only optical absorption improvement (Fig. 1e).
The two hybrid materials were developed by covering conventional Au NRs with ZnTPP. Au NRs with an aspect ratio of ∼5 were chosen as the main contrast agent, due to well matching of its absorbance at 808 nm with the PA laser irradiation wavelength.27 ZnTPP having absorbance at 675 nm was used to cover the surface of Au NRs.28 The introduction of ZnTPP could effectively broaden the absorption range of the resulting hybrid and further simplify the requirement for the PA operation. During the preparation of these hybrids, we found that the thickness of ZnTPP could be controlled by the addition of a surfactant, polyvinylpyrrolidone (PVP). The addition of PVP enables more ZnTPP to cover on the surface of Au NRs with a homogeneous thickness. To further explore the difference between the hybrid materials with and without the addition of PVP, namely Au NR@ZnTPP and Au NR@PVP + ZnTPP, we carefully characterized and compared their properties. The synthetic procedure for the preparation of Au NR@ZnTPP and Au NR@PVP + ZnTPP is shown in the ESI.† Although both Au NRs and ZnTPP have been widely acknowledged for their high performance in photothermal and photodynamic therapy,28–30 what is exciting is that the integration of Au NRs and ZnTPP offered superior performance in PA imaging, where both linear signal response and nonlinear signal feedback were enhanced and the interference of the blood background was successfully overcome.
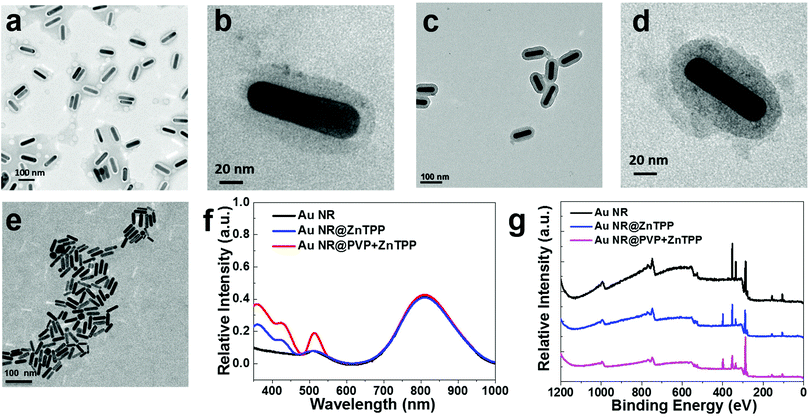
After synthesizing the two hybrid materials, we performed a series of physical characterization techniques prior to applying them for in vitro and in vivo PA imaging. The morphology, absorption range and intensity, percentage of ZnTPP in the hybrid, and toxicity are essential factors for their PA applications. Au NRs, Au NR@ZnTPP, and Au NR@PVP + ZnTPP were separately dispersed in aqueous solutions and their concentrations were standardized by their absorption spectra, where the aqueous solution of the bare Au NRs was chosen as the standard and the two hybrid materials were unified with the same absorbance intensity.31,32 The two hybrids could be dispersed in aqueous solution with a uniform size, as confirmed by transition electronic microscopy (TEM) images (Fig. 2a–d). The aspect ratio of Au NRs in the hybrids was determined to be ∼5, matching with that of the bare Au NRs. The ZnTPP layer was covered very well on the surface of Au NRs. Obviously, the addition of PVP in Au NR@PVP + ZnTPP commendably enhanced the density of the ZnTPP layer in comparison with Au NR@ZnTPP, and did not result in a thicker coverage of the ZnTPP layer (Fig. 2b and d).
