Robust electrochemical metal oxide deposition using an electrode with a superhydrophobic surface†
Jun
Zhang‡
abc,
Xia
Sheng‡
b,
Xiqing
Cheng
a,
Liping
Chen
b,
Jian
Jin
a and
Xinjian
Feng
*ab
aDivision of Nanobionic Research, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou, 215123, China
bCollege of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China. E-mail: xjfeng@suda.edu.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 24th November 2016
Abstract
Described herein is the first study of metal oxide deposition on an electrode with a solid/liquid/gas triphase reaction interface. Such an electrode enables the formation of a locally high interface pH value that is independent of the bulk conditions, and allows the deposition of a wide variety of metal oxides in a robust electrolyte. The results reveal that the control of electrode surface wettability is of significant importance for the regulation of the electrode–electrolyte local interface environment, which provides a new set of guidelines and perspective for future electrode preparation and application.
The wetting properties of an electrode surface are of significant importance in electrochemistry due to electrochemical reactions and electron transfer that take place at the electrode/electrolyte interface. Surfaces possessing superhydrophobicity have recently received substantial interest and enabled new applications in many areas,1–11 owing to their unique triphasic character: when in contact with an aqueous solution they can trap numerous gas pockets within structured gaps, thus exposing the liquid surface directly to the solid/gas interface.12–14 An electrode possessing a superhydrophobic surface will fundamentally change the electrolyte/electrode interface environment and the electrode process. Thus, exploring electrochemical reactions on such electrodes is of both theoretical and technological importance, which, nevertheless, has received limited attention.15,16
Here we report our study of metal oxide electrochemical deposition (ECD) on an electrode possessing a solid–liquid–gas triphase interface. With this electrode architecture, a large amount of oxygen (O2) from the gas phase is directly available at the electrode surface to in situ generate OH− (O2 + 2H2O + 4e− → 4OH−);17,18 and thus a locally high pH value can be achieved. This offers an ideal environment for metal oxide ECD, since it is ultimately achieved through the reactions between the OH− and the metal ion (Mn+) at the electrode/electrolyte interface.19 Unlike traditional electrodes, the triphasic electrode enables the formation of a local interface environment that is independent of the bulk conditions and allows the synthesis of metal oxide in robust electrolytes. As an example of deposition, we show that pure phase Cu2O can be readily obtained over a wide pH range of 7 to 14, which is impossible when a traditional diphase electrode is applied.20 The described principle has also been demonstrated to generally allow the deposition of other metal oxides within a new range of synthesis variables.
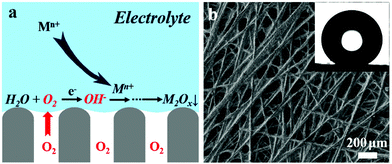
Fig. 1a is a schematic illustration of the triphasic electrode when in contact with an aqueous electrolyte. Such an electrode allows the rapid transport of oxygen via the gas phase towards the electrode/electrolyte interface and the in situ generation of needed OH− for metal oxide deposition through the oxygen reduction reaction. To fabricate such an electrode, a conducting carbon fibre substrate, with a fibre diameter of ca. 20 μm, was subjected to a polyfluorotetraethylene treatment (Fig. 1b); the micrometer-scale texture and low surface energy of the material result in superhydrophobicity on both sides of the substrate. One side of the substrate where the metal oxide is deposited is then exposed to a short-term oxygen plasma treatment, which significantly enhanced the electron transfer rate between the substrate and the electrolyte (Fig. S1, ESI†). The insert of Fig. 1b shows a spherical water droplet placed on the surface without treatment, indicating a surface superhydrophobic behaviour that can trap gas inside and prevent water from penetrating into the substrate.
