Pd-mediated cross-coupling of C-17 lithiated androst-16-en-3-ol – access to functionalized arylated steroid derivatives†
Vanessa
Koch
a and
Stefan
Bräse
 *ab
*ab
aInstitute of Organic Chemistry, Karlsruhe Institute of Technology – Campus South, Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany. E-mail: stefan.braese@kit.edu
bInstitute of Toxicology and Genetics, Karlsruhe Institute of Technology – Campus North, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 22nd November 2016
Abstract
Herein, we report on Pd-mediated cross-coupling of vinyllithium steroids and aryl bromides to introduce various substituted aryls at C-17 of steroidal frameworks based on the structure of epi-androsterone. Compared to other C–C cross-couplings, this method turned out to be an easy and competitive access to biologically interesting C-17 modified steroids.
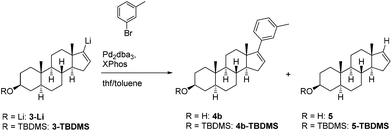
Palladium-catalysed cross-coupling reactions for C–C bond formation play a key role in all fields of organic synthesis.1–5 Among these, Suzuki–Miyaura coupling with organoboron as an organometallic nucleophile6–9 and Stille coupling (organotin reagent)10–15 are mainly used as they tolerate a huge range of substituents. However, the high toxicity of organostannanes is often undesirable in particular with regard to pharmaceutical and medical applications. To overcome this disadvantage many coupling reactions based on different “metal nucleophiles” such as magnesium (Kumada),16,17 zinc (Negishi)18 and even silicon (Hiyama-Denmark)19,20 were already examined. Although organolithium reagents are inexpensive and either commercially available or easily accessible via halogen–metal exchange or direct metallation, the application of organolithium compounds as cross-coupling reagents is not well established until now. Early studies on Pd-mediated C–C bond formation with organolithium reagents as nucleophiles by Murahashi demonstrated the limitations of this method resulting from the high reactivity and poor selectivity of organolithium reagents in general.21–23 To overcome these difficulties, Feringa et al.24,25 recently reported on a novel protocol under mild conditions avoiding the lithium halogen exchange as well as the competing homocoupling. Using Pd2dba3 and different phosphine ligands like XPhos or PtBu3 as the catalytic system enables the alkylation and arylation of different aryl halides as well as the arylation of different alkenylhalides by employing the corresponding organolithium reagents.24,25 This new methodology attracted our research interest to find a novel method for introducing various substituents at C-17 of a steroidal precursor within a few steps since many natural products based on a steroidal framework carry different residues at C-17. Applying this method should allow access to biologically active and important steroid analogues without using any toxic reagents. Herein, we present a fast and convenient method for introducing different substituted aryls at C-17 of a steroid framework via a direct cross-coupling of the easily accessible and in situ prepared vinyllithium steroid 3-Li.
The lithiated vinyl steroid 3-Li is easy accessible within 2 steps (Scheme 1) from commercially available epi-androsterone (1). Therefore epi-androsterone (1) was converted to the corresponding vinyl bromide 2-Br using first hydrazine to build the hydrazone and then NBS and pyridine yielding the vinyl bromide 2-Br in a good yield of 76%. Best results for lithiation of vinyl bromide 2-Br were achieved when using 3.1 equivalents of tert-butyllithium in dry THF.
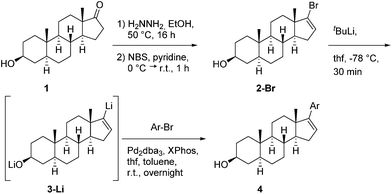 | ||
| Scheme 1 Synthesis of the lithiated steroid 3 and palladium-catalysed cross-coupling of 3 with aryl bromides. | ||
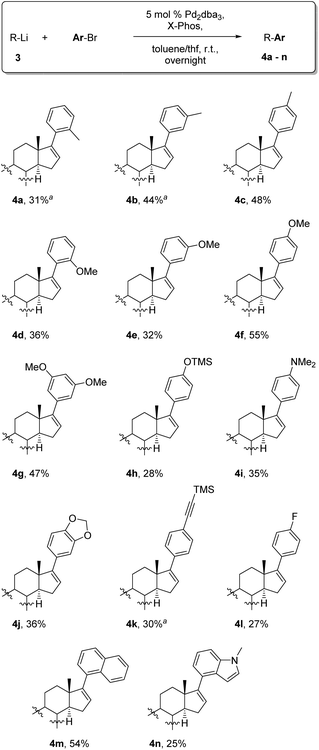
For our initial model reaction (Table 1) we selected 3-bromotoluene in a mixture of toluene and THF as the solvent system and Pd2dba3/XPhos as the catalytic system based on the work of Feringa et al.24,25 We started our investigations with the usage of a syringe pump to ensure a slow addition of the highly reactive organolithium reagent (entries 1–3) by varying the reaction time and temperature afterwards. By increasing the reaction time from 3 h to overnight, we were able to obtain at least 10% instead of traces of the desired product 4b which was isolated as a mixture with the defunctionalized steroid 5. By increasing the reaction temperature to 60 °C the ratio of the coupled product 4b and the defunctionalized steroid 5 could be drastically improved (entry 3). However, best yields were achieved if the freshly prepared lithium steroid was directly cannulated as THF solution at −78 °C (entry 4) which is reasonable due to the lower stability of 3-Li compared to the substrates of the Feringa group. Anyway, even with equimolar amounts of the catalyst system, yields could not be further improved indicating that the moderate yields are not a result of an interrupted catalytic cycle. To exclude that these rather moderate yields are due to effects caused by the deprotonated hydroxyl group of the steroid, the OH was protected with a TBDMS group and again subjected to the developed protocol giving product 4b-TBDMS in a better ratio, but unfortunately with no further enhancement of the yield (entry 5). Along with the described catalytic system, different palladium sources like Pd(PPh3)4 as well as alternative phosphine ligands like PtBu3 were tested but without further improvements. With the optimal conditions in hand, the scope of the reaction was examined (Table 2) including different substituted aryl bromides bearing electron-donating and electron-withdrawing groups as well as annulated systems. Regarding the electron-donating methyl (hyper-conjugation) and methoxy (+M-effect) group, moderate yields were achieved in the case of ortho- and meta-substituted bromotoluenes whereas para-substitution gave the arylated product in good yields of 48% (para-methyl, 4c) and 55% (para-methoxy, 4f).
| Entry | Starting material | Conditionsa | Ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Yield of 4 [%] |
|---|---|---|---|---|
| a Lithiated steroid 3/3-TBDMS (1.0 eq.) in dry THF was added to a solution of 3-bromotoluene (1.3 eq.), Pd2dba3 (5 mol%) and XPhos (20 mol%) in dry toluene and maintained at the appropriate temperature for the stated amount of time. | ||||
| 1 | 3 | Syringe pump (1 h) 3 h at r.t. | — | Traces |
| 2 | 3 | Syringe pump (1 h) overnight at r.t. | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 3.0 |
10 |
| 3 | 3 | Syringe pump (1 h) overnight at 60 °C | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.56 0.56 |
10 |
| 4 | 3 | Cannulation (30 min) overnight at r.t. | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.43 0.43 |
44 |
| 5 | 3-TBDMS | Cannulation (30 min) overnight at r.t. | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.28 0.28 |
33 |
| a This steroid could not be isolated purely (traces of the defunctionalized steroid 5 could not be completely removed via column chromatography). Yields were estimated via integration of the signals of the 1H NMR spectrum. |
|---|
|
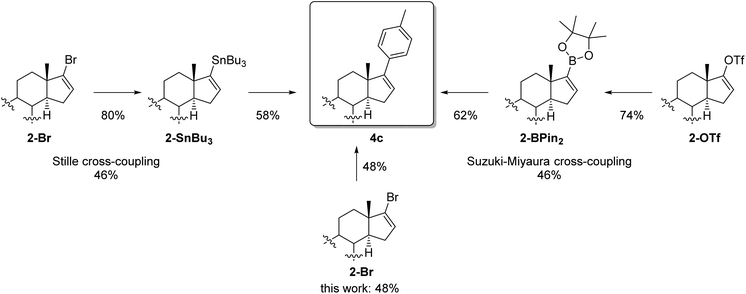
It is worth mentioning that the usage of highly polar substituted bromides like 1-bromo-3,5-dimethoxybenzene (4g) facilitates column chromatography extremely since the separation from the deprotected steroid 5 is, in particular for nonpolar aryls, challenging and in some cases not completely possible even after multiple column chromatography. Also, TMS-protected bromophenol (4h) and dimethyl protected bromoaniline (4i) could be introduced at C-17 of the steroid in moderate yields. Furthermore, TMS-protected alkynes and also acetals were tolerated giving the arylated steroids 4k and 4j in 30% and 36% yields, respectively. In accordance with the results of Feringa et al.24,25 nitriles, nitro- and keto groups were not compatible with the organolithium reagent, mainly due to the formation of 1,2-addition side products. Furthermore, it could be proven that an electron-poor aryl bromide like 1-bromo-4-fluorobenzene (4l) can be introduced successfully to the steroid pattern even if the yield is not as good as those for electron-rich aryl bromides. The arylated systems could be extended to naphthylbromides which provided the steroid 4m in a yield of 54%. Motivated by this result a heterocyclic example of an annulated system like methyl protected 4-bromoindole was also examined giving the arylated steroid 4n in 25% yield. In order to evaluate the power of this novel method Stille and Suzuki–Miyaura cross-couplings were performed for comparison starting from vinylbromide 2-Br and the analogous vinyltriflate 2-OTf (Scheme 2) whereas para-bromotoluene served as a model bromide. It is noteworthy that other arylbromides bearing electron withdrawing (e.g. para-fluorine, 4l) and electron donating substituents (e.g. para-methoxy, 4f) yield similar results. For Stille cross-coupling the vinyl stannyl derivative 2a-SnBu3 as a nucleophile had to be synthesized via lithiation with tBuLi followed by the reaction with tributyltin chloride giving 2a-SnBu3 in a good yield of 80%. Using Pd(PPh3)4 as the catalyst and LiCl and CuCl as additives the arylated product 4c was obtained in a moderate yield of 58%. For the Suzuki–Miyaura cross-coupling, we installed a pinacolatoboron moiety at C-17 of the steroid starting from vinyl triflate 2-OTf (see the ESI† for further information). Applying an optimized protocol for Suzuki cross-coupling with Pd(PPh3)4 as the catalyst and Na2CO3 as the base the arylated product was isolated in 62% yield. When comparing the yield of the cross-couplings as well as their overall yield over two steps with the herein presented method, it becomes obvious that this method is competitive with other well established methods. On top of it, considering aspects of toxicity, waste disposal and effort the shown coupling is even superior to Stille and Suzuki reactions.
Conclusions
In summary, we presented Pd-mediated cross-coupling of vinyllithium steroids based on the core structure of epi-androsterone with different substituted aryl bromides using the well-known catalytic system Pd2dba3/XPhos in toluene and THF as the solvent system in order to create new derivatives based on highly biologically active steroids. Comparing this method to the established methods like Stille or Suzuki cross-coupling reactions illustrates its competitiveness and advantages with respect to toxicity, the amount of waste and overall yields.Acknowledgements
V. K. gratefully acknowledges the Studienstiftung des deutschen Volkes for financial support.References
- C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef CAS PubMed.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS PubMed.
- X.-F. Wu, P. Anbarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047–9050 CrossRef CAS PubMed.
- E.-i. Negishi, Angew. Chem., Int. Ed., 2011, 50, 6738–6764 CrossRef CAS PubMed.
- J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651–2710 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- A. Suzuki, Acc. Chem. Res., 1982, 15, 178–184 CrossRef CAS.
- A. Suzuki, Angew. Chem., Int. Ed., 2011, 50, 6722–6737 CrossRef CAS PubMed.
- V. Farina, V. Krishnamurthy and W. J. Scott, in Organic Reactions, John Wiley & Sons, Inc., 2004 Search PubMed.
- D. Milstein and J. K. Stille, J. Am. Chem. Soc., 1978, 100, 3636–3638 CrossRef CAS.
- D. Milstein and J. K. Stille, J. Am. Chem. Soc., 1979, 101, 4992–4998 CrossRef CAS.
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508–524 CrossRef.
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 98, 504–519 CAS.
- P. Espinet and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 4704–4734 CAS.
- K. Tamao, K. Sumitani and M. Kumada, J. Am. Chem. Soc., 1972, 94, 4374–4376 CrossRef CAS.
- C. E. I. Knappke and A. Jacobi von Wangelin, Chem. Soc. Rev., 2011, 40, 4948–4962 RSC.
- E. Negishi, T. Takahashi, S. Baba, D. E. Van Horn and N. Okukado, J. Am. Chem. Soc., 1987, 109, 2393–2401 CrossRef CAS.
- S. Bracegirdle and E. A. Anderson, Chem. Soc. Rev., 2010, 39, 4114–4129 RSC.
- Y. Nakao and T. Hiyama, Chem. Soc. Rev., 2011, 40, 4893–4901 RSC.
- S. Murahashi, M. Yamamura, K. Yanagisawa, N. Mita and K. Kondo, J. Org. Chem., 1979, 44, 2408–2417 CrossRef CAS.
- S.-I. Murahashi, J. Organomet. Chem., 2002, 653, 27–33 CrossRef CAS.
- A. Nagaki, A. Kenmoku, Y. Moriwaki, A. Hayashi and J.-i. Yoshida, Angew. Chem., Int. Ed., 2010, 49, 7543–7547 CrossRef CAS PubMed.
- M. Giannerini, M. Fañanás-Mastral and B. L. Feringa, Nat. Chem., 2013, 5, 667–672 CrossRef CAS PubMed.
- C. Vila, M. Giannerini, V. Hornillos, M. Fananas-Mastral and B. L. Feringa, Chem. Sci., 2014, 5, 1361–1367 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data, 1H and 13C NMR spectra. See DOI: 10.1039/c6ob02496c |
| This journal is © The Royal Society of Chemistry 2017 |