Since the light to thermal conversion is required for PA application, the thermal stability of Au NR@ZnTPP and Au NR@PVP + ZnTPP was then characterized. In thermogravimetric analysis (TGA), the two hybrid materials presented good thermal stability (Fig. S1 in ESI†). Furthermore, the percentage of PVP and ZnTPP in the hybrids was calculated. The percentage of PVP covered on the surface of Au NRs reached ∼20%, while that of ZnTPP was ∼8% and ∼17% for Au NR@ZnTPP and Au NR@PVP + ZnTPP, respectively. Fourier transform infrared (FT-IR) spectra showed that the relative peak intensity of ZnTPP in AuNR@PVP + ZnTPP was obviously higher than that in Au NR@ZnTPP (Fig. S2 in ESI†), indicating that more ZnTPP was introduced onto Au NRs with the addition of PVP. Notably, PVP plays an important role in the process of covering ZnTPP on the surface of Au NRs, which effectively controls the density of the ZnTPP layer and contributes to the improvement of thermal confinement capability for PA applications.
The photophysical properties of the hybrids were further studied in an aqueous solution with 5% DMSO (volume ratio) as shown in Fig. 2f, where the addition of DMSO was to maintain a consistent condition with following cell culture experiments.33 Bare Au NRs with an aspect ratio of ∼5 had the major absorption peak at 808 nm. The two hybrid materials showed the same absorption as bare Au NRs around 808 nm. Additional absorption peaks at 448 nm and 512 nm from the two hybrids are ascribed to the absorption of loaded ZnTPP. Here, optical properties of ZnTPP are briefly introduced for better understanding of its utilization rationale. Generally, the metalation of the porphyrin rings such as ZnTPP leads to deprotonation. Hence, the absorption of metalloporphyrin involves the excitation of electrons from π to π* porphyrin orbitals.34,35 A typical absorption spectrum of metalloporphyrin is often comprised of a strong transition to the second excited state (S0 → S2) at ∼420 nm (so called the Soret band) and a weak transition to the first excited state (S0 → S1) at ∼520 nm (so called the Q band). Internal conversion from S2 to S1 is rapid, so the fluorescence is only detected from S1.35 Thus, the enhanced peak at 448 nm assigned to the characteristic Soret band of ZnTPP in the absorption spectra of Au NR@ZnTPP and Au NR@PVP + ZnTPP indicates the presence of ZnTPP. This peak did not match exactly with that of free ZnTPP, since the interaction between Au NRs and ZnTPP results in a red shift of 28 nm in comparison with the Soret band of free ZnTPP.36 The absorption at 512 nm is actually the Q band of ZnTPP in the two hybrids, which overlaps with the absorption of Au NRs. In order to further confirm their photophysical stability, a comparative study of the hybrids in phosphate buffered saline (PBS) solution was performed using TEM and UV-vis spectroscopy. The dispersion of the two hybrids in PBS remained unchanged with time, indicating their great stability without obvious aggregation (Fig. S3 and S4 in ESI†). Importantly, both of them exhibited excellent photostability under constant laser exposure, without losing their near-infrared absorbance after 10 min of irradiation (808 nm, 0.5 W cm−2). The stable and strong photothermal performance of the two hybrids makes them suitable agents for intracellular PA imaging.
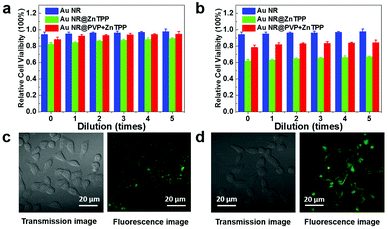
In order to confirm the biocompatibility of the two hybrids we synthesized, their cytotoxicity was investigated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, where the HeLa cell line was chosen and harvested in the logarithmic growth phase.37 The cells were incubated with Au NRs and the two hybrids in the dark for 24 and 48 hours respectively, and then tested using MTT. In this process, the initial concentration (43 μg mL−1 at 0 dilution time) of Au NRs and the two hybrids was set at the same as that in the absorption measurements. Subsequently, dilution with different times (from 1 to 5) was performed to carefully characterize cell viability. The statistical analysis (Fig. 3a and b) indicates that cell viability with the two hybrids could remain above 81% (∼84 ± 3%) after 24 h of incubation. After 48 h of incubation, cell viability with Au NR@PVP + ZnTPP was still maintained above 75% (∼78 ± 3%), and that of Au NR@ZnTPP decreased to over 60% (∼62 ± 2%). Moreover, confocal laser scanning microscopy was utilized to characterize successful cellular uptake of the two hybrids as shown in Fig. 3c and d.