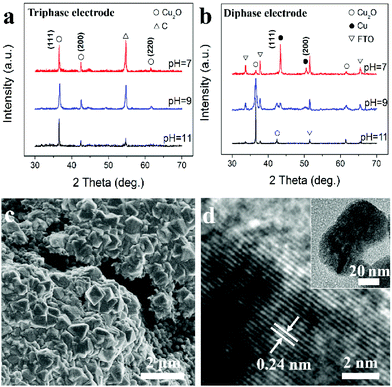
The as-prepared electrode is then used as a working electrode for metal oxide ECD. Fig. 2a shows X-ray diffraction (XRD) patterns of Cu2O samples prepared in electrolytes with pH values of 7, 9 and 11. The diffraction peaks can be respectively indexed to (111), (200) and (220) of the standard cubic Cu2O (JCPDS card no. 65-3288). The result confirms that, using the triphasic electrode, the ECD of Cu2O is independent of the pH value of the bulk electrolyte, and that pure Cu2O can be obtained even in a neutral electrolyte. In marked contrast, as shown in Fig. 2b and S2,† when ECD is carried out using a traditional diphase electrode (e.g. FTO and carbon fibre substrate without superhydrophobic treatment), Cu peaks (JCPDS card no. 02-1225) are observed in addition to the Cu2O peaks at pH values of 7 and 9. Fig. 2c is a field emission scanning electron microscopy (FE-SEM) image of Cu2O synthesized using the triphase electrode with a pH 9 electrolyte, which is composed of regular octahedron shaped nanocrystals. A transmission electron microscopy (TEM) image of a single nanocrystal, inset of Fig. 2d, further confirms the Cu2O octahedron shape. The clearly defined fringe spacing of 0.24 nm seen in the high-resolution TEM image, Fig. 2d, corresponds to the d-spacing of the (111) planes of Cu2O. These results verify the formation of well-crystallized Cu2O using the triphase electrode. When the experiments were performed using a diphase electrode under the same deposition conditions, products with no regular shape were deposited as shown in Fig. S3,† and the presence of Cu was clearly observed according to HR-TEM analysis (Fig. S3b†), a result consistent with XRD experimental results (Fig. 2b).
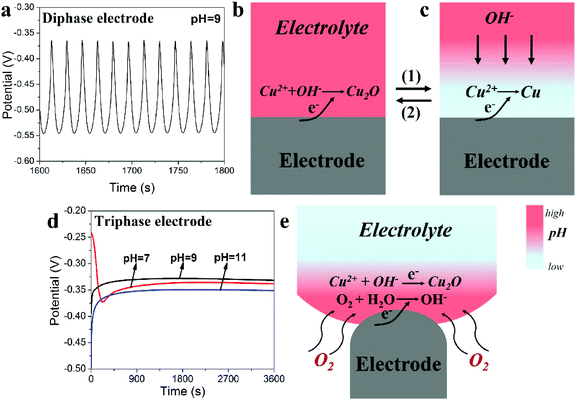
The remarkable differences seen in the ECD results using either the triphase or diphase electrodes can be explained from their electrodeposition curves (Fig. 3). For the diphase electrode, when ECD is carried out in electrolytes of relatively low pH value (e.g. 9), typical potential oscillations are observed as seen in Fig. 3a and S4,† similar to the previous reports.21–24 During the positive-going potential shift, when OH− concentration at the electrode surface is sufficient (Fig. 3b), Cu2O is deposited (2Cu2+ + 2e− + 2OH− → Cu2O + H2O). As Cu2O is being deposited, OH− at the electrode surface will be rapidly consumed but cannot be promptly supplied due to the slow diffusion rate of OH− caused by the low concentration gradient from the bulk electrolyte to the electrode surface. As a result, the interface pH value decreases (route 1, from Fig. 3b to c). Since the metal oxide is generally achieved through reactions between the OH− and the metal ion (Mn+), the low pH value at the interface surface will consequently lead to the deposition of Cu (Cu2+ + 2e− → Cu) during the negative-going potential shift. Meanwhile, the interface pH value will increase following OH− diffusion from the bulk electrolyte (route 2, from Fig. 3c to b). Potential oscillations occur as the above processes repeats, leading to deposition of a Cu2O/Cu composite.
In marked contrast, as shown in Fig. 3d, smooth and well-behaved deposition curves are recorded for different pH values using the as-constructed triphasic electrode. As illustrated in Fig. 3e, the existence of O2 at the triphase electrode/electrolyte interface allows significantly faster oxygen flow via gas phase permeation towards the electrode surface, consequently a locally high interface pH value is achieved, independent of the diffusion and concentrations of OH− in the bulk electrolyte. To confirm this, as shown in Fig. S5,† when the triphasic electrode is cathodically polarized in a neutral electrolyte in the presence of a phenolphthalein indicator, coloration of the solution (colourless to red at pH value higher than 10)25 at the electrode surface is observed within seconds, indicating a locally high pH environment. This result indicates that when ECD is carried out using the triphasic electrode, OH− consumed at the electrode surface during the deposition of Cu2O is in situ rapidly supplied via the oxygen reduction reaction, so that the ECD process proceeds with great stability in electrolytes having a wide range of pH values.
