Large photoacoustic effect enhancement for ICG confined inside MCM-41 mesoporous silica nanoparticles†
Giuseppe
Ferrauto
a,
Fabio
Carniato
b,
Enza
Di Gregorio
a,
Lorenzo
Tei
*b,
Mauro
Botta
 b and
Silvio
Aime
*a
b and
Silvio
Aime
*a
aDepartment of Molecular Biotechnology and Health Sciences, University of Turin, Via Nizza 52, 10126 Turin, Italy. E-mail: silvio.aime@unito.it
bDipartimento di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale “A. Avogadro”, Viale T. Michel 11, 15121 Alessandria, Italy. E-mail: lorenzo.tei@uniupo.it
First published on 24th November 2016
Abstract
Indocyanine green was encapsulated inside the pores of pegylated amino-functionalized MCM-41 Mesoporous Silica Nanoparticles (ICG-MSNs). In addition to a greater stability and a decrease of toxicity, the photoacoustic effect of ICG-MSNs increases by nearly 400% compared to free ICG due to fluorescence quenching and high photothermal conversion of the encapsulated dyes. Upon i.v. administration in tumor-bearing mice, an overall photoacoustic enhancement of ca. 25% was measured in the tumor region.
ICG has been extensively used in medical diagnosis for a number of applications and, above all, in preclinical optical studies.1 In particular it has been employed for rapid blood volume measurements,2 for angiography3 and ophthalmology.4 In addition, its application has also been proposed for guided surgery5 as well as in sentinel lymph node detection.6 More recently, it has been demonstrated that ICG is also an efficient dye for photoacoustic imaging (PAI).7
PAI has emerged in the last few years as an innovative imaging technique that can provide biomedical, structural and functional information on specific regions of interest.8 It effectively merges the ultrasound's advantages (spatial resolution, penetration depth, etc.) with the optical properties of absorbing molecules naturally occurring in tissues or externally administered, such as sensitivity and possibility to visualize contemporarily different molecules.8 Numerous endogenous contrast molecules (hemoglobin, melanin, lipid, water)9 have been successfully exploited as PAI contrast agents to gain physiological information about healthy and pathological tissues (vascular volume, oxygen content, etc.). More recently, the development of exogenous contrast agents has emerged as a prominent field of research.10,11 Typically, an efficient PAI contrast agent should be characterized by: (i) biological compatibility and lack of toxicity; (ii) water solubility and a sufficiently long half-life compatible with an efficient excretion from the body; (iii) high light-to-ultrasound energy conversion; (iv) suitability to chemical modifications in order to endow the agent with targeting properties; and (v) ease of preparation and good stability.10–12 Many PAI agents have been already proposed. They belong to four main classes, namely: (i) molecular dyes; (ii) fluorescent proteins; (iii) plasmonic surface resonance noble metal nanoparticles and (iv) non-plasmonic nano- or microparticles.10 Molecular dyes typically employed in optical imaging have been translated to optoacoustic applications (e.g. Methylene Blue, Congo Red, NIRF molecules, ICG, etc.) showing, in general, a good sensitivity without significant negative side effects.10 In particular, ICG is already approved by the FDA for human application and is undoubtedly safer than plasmonic surface resonance metals (Au, Ag, etc.); however, the in vivo application is hampered by a rapid protein binding, fast clearance, and instability under physiologically relevant conditions.13 Thus, the encapsulation of ICG inside nanocarriers appears to be a suitable way to improve its performance as a molecular imaging probe. Several exogenous nanocarriers have been tested including ICG-encapsulated micelles,14 ICG-doped PLGA,15 HSA (Human Serum Albumin)-ICG,16 ICG-loaded liposomes,17 lipidic particles (lipidots)18 and ICG-coated single-walled carbon nanotubes.19 Typically, they show enhanced photostability and better bioavailability but not an increased photoacoustic effect with respect to the free dye. Analogously, the encapsulation of ICG20 as well as other NIR cyanine dyes21 inside silica NPs has been demonstrated to protect the molecules from degradation.