Before using these hybrids for intracellular PA imaging, we would like to introduce the fundamental physics of the proposed quasi-continuous wave (CW) nonlinear PA technique. Different from the conventional single laser pulse induced PA effect (Fig. S5a in ESI†), the nonlinear PA method used in this work is based on the quasi-CW laser diode illumination, where multiple consecutive laser pulses are fired to induce multiple PA signals (Fig. S5b in ESI†). The linear PA signal P0 follows the conventional linear PA equation (eqn (S1)†), exhibiting that the linear PA amplitude is linearly proportional to the optical absorption coefficient of the nanorods and laser fluence. In contrast, the nth nonlinear PA signal Pn follows a more complex equation (eqn (S2)†) with nonlinear enhancement terms (Gruneisen saturation terms) due to the heat accumulation and temperature rising during n laser pulse illuminations under the thermal confinement conditions. One of the advantages of the proposed quasi-CW nonlinear PA method as compared with microbubble-induced nonlinear PA imaging is that this method requires low laser fluence and temperature rising to achieve obvious nonlinearity, which is preferred for in vivo and clinical PA imaging.38 By subtracting the linear PA equation from the nonlinear PA equation, the differential PA signal representing the nonlinear increase (nonlinear enhancement term in eqn (S2)†) could be obtained. Accordingly, the differential image could be obtained by subtracting the nonlinear PA image with a linear PA image. Thus, the PA nonlinearity is proportional to the square of the optical absorption coefficient, and related with the square of the thermal relaxation time of the nanorods. From the physical perspective, it predicts that the nonlinear PA imaging could potentially provide a higher image contrast on account of the square of the optical absorption coefficient and a higher thermal relaxation time.39–41 From the materials perspective, a better design of nanorods requires a high optical absorption coefficient, and more importantly, good thermal confinement (smaller thermal diffusivity) to improve both linear and nonlinear PA effects.42–45
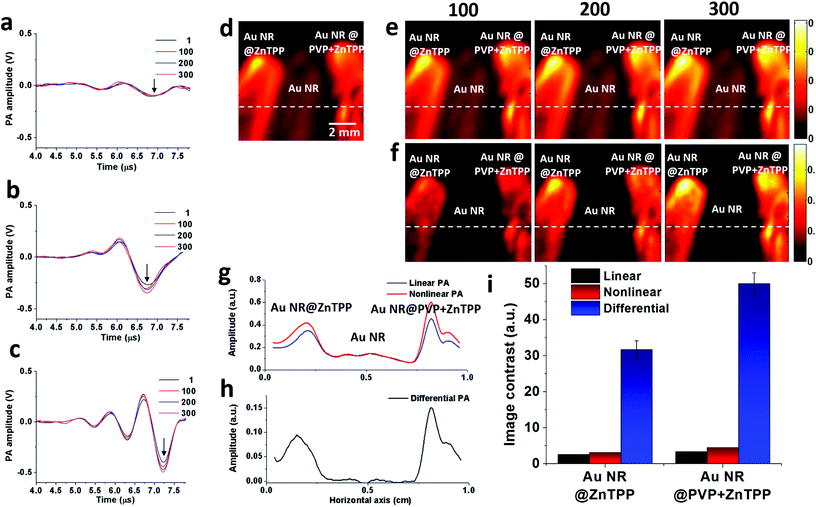
To prove the feasibility of both linear and nonlinear PA imaging using Au NR@ZnTPP and Au NR@PVP + ZnTPP as compared with Au NRs, in vitro experiments were performed by locating these three samples in microtubes with the same concentration. The experimental setup (Fig. S6 in ESI†) was similar to a PA microscopy configuration using an 808 nm pulse laser diode (Quantel Laser Diode Illuminator, tunable pulse number) and mechanical raster scanning controlled by a computer. Firstly, time-domain PA signals generated from the three samples with various laser pulse numbers (1, 100, 200, and 300) were measured