To further understand the important role that the triphasic electrode plays, ECD was performed using a diphase electrode system in an oxygen-saturated electrolyte with a pH value of 9. As can be seen from Fig. S6,† typical potential oscillations and Cu2O/Cu composites are still obtained. This result reveals that using a traditional diphase electrode, it is difficult for a constant and high concentration of OH− at the electrode/electrolyte interface to be maintained during the ECD even with an oxygen saturated electrolyte of relatively low pH value, due to the much lower diffusion coefficient of oxygen in the liquid phase (∼2.1 × 10−5 cm2 s−1) than that in the gas phase (∼2.0 × 10−1 cm2 s−1).26
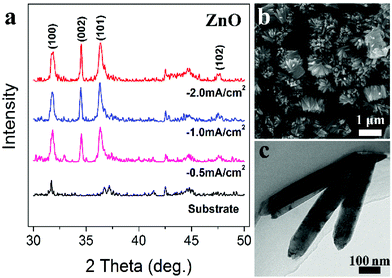
Such electrode is generally applicable for deposition of a great variety of metal oxides. In a traditional electrode system, as is well recognized, in addition to tailoring the pH value of an electrolyte, extra additives that can generate OH− at the electrode surface are required. For example, in the ECD process of ZnO, NO3− (NO3− + H2O + e− → NO2− + 2OH−) or H2O2 (H2O2 + 2e− → 2OH−) are needed in the electrolytes.27,28 However in the triphase electrode, ZnO can be easily deposited in the absence of such additives. Fig. 4a shows the XRD patterns of ZnO samples deposited at different current densities; all of these diffraction peaks can be respectively indexed as (100), (002), (101) and (102) of the standard ZnO (JCPDS card no. 79-0205). Fig. 4b and c are typical FE-SEM and TEM images of the as-deposited ZnO nanorods, with a nanorod length of about 550 nm. Similarly, CeO2 is deposited using the triphase electrode without the need of such additives, which are otherwise required with a traditional electrode. As shown in Fig. S7,† the resulting CeO2 crystalline nanoparticles have a diameter of approximately 65 nm. These results demonstrate the universality of the triphasic electrode while being applied for metal oxide ECD.
Conclusions
In summary, we have demonstrated a novel triphase electrode for simple and robust electrochemical metal oxide deposition. The unique triphasic character of the electrode enables the direct introduction of O2 to the electrode surface thus maintaining a locally high interface pH environment that is independent of that of the bulk conditions. Pure Cu2O has been successfully prepared using electrolytes over a range of pH values, including neutral conditions. Further, the triphasic electrode has also been used to synthesize ZnO and CeO2. The novel phenomenon reveals that the control of electrode surface wettability is of significant importance for the regulation of the electrode/electrolyte interface local environment, which provides a new set of guidelines and perspective for ECD. In a wider electrochemistry context, we hope the principle reported in this study will help the design of efficient electrodes for various electrochemical applications.Acknowledgements
X. F. acknowledges financial support from the National Natural Science Foundation of China (21371178), the Jiangsu Province Science Foundation for Distinguished Young Scholars (BK20150032), and the Chinese Thousand Youth Talents Program (YZBQF11001). X. S. acknowledges financial support from the National Natural Science Foundation of China (21501193).Notes and references
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132 CrossRef CAS PubMed.
- T. L. Liu and C.-J. C. Kim, Science, 2014, 346, 1096 CrossRef CAS PubMed.
- X. Deng, L. Mammen, H.-J. Butt and D. Vollmer, Science, 2011, 335, 67 CrossRef PubMed.
- L. Wen, Y. Tian and L. Jiang, Angew. Chem., Int. Ed., 2015, 54, 3387 CrossRef CAS PubMed.
- D. Aebisher, D. Bartusik, Y. Liu, Y. Zhao, M. Barahman, Q. Xu, A. M. Lyons and A. Greer, J. Am. Chem. Soc., 2013, 135, 18990 CrossRef CAS PubMed.
- J. G. Nguyen and S. M. Cohen, J. Am. Chem. Soc., 2010, 132, 4560 CrossRef CAS PubMed.
- M. Paven, P. Papadopoulos, S. Schöttler, X. Deng, V. Mailänder, D. Vollmer and H.-J. Butt, Nat. Commun., 2013, 4, 2512 Search PubMed.
- Y. Tian, B. Su and L. Jiang, Adv. Mater., 2014, 26, 6872 CrossRef CAS PubMed.
- Y. Tang, Y. Zhang, X. Rui, D. Qi, Y. Luo, W. R. Leow, S. Chen, J. Guo, J. Wei, W. Li, J. Deng, Y. Lai, B. Ma and X. Chen, Adv. Mater., 2016, 28, 1567 CrossRef CAS PubMed.
- S. Zhang, J. Huang, Y. Tang, S. Li, M. Ge, Z. Chen, K. Zhang and Y. Lai, Small, 2016 DOI:10.1002/smll.201600687.
- C. Cao, M. Ge, J. Huang, S. Li, S. Deng, S. Zhang, Z. Chen, K. Q. Zhang, S. S. Aldeyab and Y. Lai, J. Mater. Chem. A, 2016, 4, 12179 CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
- L. Feng, S. H. Li, Y. S. Li, H. J. Li, L. J. Zhang, J. Zhai, Y. L. Song, B. Q. Liu, L. Jiang and D. B. Zhu, Adv. Mater., 2002, 14, 1857 CrossRef CAS.
- A. Lafuma and D. Quéré, Nat. Mater., 2003, 2, 457 CrossRef CAS PubMed.
- Y. Lei, R. Sun, X. Zhang, X. Feng and L. Jiang, Adv. Mater., 2016, 28, 1477 CrossRef CAS PubMed.
- Y. C. Wu, K. S. Liu, B. Su and L. Jiang, Adv. Mater., 2014, 26, 1124 CrossRef CAS PubMed.
- D. Chen, C. Chen, Z. M. Baiyee, Z. Shao and F. Ciucci, Chem. Rev., 2015, 115, 9869 CrossRef CAS PubMed.
- J. Masa, W. Xia, M. Muhler and W. Schuhmann, Angew. Chem., Int. Ed., 2015, 54, 10102 CrossRef CAS PubMed.
- G. H. A. Therese and P. V. Kamath, Chem. Mater., 2000, 12, 1195 CrossRef CAS.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10, 456 CrossRef CAS PubMed.
- J. A. Switzer, C.-J. Hung, L.-Y. Huang, E. R. Switzer, D. R. Kammler, T. D. Golden and E. W. Bohannan, J. Am. Chem. Soc., 1998, 120, 3530 CrossRef CAS.
- E. W. Bohannan, L.-Y. Huang, F. S. Miller, M. G. Shumsky and J. A. Switzer, Langmuir, 1999, 15, 813 CrossRef CAS.
- J. A. Switzer, C.-J. Hung, E. W. Bohannan, M. G. Shumsky, T. D. Golden and D. C. VanAken, Adv. Mater., 2009, 21, 334 CrossRef.
- E. D. Mishina, K. Nagai and S. Nakabayashi, Nano Lett., 2001, 1, 401 CrossRef CAS.
- S. S. He and C. W. Frank, J. Mater. Chem. A, 2014, 2, 16489 CAS.
- E. L. Cussler, Diffusion: Mass Transfer in Fluid Systems, Cambridge University Press, New York, 2nd edn, 1997 Search PubMed.
- T. Yoshida and H. Minoura, Adv. Mater., 2000, 12, 1219 CrossRef CAS.
- T. Yoshida, J. Zhang, D. Komatsu, S. Sawatani, H. Minoura, T. Pauporte, D. Lincot, T. Oekermann, D. Schlettwein, H. Tada, D. Wöhrle, K. Funabiki, M. Matsui, H. Miura and H. Yanagi, Adv. Funct. Mater., 2009, 19, 17 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nr07421a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |