Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Can the analyte-triggered asymmetric autocatalytic Soai reaction serve as a universal analytical tool for measuring enantiopurity and assigning absolute configuration?
Christopher J.
Welch
*a,
Kerstin
Zawatzky
a,
Alexey A.
Makarov
a,
Satoshi
Fujiwara
b,
Arimasa
Matsumoto
c and
Kenso
Soai
*c
aDepartment of Process Research & Development, Merck Research Laboratories, Rahway, NJ, USA. E-mail: christopher_welch@merck.com
bDepartment of Applied Chemistry, Tokyo University of Science Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan
cResearch Center for Chirality, Research Institute for Science and Technology, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp
First published on 28th September 2016
Abstract
An investigation is reported on the use of the autocatalytic enantioselective Soai reaction, known to be influenced by the presence of a wide variety of chiral materials, as a generic tool for measuring the enantiopurity and absolute configuration of any substance. Good generality for the reaction across a small group of test analytes was observed, consistent with literature reports suggesting a diversity of compound types that can influence the stereochemical outcome of this reaction. Some trends in the absolute sense of stereochemical enrichment were noted, suggesting the possible utility of the approach for assigning absolute configuration to unknown compounds, by analogy to closely related species with known outcomes. Considerable variation was observed in the triggering strength of different enantiopure materials, an undesirable characteristic when dealing with mixtures containing minor impurities with strong triggering strength in the presence of major components with weak triggering strength. A strong tendency of the reaction toward an ‘all or none’ type of behavior makes the reaction most sensitive for detecting enantioenrichment close to zero. Consequently, the ability to discern modest from excellent enantioselectivity was relatively poor. While these properties limit the ability to obtain precise enantiopurity measurements in a simple single addition experiment, prospects may exist for more complex experimental setups that may potentially offer improved performance.
Introduction
Measurement technologies are vitally important for the progress and development of chemistry. The preferred enabling analytical technologies for organic synthesis, e.g. nuclear magnetic resonance spectroscopy (NMR) and reversed phase high performance liquid chromatography-mass spectrometry (RP-HPLC-MS) are highly universal and user-friendly, requiring only minimum assay development and optimization for particular molecules. On the other hand, stereochemical analysis is currently carried out by enantioselective supercritical fluid chromatography (SFC),1 vibrational circular dichroism2 and other techniques, where, despite recent improvement in speed and simplification, considerable method development1 or computational analysis3 is still required to achieve comprehensive stereochemical analysis of organic molecules. Ideally, stereochemical analysis for supporting synthetic chemistry would become as universal and streamlined as achiral analysis.The Soai reaction is an extraordinary asymmetric autocatalytic system4 that has been shown to be ‘triggerable’ by a wide variety of enantioenriched substances5 ranging from chiral hydrocarbons6 to compounds that are chiral by virtue of isotopic substitution7 to macroscopic chiral objects comprised of achiral materials8,9 (Fig. 1). This remarkable property has led to the recognition that the Soai reaction could serve as a generic enantioenrichment detector that could be useful for robotic planetary exploration.10
The ability of a reporter reaction to relay stereochemical information from any given molecule would be a significant breakthrough, offering researchers a universal stereochemical translator akin to the Babel fish described in Douglas Adams’, The Hitchhiker's Guide to the Galaxy11 (“…if you stick one in your ear, you can instantly understand anything said to you in any form of language…”). In such a system, dedicated instrumentation optimized for rapid analysis of the enantiopurity of the product of the reporter reaction would enable enantiopurity analysis for any substance, thereby avoiding the need for the development of tailor-made methods for individual compounds. Such an approach could potentially lead to powerful, low cost, robust and miniaturized ‘stereochemistry detectors’ that could be integrated into other analytical devices (it is important to point out that in order to determine the enantiomeric excess for a given compound, the measurement of bulk ‘stereochemistry’ provided by such a detector would have to be combined with an independent measurement of the concentration of the compound under study).