as shown in Fig. 4a–c. PA signals with a single laser pulse (pulse number = 1) are linear PA signals, while the PA signals with multiple laser pulses (pulse numbers = 100, 200, and 300) are quasi-CW nonlinear PA signals. It was observed that the linear PA signals of Au NR@ZnTPP and Au NR@PVP + ZnTPP (Fig. 4b and c) showed much stronger amplitudes (×4.1 and ×6.3, respectively) than that of Au NRs (Fig. 4a). When exploring the nonlinearity of the PA signals at 100, 200 and 300 laser pulses, Au NR@ZnTPP and Au NR@PVP + ZnTPP demonstrated a clear amplitude enhancement upon increasing the laser pulse number. On the other hand, the Au NRs presented no obvious nonlinear PA improvement. Secondly, the PA images were acquired by raster-scanning the samples in two dimensions and mapping the peak-to-peak amplitude of each A-line signal. In linear PA imaging (Fig. 4d), it once again exhibited that Au NR@ZnTPP and Au NR@PVP + ZnTPP could significantly improve the linear PA imaging contrast. From the nonlinear PA imaging results (Fig. 4e), it could be seen that, upon increasing the laser pulse number, the image contrast from Au NR@ZnTPP and Au NR@PVP + ZnTPP was improved accordingly due to the induced stronger PA nonlinearity. By subtracting the nonlinear PA images from the linear PA images, differential PA images were obtained (Fig. 4f), showing that the image contrast of Au NR@ZnTPP versus Au NRs as well as Au NR@PVP + ZnTPP versus Au NRs obviously improved. On account of sufficient suppression of a linear absorption background in the differential PA images, Au NR@ZnTPP and Au NR@PVP + ZnTPP achieved a much higher image contrast over Au NRs. To quantitatively characterize the contrast improvement, the intensity cross-section lines obtained from the white dotted lines of the linear PA image, the nonlinear PA image and the differential PA image with a 300 laser pulse number in Fig. 4d–f are plotted in Fig. 4g and h. It was observed from Fig. 4g that obvious nonlinear enhancement was achieved for both Au NR@ZnTPP and Au NR@PVP + ZnTPP, while almost no nonlinear improvement was found for Au NRs. After the subtraction as shown in Fig. 4h, the image contrast further improved. The linear, nonlinear and differential PA image contrast for Au NR@ZnTPP was 2.6, 3.1, and 31.7 (Fig. 4i), which was calculated by using the ratios between the amplitudes of Au NR@ZnTPP and Au NRs in Fig. 4g and h. For Au NR@PVP + ZnTPP, the corresponding contrast was 3.4, 4.5, and 50.1, which was calculated by using the ratios between the amplitudes of Au NR@PVP + ZnTPP and Au NRs in Fig. 4g and h. Therefore, we achieved 12 times (31.7/2.6 = 12.2) and 15 times (50.1/3.4 = 14.7) image contrast improvement for Au NR@ZnTPP and Au NR@PVP + ZnTPP by comparing the nonlinear PA image with the conventional linear PA image, respectively.