Since MSNs are highly stable and versatile, we designed a new PAI probe by loading ICG dyes inside the pores of pegylated MCM-41 MSNs (Fig. 1) with the aim to increase both the stability of the dye and its photoacoustic effect.22 In this work, MCM-41 NPs were prepared through a typical sol–gel procedure by using a block-copolymer and a surfactant-assisted co-assembly method.23
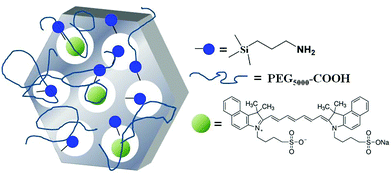 | ||
| Fig. 1 A schematic view of the distribution of ICG molecules inside functionalized mesoporous silica NPs. | ||
Physico-chemical characterization of MSNs (HR-TEM, XRD, N2 physisorption analysis) is reported in the ESI (Fig. S1–S3†). The results show a system composed of spherical particles with size in the 20–50 nm range and a regular hexagonal pore array of 2.5 nm diameter. Then, the surface of the MSNs was decorated with amino propyl groups24 in order to: (i) allow the grafting of PEG-COOH molecules on the surface and (ii) promote the ionic interaction between the ammonium ions on the MSNs and the sulfonates of the ICG molecules, as previously reported in the case of ICG-loaded trimethylammonium modified MSNs.25 The amount of –NH2 groups present on the surface was calculated by thermogravimetric analysis under oxygen flow and resulted to be 1.3 mmol g−1 (Fig. S4†).
In the second step, organo-modified MCM-41 NPs were suspended in a MeOH solution of ICG (0.07 μM) for 8 h at room temperature to allow the impregnation of the dye into the silica channels. After washing with MeOH, the ICG loading in ICG-MSNs was 0.80 μmol g−1 (ca. 25 ICG molecules per particle, see ESI† for details), assessed by measuring the amount of free dye in the washing medium using UV-visible spectroscopy at 785 nm (Fig. S5†). To evaluate the result of the amount of dye loaded in MSN pores on the photoacoustic effect, samples containing different amounts of ICG (1.7 and 3.7 μmol g−1) were also prepared.
Finally, a fraction of the –NH2 groups exposed on the silica surface was reacted with polyethylene glycol (PEG5000-COOH) via amide-coupling methods to improve particle stability and to confer a stealth character towards the immune system.26 PEG moieties located on the MSNs’ external surface ensured good compatibility in aqueous solution preventing particle aggregation. The NPs appeared substantially monodispersed in water with a hydrodynamic diameter of ca. 60 nm (Fig. S6†) and a surface ζ-potential of +21.4 mV at 25 °C (Fig. S7,† data unchanged with respect to the MSN-NH2 nanoparticles). The IR spectrum of the pegylated NPs showed an additional absorption at 1650 cm−1, assigned to the stretching of amide groups formed by the coupling of PEG to the surface (Fig. S8†). It is worth noting that, no release of ICG molecules was detected during the reaction.
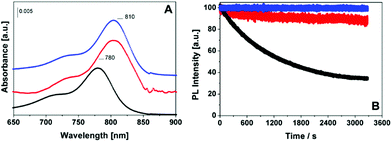
ICG-MSNs were also characterized by UV-visible and photoluminescence spectroscopy. As reported in Fig. 2A, ICG-MSNs show a main absorption at 810 nm, promoted by the π → π* transitions, slightly shifted to higher wavelengths with respect to the isolated dye, as a consequence of a different local chemical environment. The excitation of the ICG-MSN at 780 nm promotes a strong emission in the 810–840 nm range (Fig. S9†). A quenching of the fluorescence emission for ICG-MSNs was observed when compared to that of equivalent concentrations of the free dye resulting in a ca. 2.2 fold decrease of quantum yield (Table 1).
| System | ICG stability (irradiation at λ = 750 nm, 1 h) | Quantum efficiency | Photoacoustic effect |
|---|---|---|---|
| ICG | 35% | 2.58%31 | 1 |
| ICG-MSNs | 90% | 1.13% | +370% |
| ICG-liposomes | 100% | 5.50% | +25% |
The fluorescence quenching of ICG loaded inside the MSN pores was comparable to that of gold nanostructures27 and can be attributed to dye self-aggregation.28
Remarkably, photodegradation (bleaching tests) of ICG inside silica channels is almost completely absent after 1 hour of light irradiation (Fig. 2B and Table 1) and no release in solution of ICG was detected during the experiment. This behavior appears very similar to that measured for pegylated liposomes incorporating ICG, prepared as previously reported.17 In contrast, the free dye showed a degradation of ca. 60% under identical experimental conditions.
It is known that, when ICG molecules are loaded inside nanocarriers, their accessibility to water is limited and their stability is enhanced.17,20 The cyanine photobleaching is mainly caused by the generation of reactive oxygen species (ROS) triggered by photoexcitation of fluorophores.29 Therefore, molecular oxygen dissolved in water (0.3 mM) has a central role and when ICG is free in aqueous solution its stability is strongly affected. Conversely, when such molecules are partially shielded from water solvent their stability is strongly enhanced. In the herein described MSNs, the ICG molecules are confined inside the silica pores where the water diffusion is markedly slowed down,30 thus reducing the negative effect of ROS on ICG stability.
It is noteworthy that, the absorption spectrum and the photodegradation pattern of ICG-MSNs and ICG-liposomes are almost identical (Fig. S9† and Fig. 2B). Nevertheless, in the case of ICG loaded into liposomes, the quantum efficiency in water resulted higher than that of free ICG (Table 1), as also observed in the case of ICG encapsulated into micellar systems.31
Moreover, ICG-MSNs are stable in physiological media for over 24 h (Fig. S10†), whereas release tests carried out in Seronorm™ indicated a progressive loss of ICG from the silica NPs, likely caused by the strong affinity of ICG for HSA.32 The test was performed by dispersing 6 mg of ICG-MSN in 3 mL of a Seronorm™ solution and monitoring the UV-Vis absorbance of the dye over time. 10 wt% and 60 wt% of ICG molecules were released after 15 min and 1 h, respectively. This result highlights the need of a further improvement of the system to prevent release of ICG from MSN's pores before an extended in vivo translation.
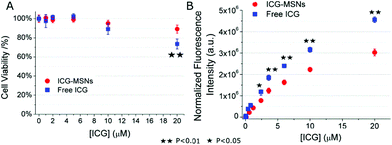
Prior to ICG-MSNs in vivo application, cell toxicity studies were carried out in vitro in J774A.1 murine macrophage cultures. 1.5 × 106 cells were plated in 6 cm Petri dishes overnight and successively incubated for 3 h at 37 °C, 5% CO2 in the presence of different amounts of ICG-MSNs (in a range corresponding to 0–20 μM of ICG). As a control, J774A.1 cells were incubated in the presence of free ICG at the same concentrations present in MSNs. After the incubation, cells were extensively washed with fresh PBS, detached by means of a scraper and counted by using a trypan blue exclusion assay. Cell viability was evaluated with respect to control unlabeled samples. As reported in Fig. 3A, cell viability is ca. 100% up to a [ICG] = 6 μM for both free ICG and ICG-MSNs. At higher concentrations, ICG-MSNs showed a lower toxicity with respect to free ICG (e.g. 11% vs. 27% at 20 μM, Fig. 3A).
The experiments were repeated in quadruplicate and mean ± SD values are reported. A two-tailed Student's t-test was performed. Furthermore, to double check count results, the cells were centrifuged and the pellets suspended into 250 μL of PBS and lysed by sonication (30 s, power 30%, 20 kHz). Then, the total protein content was evaluated by the Bradford assay and used as an indirect evaluation of cell number in each sample. The same samples were used to evaluate the uptake efficiency. In cell lysates, the fluorescence was measured by using a Horiba FluoroMax Fluorimeter and normalized by considering the relative cell number of each sample. Normalized fluorescence intensity (a.u.) was plotted against the concentration of ICG-MSNs or free ICG values in the incubation medium (Fig. 3B), showing that ICG entrapped inside MSNs is less efficiently sequestered by macrophages with respect to free ICG (at the tested concentrations). Consequently, ICG-MSN blood availability is longer and they can be detected in vivo for a longer time compared to free ICG.
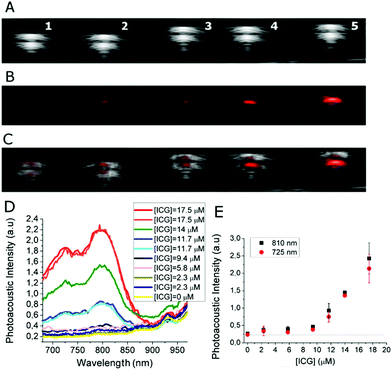
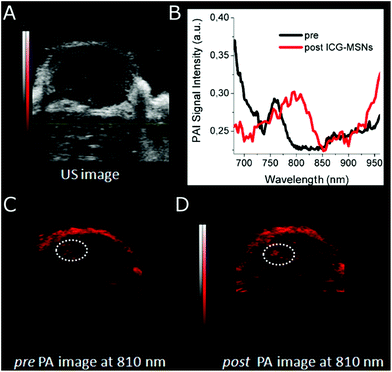
Then, photoacoustic properties of ICG-MSNs were analyzed by using a VisualSonics Vevo 2100 LAZR Imaging Station (VisualSonics, Inc., Toronto, Canada) equipped with a LZ250 transducer operating at 21 MHz. For in vitro PAI, ICG-MSN suspensions were loaded onto plastic capillaries surrounded by 1% agarose gel (Fig. 4A–C). The acquisition of PAI spectra shows that these nanoparticles display two PA peaks at 725 and 810 nm (Fig. 4D). For this system the detection threshold (i.e. the minimum concentration of probe needed to have a PA signal higher than the background, herein represented by MSNs w/o ICG) corresponds to ca. 5–6 μM of ICG (Fig. 4E). Remarkably, when ICG is localized inside MSNs the PAI efficiency increases by almost 4 times compared to that of free ICG (+370% signal intensity; ca. one order of magnitude considering the area under curve, Fig. 5).
The strong intermolecular interaction among ICG molecules inside MSN pores, responsible for the fluorescence quenching, translates into photothermal conversion by non-radiative decay and thus to an increase of the photoacoustic effect. This explanation finds support in the behavior of ICG-loaded liposomes that generate a higher quantum yield (Table 1) and then do not increase the PAI effect with respect to free ICG.
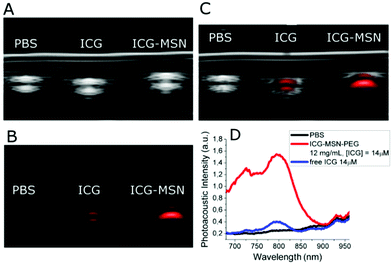
Interestingly, a clear role in the generation of an efficient PAI effect is played by the amount of ICG molecules present in the MSNs. It is known that the molar absorption extinction coefficient (ε) of ICG in solution decreases at high concentrations as a consequence of aggregation phenomena,13 in our case, an analogous effect is observed by comparing three ICG loadings in MSN (0.8, 1.7 and 3.7 μmol g−1, respectively, Table S1†). When the amount of ICG encapsulated inside MSN pores is too high, the ε is very low and the PAI response is weak as a consequence of the low absorption capability (Fig. S11†). Finally, as a proof of concept of the in vivo applicability of this probe, mice bearing subcutaneous tumors (US image in Fig. 6A) were i.v. injected with ICG-MSNs (at the dose of 120 mg MSNs per kg) and PA images of the tumor region were acquired ca. 20 min post injection. For this system, as previously reported,25 a hepatic accumulation and excretion is expected.
Upon ICG-MSNs administration, an overall PAI (λ = 810 nm) signal enhancement was observed inside the tumor region (Fig. 6C and D). A quantitative calculation of the signal enhancement was carried out in a small portion of the tumor (ROI manually drawn and highlighted by dotted line in Fig. 6C and D) that corresponds to ca. 25%. The differences between pre and post ICG-MSN administration were clearly detectable by looking at the PA spectra inside dotted ROI. Before the administration of the nanoparticles, a typical PA spectrum of hemoglobin was observed (Fig. 6B). In particular, a large amount of deoxygenated hemoglobin was present, as typical of tumors at late development stages. Conversely, the PA spectrum acquired after the administration of the photoacoustic nanoprobe, showed the expected signal due to the presence of ICG-particles entrapped in the MSN channels.
Conclusions
In summary, it has been shown that ICG can dramatically change its optical and PA properties depending on its localization (e.g. in solution or inside MSN pores or within the inner cavity of a liposome) and on its actual concentration inside the pores of the nanoparticle. Thus, the confinement of ICG molecules into MSNs allowed to obtain a PAI effect much greater (ca. 400% higher) than free ICG and ICG-loaded liposomes. This property is likely to be associated with the strong interaction of ICG molecules inside MSNs that quenches the fluorescence by increasing the photothermal conversion of the absorbed light. However, we observed that, when the amount of encapsulated ICG is too high (e.g. in the 3.7 μmol g−1 sample), the molar absorption extinction coefficient becomes too low thus making the system not suitable to generate a PAI signal.Moreover, the reported ICG-MSNs efficiently stabilize ICG and reduce cell toxicity and uptake by macrophages, being thus more suitable for in vivo experiments. Further studies will be directed to improve the ICG confinement into the silica pores, thereby preventing partial release when the particles are in contact with serum proteins (HSA). Then, with the optimized system, a combination of the enhanced photostability and photoacoustic signal with the ability of MSNs to be functionalized with targeting vectors or other imaging reporters, would allow the future development of efficient molecular imaging PAI probes.
Acknowledgements
We gratefully acknowledge the support of AIRC (IG-GRANT, S. A.), MIUR (PRIN 2012; project 2012SK7ASN_002) and Compagnia di San Paolo (CSP-2014 THERASIL Project, F. C., L. T.). G. F. and E. D. G. were supported by FIRC (Fondazione Italiana per la Ricerca sul Cancro AIRC) fellowships.Notes and references
- J. Müller, A. Wunder and K. Licha, Recent Results Cancer Res., 2013, 187, 221–246 CrossRef.
- S. Henschen, M. W. Busse, S. Zisowsky and B. Panning, J. Med., 1993, 24, 10–27 CAS.
- A. Scerrati, G. M. Della Pepa, G. Conforti, G. Sabatino, A. Puca, A. Albanese, G. Maira, E. Marchese and G. Esposito, Clin Neurol. Neurosurg., 2014, 124, 106–113 CrossRef CAS PubMed.
- P. E. Stanga, J. I. Lim and P. Hamilton, Ophthalmology, 2003, 110, 15–21 CrossRef PubMed.
- W. Polom, M. Markuszewski, Y. S. Rho and M. Matuszewski, Cent. Eur. J. Urol., 2014, 67, 142–148 Search PubMed.
- (a) H. Takeuchi and Y. Kitagawa, Dig. Surg., 2013, 30, 104–111 CrossRef PubMed; (b) D. Pan, X. Cai, C. Yalaz, A. Senpa, K. Omanakuttan, S. A. Wickline, L. V. Wang and G. M. Lanza, ACS Nano, 2012, 6, 1260–1267 CrossRef CAS PubMed.
- V. Ntziachristos and D. Razansky, Chem. Rev., 2010, 110, 2783–2794 CrossRef CAS PubMed.
- (a) L. V. Wang and J. Yao, Nat. Methods, 2016, 17, 627 CrossRef PubMed; (b) L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS PubMed; (c) S. Zackrisson, S. M. W. Y. van de Ven and S. S. Gambhir, Cancer Res., 2014, 74, 979–1004 CrossRef CAS PubMed; (d) V. Ntziachristos and D. Razansky, Recent Results Cancer Res., 2013, 187, 133–150 Search PubMed; (e) A. Taruttis, G. M. van Dam and V. Ntziachristos, Cancer Res., 2015, 75, 1548–1559 CrossRef CAS PubMed; (f) G. M. Lanza, Contrast Media Mol. Imaging, 2011, 6, 331 CrossRef CAS PubMed.
- (a) S. Hu and L. V. Wang, J. Biomed. Opt., 2010, 011101 CrossRef PubMed; (b) G. C. Langhout, D. J. Grootendorst, O. E. Nieweg, M. W. Wouters, J. A. van der Hage, J. Jose, H. van Boven, W. Steenbergen, S. Manohar and T. J. Ruers, Int. J. Biomed. Imaging, 2014, 163652 Search PubMed; (c) K. Jansen, M. Wu, A. F. van der Steen and G. van Soest, Opt. Express, 2013, 21, 21472–21484 CrossRef PubMed.
- J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639 CrossRef CAS PubMed.
- (a) G. P. Luke, D. Yeager and S. Emelianov, Ann. Biomed. Eng., 2012, 40, 422–437 CrossRef PubMed; (b) D. Wu, L. Huang, M. S. Jiang and H. Jiang, Int. J. Mol. Sci., 2014, 15, 23616–23639 CrossRef PubMed; (c) D. Pan, B. Kim, L. V. Wang and G. M. Lanza, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2013, 5, 517–543 CrossRef CAS PubMed.
- D. Wu, L. Huang, M. S. Jiang and H. Jiang, Int. J. Mol. Sci., 2014, 15, 23616–23639 CrossRef PubMed.
- M. L. J. Landsman, G. Kwant, G. A. Mook and W. G. Zijlstra, J. Appl. Phys., 1976, 40, 575 CAS.
- S. Uthaman, J. Bom, H. S. Kim, J. V. John, H. Bom, S. Kim, J. Min, I. Kim and I. Park, J. Biomed. Mater. Res., Part B, 2016, 104, 825–834 CrossRef CAS PubMed.
- (a) C. Zheng, M. Zheng, P. Gong, D. Jia, P. Zhang, B. Shi, Z. Sheng, Y. Ma and L. Cai, Biomaterials, 2012, 33, 5603–5609 CrossRef CAS PubMed; (b) N. H. Wang, G. B. Liu, X. J. Gong, D. H. Hu, R. D. Lin, Z. H. Sheng, C. F. Zheng, M. Yan, J. Q. Chen and L. T. Cai, Nanoscale, 2014, 6, 14270–14279 RSC.
- Z. Sheng, D. Hu, M. Zheng, P. Zhao, L. Huilong, D. Gao, P. Gong, G. Gao, P. Zhang, Y. Ma and L. Cai, ACS Nano, 2014, 8, 12310–12322 CrossRef CAS PubMed.
- N. Beziere, N. Lozano, A. Nunes, J. Salichs, D. Queiros, K. Kostarelos and V. Ntziachristos, Biomaterials, 2015, 37, 415–424 CrossRef CAS PubMed.
- P. Fabrice, P. Navarro, T. Delmas, F. Mittler, A. C. Couffin, F. Vinet and I. Texier, J. Biomed. Opt., 2011, 16, 096013 CrossRef PubMed.
- A. de la Zerda, S. Bodapati, R. Teed, S. Y. May, S. M. Tabakman, Z. Liu, B. T. Khuri-Yakub, X. Chen, H. Dai and S. S. Gambhir, ACS Nano, 2012, 6, 4694–4701 CrossRef CAS PubMed.
- P. Sharma, N. E. Bengtsson, G. A. Walter, H. B. Sohn, G. Zhou, N. Iwakuma, H. Zeng, S. R. Grobmyer, E. W. Scott and B. M. Moudgil, Small, 2012, 8, 2856–2868 CrossRef CAS PubMed.
- L. Zhiguo, P. Rong, Y. Lun, X. Zhang, C. Yang, F. Guo, Y. Zhao, K. Zhou, W. Wang and W. Zeng, Mol. Pharmaceutics, 2015, 12, 3119–3128 CrossRef PubMed.
- S. Aime, G. Ferrauto, E. Di Gregorio, L. Tei, F. Carniato and M. Botta, IT102016000023103, 2016 Search PubMed.
- K. Suzuki, K. Ikari and H. J. Imai, J. Am. Chem. Soc., 2004, 126, 462–463 CrossRef CAS PubMed.
- F. Carniato, L. Tei, M. Cossi, L. Marchese and M. Botta, Chem. – Eur. J., 2010, 16, 10727–10734 CrossRef CAS PubMed.
- H. Lee, S. H. Cheng, Y. J. Wang, Y. C. Chen, N. T. Chen, J. Souris, C. T. Chen, C. Y. Mou, C.-S. Yang and L.-W. Lo, Adv. Funct. Mater., 2009, 19, 215–222 CrossRef.
- I. M. Rio-Echevarria, F. Selvestrel, D. Segat, G. Guarino, R. Tavano, V. Causin, E. Reddi, E. Papini and F. J. Mancin, J. Mater. Chem., 2010, 20, 2780–2787 RSC.
- Y. Li, T. Wen, R. Zhao, X. Liu, X. T. Ji, H. Wang, X. Shi and J. Shi, ACS Nano, 2014, 8, 11529–11542 CrossRef CAS PubMed.
- I. Miletto, E. Bottinelli, G. Caputo, S. Coluccia and E. Gianotti, Phys. Chem. Chem. Phys., 2012, 14, 10015–10021 RSC.
- Q. Zheng, S. Jochusch, Z. Zhou and S. C. Blanchard, Photochem. Photobiol., 2014, 90, 448–454 CrossRef CAS PubMed.
- (a) A. Faraone, L. Liu, C. Y. Mou, P. C. Shih, J. R. D. Copley and S. H. Chen, J. Chem. Phys., 2003, 119, 3963–3971 CrossRef CAS; (b) F. Carniato, L. Tei, W. Dastrù, L. Marchese and M. Botta, Chem. Commun., 2009, 1246–1247 RSC.
- A. K. Kirchherr, A. Briel and K. Mäder, Mol. Pharmaceutics, 2009, 6, 480–491 CrossRef CAS PubMed.
- E. D. Moody, P. J. Viskari and C. L. Colyer, J. Chromatogr., B: Biomed. Appl., 1999, 729, 55–64 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Methods (synthesis of mesoporous support (MSNs), impregnation of ICG in the silica nanoparticles and PEG anchoring, tools for MSN characterization, ICG-loaded liposomes, cell cultures, mouse models, in vitro and in vivo photoacoustic experiments), supplementary results (HR-TEM micrographs, DLS hydrodynamic radius, surface ζ-potential, X-ray pattern, pore diameter distribution, thermogravimetric analysis, UV-visible, FT-IR, photoluminescence spectra of MSNs, and relative PAI signal of ICG-MSNs with a different ICG loading) and supplementary references. See DOI: 10.1039/c6nr08282c |
| This journal is © The Royal Society of Chemistry 2017 |