In order to fulfill the function of a universal stereochemistry detector, several important criteria should be met:
- the stereochemistry indicator reaction should be truly universal, being triggerable by any enantioenriched compound to a similar degree, such that the outcome of triggering is not dominated by trace impurities;
- the triggered response should be relatable to the amount of enantioenriched compound present in the sample, thereby allowing for the measurement of enantiopurity;
- the absolute stereochemical sense of the reaction (tendency to form either the R or S product for a given enantioenriched analyte) should be reproducible, and should, ideally, follow rules such that unknown compounds belonging to certain classes or families of molecules would behave in a predictable fashion;
- the reaction should be easy to perform for non-specialist users, ideally being amenable to an automated approach using stable, prepackaged reagents that can be dispensed in a single addition without the use of cryogenics, inertion or special handling;
- ideally, a single analysis would be used to make a stereochemical determination, avoiding the requirement for replicate experiments for each determination.
Although the Soai reaction has been intensively investigated in many laboratories around the world,12–14 several outstanding questions remain for its practical utilization as a stereochemistry indicator reaction. In this study we investigate the potential of the Soai reaction to be used as a general reporter reaction for routine laboratory tasks associated with the study of stereochemistry, namely the determination of absolute configuration and the measurement of enantiopurity.
Results and discussion
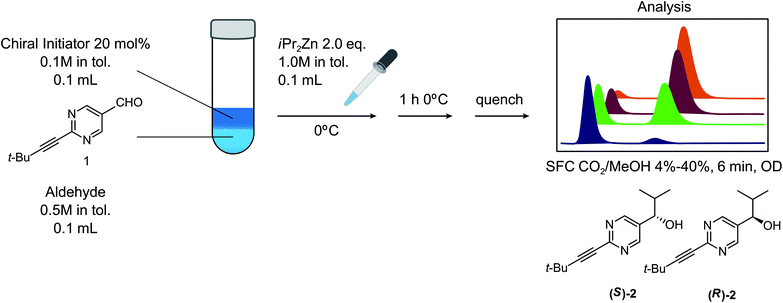
Based upon literature reports, it is already known that the Soai reaction fulfills many of the requirements for a universal stereochemistry indicator reaction that can be triggered by a variety of enantioenriched analytes. There are actually a number of different Soai reactions, with several combinations of starting materials, reagents and conditions known to lead to the triggerable autocatalysis with asymmetric amplification behavior that is crucial for the desired effect.15,16 We chose the reagents and conditions illustrated in Fig. 1 for our study. While cryogenic conditions are sometimes used for the Soai reaction, the reaction has been performed typically at 0 °C.15,17 Several solvent systems have been used for the Soai reaction, including toluene, hexane and diethylether. For the purpose of our investigation, we confined our investigations to toluene. We chose a concentration of 0.5 M for the substrate, with the addition of 2.0 eq. of Zn(iPr)2 and the addition of 20 mol% of the triggering reagent, in keeping with previous reports. We investigated the order of addition, finding similar results when Zn(iPr)2 was added to the chiral initiator followed by aldehyde, 1, or when Zn(iPr)2 was added to a mixture of initiator and aldehyde. The use of the reagent, Zn(iPr)2, would seem to defy one of our stated criteria – the preference for reaction conditions that avoid the use of cryogenics, inertion and special handling. While a reagent with a propensity to burst into flames when exposed to a humid atmosphere does not at first seem to meet these criteria, we reasoned that an approach where this highly sensitive reagent could be packaged within breakable microampules18 could potentially be used, provided other criteria were met. The reaction protocol used by Soai and co-workers has generally involved multiple reagent additions over time, in order to maximize the extent of asymmetric autocatalytic amplification through multiple ‘feedback’ cycles. We decided to confine our investigations to a single addition of reagents, which, while affording lower enantioenrichment in the Soai product, 2, is more compatible with the objective of obtaining results where some possibility exists for accurate determination of the amount of the enantioenriched triggering substances present in the sample.A variety of methods have been reported for the analysis of the enantiopurity of product alcohol, 2, obtained in these studies.19 We opted to use an SFC method with an analysis time of 6 min, where the two enantiomeric alcohols are well separated from the starting material and byproducts. Faster methods have been previously reported, but require sample pretreatment to remove the residual starting material. If warranted, a sub-minute assay time could likely be developed, but we reasoned that the current method would be suitable for investigating proof of principle for the use of the Soai reaction as a general stereochemical indicator.
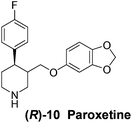
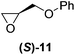
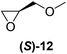
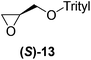
With our experimental protocol in hand, we carried out an investigation of the generality of triggering of the Soai reaction. We explored the ability of a variety of different enantioenriched substances to trigger the reaction, by carrying out each reaction in duplicate or triplicate, and using 20 mol% of the triggering substance (Table 1). We evaluated the triggering strength for a variety of compounds, including species expected to survive the relatively harsh reaction conditions unscathed (e.g. amino alcohols) as well as other compounds that might prove more vulnerable to the reaction or degradation during the course of the reaction (e.g. ketones, esters and sulfoxides). In addition, several members from particular compound families were chosen to determine general trends in the absolute sense of stereochemical induction.
| Chiral initiator | Yielda (%) | eeb (%) | Config. |
|---|---|---|---|
| a Yields were determined by GC using n-tridecane as an internal standard. b ee of the Soai product alcohol 2. Determined by enantioselective SFC. | |||
|
>99 | 87 | S |
| >99 | 86 | S | |
| >99 | 87 | S | |
|
76 | 7 | R |
| 88 | 16 | R | |
|
84 | 58 | S |
| 89 | 60 | S | |
|
81 | 84 | S |
| 84 | 76 | S | |
|
97 | 72 | S |
| 96 | 77 | S | |
|
97 | 50 | R |
| 94 | 50 | R | |
|
98 | 92 | R |
| 92 | 92 | R | |
|
>99 | 61 | R |
| 97 | 38 | R | |
|
>99 | 38 | S |
| >99 | 36 | S | |
| >99 | 44 | S | |
|
>99 | 90 | R |
| >99 | 84 | R | |
| >99 | 90 | R | |
|
97 | 55 | S |
| >99 | 48 | S | |
| >99 | 53 | S | |
|
>99 | 48 | R |
| >99 | 57 | R | |
| >99 | 64 | R | |
|
>99 | 19 | S |
| >99 | 26 | S | |
|
99 | 85 | R |
| 95 | 86 | R | |
As the exact mechanism whereby enantioenriched triggering molecules lead to asymmetric induction in the Soai reaction is largely unknown, we were uncertain of whether trends could be observed for the absolute sense of stereochemical induction for different types of triggering molecules, i.e. whether (R) triggering agents from particular families would give rise to the (R) or (S) Soai product, 2, or whether every unique molecule would behave independently.
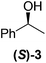
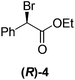
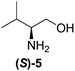
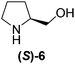
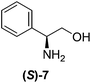
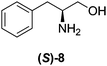
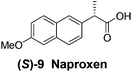
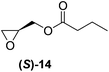
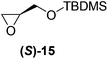
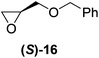
Although a more comprehensive study with a larger sample set would be required to establish conclusively, there does seem to be a trend for compounds within the same family to bias the absolute configuration of product formation in the same direction. For example, the amino alcohols valinol 5, prolinol 6 and phenylglycinol 7 all lead to the enantioselective formation of the Soai product, 2, with the same absolute configuration as the triggering substance (i.e. S-amino alcohol gives rise to S-2). Interestingly, phenylalaninol 8, affords the opposite enantiomer in excess. The glycidol ethers and esters (compounds 11–16) show a more complicated relationship, with some members affording heterochiral enantioenrichment and others showing homochiral enrichment. Consequently, for any given new compound it is difficult to predict with certainty whether the Soai reaction will afford heterochiral or homochiral enantioenrichment, but once this is known, the Soai reaction can be used for rapid screening applications to identify reactions producing the desired enantiomer. The results show that while most of the tested initiators afford some enantioenrichment in the Soai reaction, the degree of enantioenrichment can vary considerably, ranging from a high degree of about 90% in the best cases (naproxen 9 and 2-phenyl ethanol 3) to some instances where very little asymmetric induction in product formation was obtained (e.g., compound 4). The reproducibility of the reaction in parallel runs is generally good, suggesting that a single test reaction could potentially be sufficient for assessing the triggering strength.
While we were pleased with the apparent universality of enantioselective triggering of the Soai reaction within our small set of analytes, we were somewhat disappointed with the apparent variation in triggering strength among these different test compounds. Ideally, an indicator reaction would work equally for the vast majority of enantioenriched compounds of interest. It could be possible that further optimization of reaction conditions or solvents could lead to improved performance, but we reasoned that since no analytical method is 100% universal, acceptance of some variation in triggering strength could be tolerated.
We next investigated how the ee of the Soai product, 2, depends upon the mol% of the initiator to determine if the 20 mol% range chosen for our initial experiments could be improved. We investigated three chiral initiators with medium to strong triggering efficiency (valinol 5, prolinol 6, and methyl glycidyl ether 12). Fig. 2 shows plots of the %ee of the Soai product, 2, vs. concentration of the enantiopure initiator. It can be seen that for all the chosen compounds the curve follows a similar shape with almost no enrichment at 0.1 mol% modest enrichment at 1 mol% and nearly the same enrichment at 5, 10 or 20 mol%. This result suggests that for stronger initiators, the level of the triggering agent could perhaps be reduced – a good thing since material demand for an analytical reporter assay should be as small as possible. It also illustrates that below a certain concentration, the amount of enantioenrichment afforded by even a strong triggering agent can be quite low.
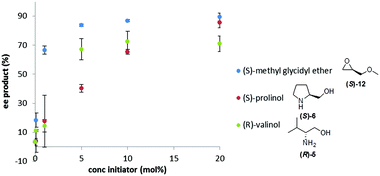 | ||
| Fig. 2 Influence of mol% of the enantiopure initiator on %ee of the Soai product, 2, formed in the reaction. Conditions as described in Fig. 1. | ||
Ideally, a general reporter assay would have the ability to differentiate samples of known concentration but unknown enantiopurity. We therefore investigated the effect of the enantiopurity of the initiator (at 20 mol%) on the enantiopurity of the product, 2, produced in the reaction. We investigated both methyl glycidyl ether 12 and prolinol 6 as initiators, by varying enantiopurity as follows: (100%(R), 95%, 80%, 40%, 20%, 10%(R), 10%(S), 20%, 40%, 80%, 95%, 100%(S)), and performing each of the reactions in triplicate (Fig. 3).
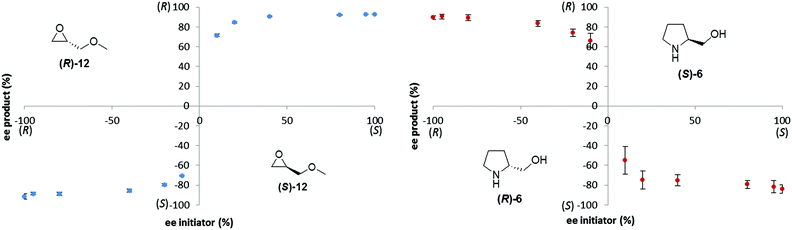 | ||
| Fig. 3 Influence of enantiopurity of the triggering substance (at 20 mol%) on the enantiopurity of the Soai product, 2, formed in the reaction. | ||
Both plots show good reproducibility and very similar shape of the curves, however even very low ee's of the triggering reagent provide relatively strong enantioenrichment of the Soai product (2). Consequently, the ability to discriminate a modest enantioenrichment (e.g. 50% ee) from a very good enantioenrichment (e.g. 98% ee) would be quite limited using this method. The results show an ‘all or nothing’ behavior that is driven by asymmetric autocatalytic amplification of chirality20 and that is similar to the ‘majority rules’ phenomenon of polymer helicity originally reported by Green21,22 and subsequently studied by Yashima23 and others. While some lack of precision in measuring enantiopurity can often be sacrificed for speed and convenience in high throughput screening applications, the inability to discriminate excellent from average enantiopurity would be a very serious drawback. A possible mitigation strategy could involve the ‘titrating with the opposite enantiomer’ strategy described by Seifert and Anslyn.24 In this approach, addition of known amounts of the opposite enantiomer of the product can be used to titrate to a null signal, thereby allowing precise estimation of enantiopurity using what is essentially a ‘not racemic’ sensor.
To investigate the interplay between the concentration and %ee of the triggering substance on the outcome of the Soai reaction, reactions were carried out in duplicate at a variety of (S)-prolinol concentrations and enantiopurities (Fig. 4). The response of the %ee curves is extremely sensitive to the concentration, showing an ‘all or nothing’ type of response that again makes the application of the method to precisely determine the enantiopurity somewhat difficult.
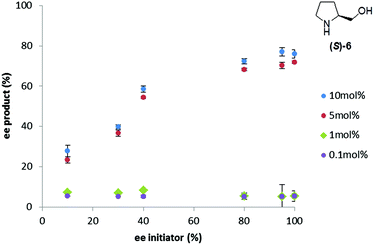 | ||
| Fig. 4 Influence of the prolinol concentration and enantiopurity on the enantiopurity of the Soai product, 2, formed in the reaction. | ||
While these studies show a good degree of generality in the use of the Soai reaction as a general indicator reaction for the study of enantioenrichment and absolute configuration, it is clear that the ‘hair trigger’ responsiveness of the reaction makes the prospects for obtaining a precise measurement of enantioenrichment in a single measurement quite challenging. While more complex experimental approaches involving the analysis of multiple reactions to titrate enantiopurity are beyond the scope of the present study, there is some prospect that such an approach could be both successful and amenable to automation. For example, one could imagine a converted HPLC apparatus where the flow of the reagents for the Soai reaction would be combined with the injected samples, alone or in combination with the standards of a known composition, enabling the automated titration that would be necessary to precisely measure the enantiopurity.
Experimental
General
Unless specified, all reagents and starting materials were purchased from commercial sources and used as received without further purification.General procedure for asymmetric autocatalysis
A solution of pyrimidine-5-carbaldehyde 1 and internal standard n-tridecane (0.5 M in toluene, 0.05 mmol) was added to the solution of the chiral initiator (0.1 M in toluene, 0.1 mL) in a dry glass tube. After cooling to 0 °C, a solution of i-Pr2Zn (0.1 mmol, 0.1 mL of 1 M toluene solution) was added and the mixture was stirred for 1 h. Subsequently, the reaction was quenched using 1 M NH4Cl/NH4OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (0.5 mL), the phases were separated and the organic layer was analyzed by GC and chiral SFC.
1) (0.5 mL), the phases were separated and the organic layer was analyzed by GC and chiral SFC.
Instrumentation
GC analysis was performed on a Shimadzu GC-2010 with FID detector using N2 as a carrier gas. The aldehyde and alkanol were separated by using an ZB-5HT capillary column (30 m × 0.25 mm) with the temperature program ranging from 50 to 300 °C in 10 °C per min.Chiral SFC analysis was performed on Waters Acquity UPC2 (Waters Milford, MA, USA) systems equipped with a fluid delivery module (a liquid CO2 pump and a modifier pump), a sample manager, an FL autosampler, a photodiode array detector, and MassLynx software.
The two enantiomers were separated on a Chiralpak OD-3 (100 mm × 3.0 mm I.D., 3 μm) by gradient elution at a flow rate of 3 mL min−1. The SFC eluents were solvent A (CO2) and solvent B (25 mM isobutylamine in MeOH). The mobile phases were programmed as follows: linear gradient from 1% to 10% B in 3 min and from 10 to 40% B until 6 min. The column and samples were maintained at a temperature of 40 °C and 20 °C, respectively. Retention times for the product alcohols were 2.78 min for (S)-2 and 3.06 min for (R)-2.
Conclusion
In this study we have investigated the potential utility for the enantioselective autocatalytic Soai reaction to be exploited for the generic measurement of enantiopurity and absolute configuration. Good generality for the reaction across a small group of test analytes was observed, consistent with literature reports suggesting a diversity of compound types that can influence the stereochemical outcome of this reaction. Some trends in the absolute sense of stereochemical enrichment were noted, suggesting the possible utility of the approach for assigning absolute configuration to unknown compounds, by analogy with closely related species with known outcomes. Considerable variation in triggering strength was observed, an undesirable characteristic when dealing with mixtures containing minor impurities with strong triggering strength in the presence of major components with weak triggering strength. The strong tendency of the reaction toward a ‘majority rules’ type of behavior means that the reaction is the most sensitive for detecting enantioenrichment close of zero, while the ability to discern modest from excellent enantioselectivity is relatively poor. While these properties will limit the ability to obtain precise enantiopurity measurements in a simple single addition experiment, prospects exist for more complex experimental setups involving multiple measurements with addition of standards that may potentially offer improved performance.Acknowledgements
We thank the MRL Postdoctoral Research Fellows Program for a fellowship (K. Z.), Grant-in-aid for Scientific Research (JSPS KAKENHI) and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016.Notes and references
- W. Schafer, T. Chandrasekaran, Z. Pirzada, C. Zhang, X. Gong, M. Biba, E. Regalado and C. J. Welch, Chirality, 2013, 25, 799–804 CrossRef CAS PubMed.
- T. B. Freedman, X. Cao, R. K. Dukor, L. A. Nafie and A. Laurence, Chirality, 2003, 15, 743–758 CrossRef CAS PubMed.
- E. C. Sherer, C. Heechon-Lee, J. Shpungin, J. F. Cuff, C. Da, R. Ball, R. Bach, A. Crespo, X. Gong and C. J. Welch, J. Med. Chem., 2014, 57, 477–494 CrossRef CAS PubMed.
- K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767–768 CrossRef CAS.
- (a) K. Soai, T. Shibata and I. Sato, Acc. Chem. Res., 2000, 33, 382–390 CrossRef CAS PubMed; (b) M. Avalos, R. Babiano, P. Cintas, J. L. Jiménez and J. C. Palacios, Chem. Commun., 2000,(no. 11), 887–892 RSC; (c) J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776–5777 CrossRef CAS PubMed; (d) B. Barabás, J. Tóth and G. Pályi, J. Math. Chem., 2010, 48, 457–489 CrossRef; (e) G. Lente, Symmetry, 2010, 2, 767–798 CrossRef CAS; (f) J.-C. Micheau, C. Coudret and T. Buhse, in The Soai Reaction and Related Topic, ed. G. Palyi, C. Zucchi and L. Caglioti, Accad. Nazl. Sci. Lett. Arti, Editioni Artestampa, Modena, 2012, p. 169 Search PubMed; (g) A. F. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800–12826 CrossRef CAS PubMed; (h) O. Fülöp and B. Barabás, J. Math. Chem., 2016, 54, 10–17 CrossRef; (i) T. Gehring, M. Quaranta, B. Odell, D. G. Blackmond and J. M. Brown, Angew. Chem., Int. Ed., 2012, 51, 9539–9542 CrossRef CAS PubMed; (j) I. D. Gridnev and A. K. Vorobiev, Bull. Chem. Soc. Jpn., 2015, 88, 333–340 CrossRef; (k) K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1–31 CrossRef CAS; (l) T. Kawasaki and K. Soai, Bull. Chem. Soc. Jpn., 2011, 84, 879–892 CrossRef CAS; (m) T. Kawasaki and K. Soai, Isr. J. Chem., 2012, 52, 582–590 CrossRef CAS; (n) T. Kawasaki, I. Sato, H. Mineki, A. Matsumoto and K. Soai, J. Synth. Org. Chem. Jpn., 2013, 71, 109–123 CrossRef CAS; (o) K. Soai and T. Kawasaki, Top. Organomet. Chem., 2013, 44, 261–279 CrossRef CAS; (p) A. Matsumoto, T. Abe, A. Hara, T. Tobita, T. Sasagawa, T. Kawasaki and K. Soai, Angew. Chem., Int. Ed., 2015, 54, 15218–15221 CrossRef CAS PubMed.
- I. Sato, R. Yamashima, K. Kadowaki, J. Yamamoto, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2001, 40, 1096–1098 CrossRef CAS.
- T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492–495 CrossRef CAS PubMed.
- I. Sato, K. Kadowaki, H. Urabe, J. H. Jung, Y. Ono, S. Shinkai and K. Soai, Tetrahedron Lett., 2003, 44, 721–724 CrossRef CAS.
- K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235–11236 CrossRef CAS.
- C. J. Welch and J. I. Lunine, Enantiomer, 2001, 6, 69 CAS.
- Douglas Adams, The Ultimate Hitchhiker's Guide to the Galaxy, Random House, New York, 2002 Search PubMed.
- D. G. Blackmond, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5732–5736 CrossRef CAS PubMed.
- I. D. Gridnev, J. M. Serafimov and J. M. Brown, Angew. Chem., Int. Ed., 2004, 43, 4884–4887 CrossRef CAS PubMed.
- M. Funes-Maldonado, B. Sieng and M. Amedjkouh, Org. Lett., 2016, 18, 2536–2539 CrossRef CAS PubMed.
- K. Soai, T. Kawasaki and A. Matsumoto, Chem. Rec., 2014, 14, 70–83 CrossRef CAS PubMed.
- K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643–3654 CrossRef CAS PubMed.
- T. Gehring, M. Busch, M. Schlageter and D. Weingand, Chirality, 2010, 22, E173–E182 CrossRef CAS PubMed.
- A. C. Sather, H. G. Lee, J. R. Colombe, A. Zhang and S. L. Buchwald, Nature, 2015, 524, 208–211 CrossRef CAS PubMed.
- C. J. Welch, M. Biba and P. Sajonz, Chirality, 2007, 19, 34–43 CrossRef CAS PubMed.
- T. Satyanarayana, S. Abraham and H. B. Kagan, Angew. Chem., Int. Ed., 2009, 48, 456–494 CrossRef CAS PubMed.
- M. M. Green, B. A. Garetz, B. Munoz, H. Chang, S. Hoke and R. G. Cooks, J. Am. Chem. Soc., 1995, 117, 4181–4182 CrossRef CAS.
- M. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook and S. Lifson, Science, 1995, 268, 1860–1866 CAS.
- R. Nonokawa and E. Yashima, J. Am. Chem. Soc., 2003, 125, 1278–1283 CrossRef CAS PubMed.
- H. M. Seifert, Y. B. Jiang and E. V. Anslyn, Chem. Commun., 2014, 50, 15330–15332 RSC.
| This journal is © The Royal Society of Chemistry 2017 |