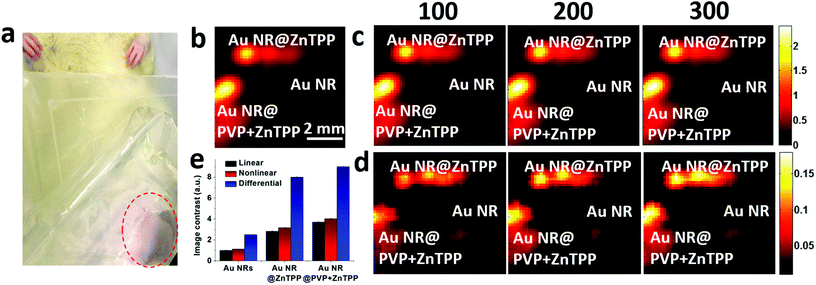
Then, we performed the in vivo linear and nonlinear PA imaging using Au NRs, Au NR@ZnTPP, and Au NR@PVP + ZnTPP in a rat. The system setup was similar to the one shown in Fig. S6 in ESI,† with a modification of the water tank. A hole was cut at the bottom of the tank, and then sealed by using a thin transparent polyethylene membrane (Fig. S7 in ESI†). The membrane was directly attached on top of the rat's abdomen skin. The hair of the ten-week-old rat was removed by hair removal lotion treatment before the experiments. The rat was anesthetized with isoflurane (2%) mixed with oxygen during the measurements. After that, the three samples were injected into the subcutaneous tissue in the abdomen close to the leg of the rat at different locations (Fig. 5a). After applying the ultrasound gel on the skin to optimize the acoustic coupling, the water tank was placed tightly on top of the rat's abdomen to ensure both optical and acoustic coupling by squeezing air out of the interface. The linear and nonlinear PA images were acquired by raster-scanning the laser and transducer over a membrane window of 1 cm × 1 cm with a step size of 100 μm. PA signals at each scanning point were recorded to extract the peak-to-peak values and employed to reconstruct the linear and nonlinear PA images. The subtraction was conducted to afford the differential PA images by subtracting each pixel value of linear images from the nonlinear PA images.
The linear PA imaging using only single laser pulse is shown in Fig. 5b, where both Au NR@ZnTPP and Au NR@PVP + ZnTPP demonstrated a clear contrast enhancement over the conventional Au NRs. Moreover, the nonlinear PA imaging was also performed using three different laser pulse numbers (100, 200, and 300). The nonlinear image results in Fig. 5c exhibited contrast improvement as compared with the linear PA imaging. Upon increasing the laser pulse number, the PA nonlinearity became more significant, leading to a further improved PA signal amplitude and image contrast. By performing differential imaging (subtracting the nonlinear PA images with the linear PA images), the background PA signals with negligible nonlinear enhancement were suppressed, generating differential images with a greatly improved image contrast for both Au NR@ZnTPP and Au NR@PVP + ZnTPP (Fig. 5d). The quantitative comparison of the image contrast for these three samples is shown in Fig. 5e, indicating that both Au NR@ZnTPP and Au NR@PVP + ZnTPP demonstrated enhanced image contrast of linear and nonlinear PA imaging as compared with conventional Au NRs (8–9 for Au NR@ZnTPP and Au NR@PVP + ZnTPP versus 2.5 for Au NRs). It should be noted that in vivo PA enhancement of Au NR@ZnTPP and Au NR@PVP + ZnTPP over Au NRs was not as significant as the in vitro enhancement, which is mainly caused by heterogeneous biological tissues surrounding the injected samples.
Here, the subcutaneous model used in this work may cause a few queries due to the limited penetration depth and the interference from the blood, although it shows the advantage of using low pulse energy (1 mJ) of the laser diode. The major significance of developing contrast-enhanced PA materials is to overcome the restricted requirements for equipment and operation conditions. Thus, the abovementioned subcutaneous model for realizing PA imaging application in a relatively simpler environment is a quite conventional mode. Supported by our experiments, a strong background linear PA signal from the subcutaneous capillaries proves the feasibility of the designed hybrid materials for enhanced linear and nonlinear PA imaging. Another note is that there are a few variations in the PA signal amplitudes as shown in Fig. 4i and 5e, which are mainly caused by the laser output energy fluctuation and environmental stability during the experiments. Less than 5% variation intensity is tolerable as compared to the linear and nonlinear PA signal enhancement. This kind of variation could be minimized by averaging more data at each scanning point, which however will sacrifice the imaging speed. In addition, the effect of PVP-induced ZnTPP layer in the PA enhancement may be another concern. Actually, we tried to explore different PA enhancements caused by different ZnTPP densities. Unfortunately, the density of ZnTPP was hard to be exactly quantified and its contrast under TEM was not obvious. Based on these reasons, we were only able to investigate the three discussed objects, i.e., the naked Au NRs, Au NR@ZnTPP and Au NR@PVP + ZnTPP. In the process of covering ZnTPP on the surface of Au NRs, we found that the signal of the acoustic wave increased upon enhancing the density of the ZnTPP layer. However, the density of ZnTPP coverage could reach its maximum, even when the concentration was further enhanced and the synthesis time was prolonged. Thus, the optimized Au NR@PVP + ZnTPP (Fig. 2d) was used in this work.
Based on the abovementioned results, it was concluded that the PA generation efficiency in this work is directly related to the size of Au NRs and the density of the ZnTPP layer, because Au NRs and ZnTPP are the sources of thermal effect and expansion, respectively. According to the physical fundamentals of PA generation (eqn (S1) and (S2) in ESI†), PA generation is actually determined by multiple physical parameters, such as thermal expansion coefficient, heat capacity, thermal confinement, and acoustic velocity. Specifically for nanorod design in this study, we found that by wrapping Au NRs with the ZnTPP layer, the obtained hybrids showed better thermal confinement, leading to enhanced linear and nonlinear PA generation efficiency. The aspect ratio of Au NRs is capable of being fine-tuned, which enables its absorbance to match well with the wavelength of external light irradiation for achieving the maximum energy conversion. In addition, the encapsulation thickness less than 30 nm has no obvious influence on the transportation of the thermal effect and subsequent expansion. This conclusion was also supported by a recent report,21 where a more than 60 nm silica layer was covered on Au NRs for PA imaging.
In conclusion, we have successfully developed ZnTPP-encapsulated Au NRs (Au NR@ZnTPP and Au NR@PVP + ZnTPP) for both linear and nonlinear PA imaging contrast enhancement. To the best of our knowledge, this is the first material design for enhancing both linear and nonlinear PA image contrast without the need of high laser fluence for bubble generation. Both in vitro and in vivo experiments have been performed to validate the enhanced PA imaging performance. Significant contrast improvements by 12 and 15 times in vitro as well as 4 and 6 times in vivo for Au NR@ZnTPP and Au NR@PVP + ZnTPP respectively have been achieved based on quasi-CW nonlinear PA imaging as compared with conventional linear PA imaging modality. In vivo experiments in rats have demonstrated a high potential of these new systems for future preclinical and clinical applications, such as targeted tumor detection and PA imaging-guided drug delivery. The present work would definitely inspire further design and synthesis of nanosystems for both linear and nonlinear PA imaging contrast improvement.
This work is supported by the NTU-Northwestern Institute for Nanomedicine and the NTU iFood Program under program grant no. 4081455.
Notes and references
- L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS PubMed.
- X. Wang, Y. Pang, G. Ku, X. Xie, G. Stoica and L. V. Wang, Nat. Biotechnol., 2003, 21, 803–806 CrossRef CAS PubMed.
- M. Xu and L. V. Wang, Rev. Sci. Instrum., 2006, 77, 041101 CrossRef.
- C. G. A. Hoelen, F. F. M. de Mul, R. Pongers and A. Dekker, Opt. Lett., 1998, 23, 648–650 CrossRef CAS PubMed.
- Z. Yuan, C. Wu, H. Zhao and H. Jiang, Opt. Lett., 2005, 30, 3054–3056 CrossRef PubMed.
- S. Sreejith, J. Joseph, M. Lin, N. V. Menon, P. Borah, H. J. Ng, Y. X. Loong, Y. Kang, S. W.-K. Yu and Y. Zhao, ACS Nano, 2015, 9, 5695–5704 CrossRef CAS PubMed.
- A. De La Zerda, C. Zavaleta, S. Keren, S. Vaithilingam, S. Bodapati, Z. Liu, J. Levi, B. R. Smith, T.-J. Ma, O. Oralkan, Z. Cheng, X. Chen, H. Dai, B. T. Khuri-Yakub and S. S. Gambhir, Nat. Nanotechnol., 2008, 3, 557–562 CrossRef CAS PubMed.
- F. Gao, X. Feng and Y. Zheng, J. Optom., 2016, 18, 074006 CrossRef.
- X. Feng, F. Gao, C. Xu, L. Gaoming and Y. Zheng, Opt. Lett., 2015, 40, 4492–4495 CrossRef PubMed.
- F. Gao, X. Feng, Y. Zheng and C.-D. Ohl, J. Biomed. Opt., 2014, 19, 067006 CrossRef PubMed.
- F. Gao, L. Bai, X. Feng, H. P. Tham, R. Zhang, Y. Zhang, S. Liu, L. Zhao, Y. Zheng and Y. Zhao, Small, 2016, 12, 5239–5244 CrossRef CAS PubMed.
- L. Bai, L. Zhu, C. Y. Ang, X. Li, S. Wu, Y. Zeng, H. Ågren and Y. Zhao, Chem. – Eur. J., 2014, 20, 4032–4037 CrossRef CAS PubMed.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef CAS PubMed.
- M. Prabaharan, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, Biomaterials, 2009, 30, 6065–6075 CrossRef CAS PubMed.
- J. A. Webb and R. Bardhan, Nanoscale, 2014, 6, 2502–2530 RSC.
- X. Yang, M. Yang, B. Pang, M. Vara and Y. Xia, Chem. Rev., 2015, 115, 10410–10488 CrossRef CAS PubMed.
- Y.-S. Chen, W. Frey, S. Kim, P. Kruizinga, K. Homan and S. Emelianov, Nano Lett., 2011, 11, 348–354 CrossRef CAS PubMed.
- S. Mallidi, T. Larson, J. Tam, P. P. Joshi, A. Karpiouk, K. Sokolov and S. Emelianov, Nano Lett., 2009, 9, 2825–2831 CrossRef CAS PubMed.
- Q. Zhang, N. Iwakuma, P. Sharma, B. M. Moudgil, C. Wu, J. McNeill, H. Jiang and S. R. Grobmyer, Nanotechnology, 2009, 20, 395102 CrossRef CAS PubMed.
- F. Gao, Y. H. Ong, G. Li, X. Feng, Q. Liu and Y. Zheng, Opt. Lett., 2015, 40, 3568–3571 CrossRef PubMed.
- J. Wang, J. Liu, Y. Liu, L. Wang, M. Cao, Y. Ji, X. Wu, Y. Xu, B. Bai, Q. Miao, C. Chen and Y. Zhao, Adv. Mater., 2016, 28, 8950–8958 CrossRef CAS PubMed.
- S. Sreejith, J. Joseph, K. T. Nguyen, V. M. Murukeshan, S. W. Lye and Y. Zhao, ChemNanoMat, 2015, 1, 39–45 CrossRef CAS.
- J. Sjollema, P. K. Sharma, R. J. B. Dijkstra, G. M. van Dam, H. C. van der Mei, A. F. Engelsman and H. J. Busscher, Biomaterials, 2010, 31, 1984–1995 CrossRef CAS PubMed.
- K. Pu, A. J. Shuhendler, J. V. Jokerst, J. Mei, S. S. Gambhir, Z. Bao and J. Rao, Nat. Nanotechnol., 2014, 9, 233–239 CrossRef CAS PubMed.
- X. Yang, E. W. Stein, S. Ashkenazi and L. V. Wang, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 360–368 CrossRef CAS PubMed.
- R. Zhang, K. Cheng, A. L. Antaris, X. Ma, M. Yang, S. Ramakrishnan, G. Liu, A. Lu, H. Dai, M. Tian and Z. Cheng, Biomaterials, 2016, 103, 265–277 CrossRef CAS PubMed.
- A. Gole and C. J. Murphy, Langmuir, 2008, 24, 266–272 CrossRef CAS PubMed.
- H. P. Tham, H. Chen, Y. H. Tan, Q. Qu, S. Sreejith, L. Zhao, S. S. Venkatraman and Y. Zhao, Chem. Commun., 2016, 52, 8854–8857 RSC.
- M. Li, H. Yan, C. Teh, V. Korzh and Y. Zhao, Chem. Commun., 2014, 50, 9745–9748 RSC.
- K. Cheng, S.-R. Kothapalli, H. Liu, A. L. Koh, J. V. Jokerst, H. Jiang, M. Yang, J. Li, J. Levi, J. C. Wu, S. S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 3560–3571 CrossRef CAS PubMed.
- J. V. Jokerst, A. J. Cole, D. Van de Sompel and S. S. Gambhir, ACS Nano, 2012, 6, 10366–10377 CrossRef CAS PubMed.
- J. V. Jokerst, M. Thangaraj, P. J. Kempen, R. Sinclair and S. S. Gambhir, ACS Nano, 2012, 6, 5920–5930 CrossRef CAS PubMed.
- L. Bai, S. Z. F. Phua, W. Q. Lim, A. Jana, Z. Luo, H. P. Tham, L. Zhao, Q. Gao and Y. Zhao, Chem. Commun., 2016, 52, 4128–4131 RSC.
- R. Dong, Y. Bo, G. Tong, Y. Zhou, X. Zhu and Y. Lu, Nanoscale, 2014, 6, 4544–4550 RSC.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, F. E. CurchodBasile, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, K. NazeeruddinMd and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- S. Wang, P. Huang, L. Nie, R. Xing, D. Liu, Z. Wang, J. Lin, S. Chen, G. Niu, G. Lu and X. Chen, Adv. Mater., 2013, 25, 3055–3061 CrossRef CAS PubMed.
- Y. Zhao, M. Cao, J. F. McClelland, Z. Shao and M. Lu, Biosens. Bioelectron., 2016, 85, 261–266 CrossRef CAS PubMed.
- J. Xia, J. Yao and L. V. Wang, Electromagn. Waves, 2014, 147, 1–22 CrossRef.
- C.-w. Wei, M. Lombardo, K. Larson-Smith, I. Pelivanov, C. Perez, J. Xia, T. Matula, D. Pozzo and M. O'Donnell, Appl. Phys. Lett., 2014, 104, 033701 CrossRef PubMed.
- Z. Sheng, L. Song, J. Zheng, D. Hu, M. He, M. Zheng, G. Gao, P. Gong, P. Zhang, Y. Ma and L. Cai, Biomaterials, 2013, 34, 5236–5243 CrossRef CAS PubMed.
- D. Zhu, K. V. Larin, Q. Luo and V. V. Tuchin, Laser Photonics Rev., 2013, 7, 732–757 CrossRef CAS PubMed.
- G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed.
- A. A. Appel, M. A. Anastasio, J. C. Larson and E. M. Brey, Biomaterials, 2013, 34, 6615–6630 CrossRef CAS PubMed.
- L. Nie and X. Chen, Chem. Soc. Rev., 2014, 43, 7132–7170 RSC.
- S. K. Maji, S. Sreejith, J. Joseph, M. Lin, T. He, Y. Tong, H. Sun, S. W.-K. Yu and Y. Zhao, Adv. Mater., 2014, 26, 5633–5638 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional synthesis and characterization data. See DOI: 10.1039/c6nr07528b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |