Optical control of GIRK channels using visible light†
Julie B.
Trads
ab,
Jessica
Burgstaller
c,
Laura
Laprell
a,
David B.
Konrad
a,
Luis
de la Osa de la Rosa
a,
C. David
Weaver
d,
Herwig
Baier
c,
Dirk
Trauner
*a and
David M.
Barber
*a
aDepartment of Chemistry and Center for Integrated Protein Science, Ludwig Maximilians University Munich, Butenandtstraße 5-13, 81377 Munich, Germany. E-mail: dirk.trauner@lmu.de; david.barber@cup.uni-muenchen.de
bCenter for DNA Nanotechnology, Department of Chemistry and iNANO, Aarhus University, Gustav Wieds Vej 14, 8000 Aarhus C, Denmark
cMax Planck Institute of Neurobiology, Am Klopferspitz 18, 82152 Martinsried, Germany
dDepartment of Pharmacology and Institute of Chemical Biology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232, USA
First published on 30th November 2016
Abstract
G-protein coupled inwardly rectifying potassium (GIRK) channels are an integral part of inhibitory signal transduction pathways, reducing the activity of excitable cells via hyperpolarization. They play crucial roles in processes such as cardiac output, cognition and the coordination of movement. Therefore, the precision control of GIRK channels is of critical importance. Here, we describe the development of the azobenzene containing molecule VLOGO (Visible Light Operated GIRK channel Opener), which activates GIRK channels in the dark and is promptly deactivated when illuminated with green light. VLOGO is a valuable addition to the existing tools for the optical control of GIRK channels as it circumvents the need to use potentially harmful UV irradiation. We therefore believe that VLOGO will be a useful research tool for studying GIRK channels in biological systems.
Introduction
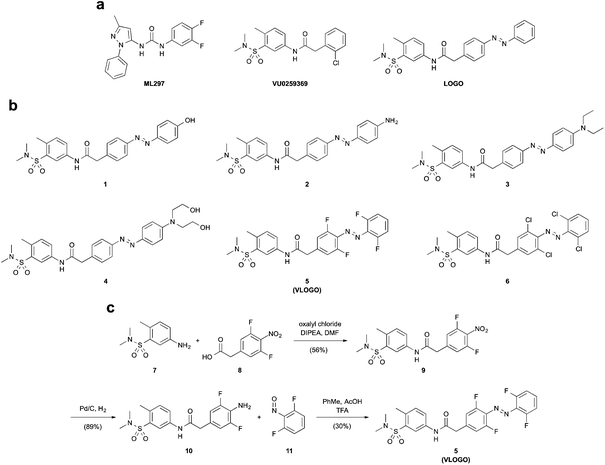
G-protein coupled inwardly rectifying potassium (GIRK) channels are a family of potassium channels that are opened via interactions with Gβγ protein complexes.1 Upon channel opening, GIRK channels hyperpolarize cell membranes causing a reduction in the activity of excitable cells. Therefore, GIRK channels are involved in processes such as nociception, cognition and cardiac output2 and are associated with numerous neurological and cardiovascular conditions.3GIRK channels exist as either homo- or heterotetramers comprised of the four subunits GIRK1-4, which exhibit distinct expression patterns throughout the body.4 For example, GIRK1/2 channels are typically found in the central nervous system (CNS) and GIRK1/4 channels are characteristic of the cardiovascular system.5 It has been shown that GIRK channels are activated by a variety of small molecules, albeit with varying degrees of selectivity.6 The compounds ML297 and VU0259369 are exceptions as they are potent and selective activators of GIRK channels bearing the GIRK1 subunit (Fig. 1a).7
We recently demonstrated that GIRK channels can be endowed with light control using photopharmacology.8 Our freely diffusible photoswitch LOGO, is a derivative of VU0259369 that contains an azobenzene moiety as the photoresponsive element (Fig. 1a).9 It harnesses the precision of light to accurately control the spontaneous action potential firing of dissociated hippocampal neurons and the swimming behaviour of zebrafish larvae. However, a significant drawback of LOGO is that UV and blue light are required for its cis/trans photoisomerisation. This results in increased levels of phototoxicity10 and limits the amount of tissue penetration.11 By employing photoswitches that respond to red-shifted wavelengths of light we would be able to improve these properties.12
Herein, we present VLOGO (Visible Light Operated GIRK channel Opener), which is an ortho-fluorinated azobenzene photoswitch that enables the optical control of GIRK channels using violet and green light. The red-shifted properties of VLOGO improve its suitability for experiments in vivo and we therefore envisage that VLOGO will be a valuable tool for GIRK channel research.
Results and discussion
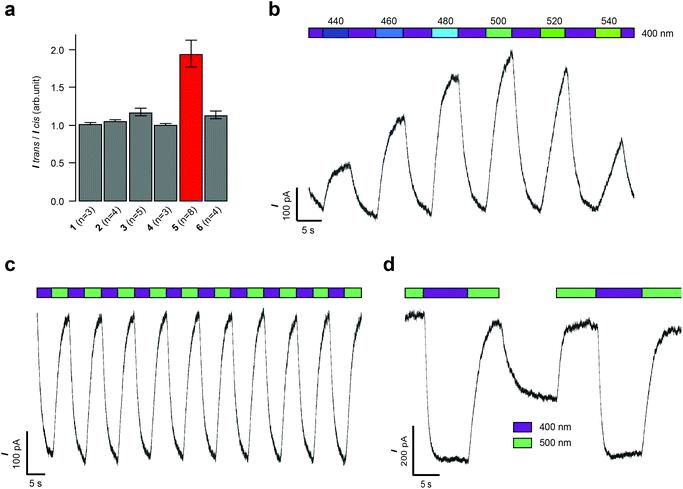
Many methods can be employed to create azobenzene photoswitches that isomerise using visible light.13 One of the simplest methods is to increase the electron density of the azobenzene moiety by incorporating electron-donating groups in its para-position.14 We initially investigated this avenue by preparing a series of photoswitchable GIRK channel openers (1–4) that had electron-donating groups in the 4′-position of the azobenzene (Fig. 1b). This was achieved by forming the diazonium salt of an intermediate aniline and trapping it with a range of electron rich aromatic compounds (Fig. S1†). More recently, several research groups have shown that exchanging the ortho-positions of the azobenzene with halogen substituents can create photoswitches that undergo cis/trans isomerisation when exposed to long wavelength visible light.15 We therefore became interested in preparing GIRK channel openers bearing ortho-fluoro and ortho-chloro substituted azobenzenes. An amide coupling between aniline 7 and acid 8 furnished amide 9, which was subsequently reduced in the presence of palladium on carbon to afford difluoro aniline 10. A Mills reaction between difluoro aniline 10 and difluoro nitrosobenzene 11 furnished VLOGO in a moderate 30% yield (Fig. 1c). In contrast, the ortho-chloro azobenzene 6 was synthesised using a recently reported C–H activation strategy (Fig. S1†).16With our small library of compounds in hand, we determined their optimum photoswitching properties using UV-Vis spectroscopy (Fig. S2†). We then applied this information to our initial compound analysis using patch clamp electrophysiology of GIRK1/2 channels expressed in HEK293T cells (Fig. 2a). Starting with the 4′-substituted azobenzenes 1–4 (Fig. 2a), we observed small amounts of GIRK1/2 channel opening and moderate levels of current modulation upon photoswitching with the 4′-amino azobenzene 2 and the 4′-N,N-diethyl azobenzene 3. In contrast, almost no change in current was observed upon photoswitching using azobenzenes 1 and 4. We next evaluated VLOGO and found it to be an excellent photoswitchable opener of GIRK1/2 channels using 400 and 500 nm light (Fig. 2a). Spurred on by this result we assessed ortho-chloro azobenzene 6. However, we discovered that this photoswitch could not endow GIRK1/2 with significant amounts of light control. This was an unfortunate result, as azobenzene 6 would have allowed longer wavelengths of light to be used for the optical control of GIRK channels. When examining all of the results, it becomes clear that only small modifications to the azobenzene photoswitch are tolerated in order to retain both potent agonism and good photoswitching behaviour at GIRK1/2 channels.
With ortho-fluoro azobenzene VOLOGO identified as our optimal red-shifted photoswitch for GIRK1/2 channels, we further investigated its properties using patch clamp electrophysiology. Firstly, we investigated the action spectrum of VLOGO by switching the wavelength between violet light (400 nm) and different wavelengths of blue/green light (440–540 nm), we observed large differences in current (Fig. 2b). The minimal inward current was exhibited when illuminating at 500 nm. In contrast, 440, 460, 480, 520 and 540 nm resulted in more inward current (Fig. S3†). We then established that reversible photoactivation of VLOGO (10 μM) is very robust, with almost no loss of photocurrent over many switching cycles (Fig. 2c). The trans-isomer of VLOGO, which predominates in the dark and under violet light (400 nm) illumination, is the most active form of VLOGO. Upon photoisomerisation using green light (500 nm), the considerably less active cis-isomer of VLOGO is formed, causing a reduction in the observed current. When operating in current clamp mode the photoswitching of VLOGO was also found to be highly reproducible (Fig. S4†). Lastly, we examined the stability of cis-VLOGO in the dark (Fig. 2d). After the full cis-isomer content had been reached under 500 nm light illumination, VLOGO was exposed to dark conditions, quickly resulting in increased inward current. The minimal inward current was achieved again using 500 nm light. This result demonstrates that constant illumination of VLOGO is required to maintain the maximum cis-isomer content.
Having characterised VLOGO using patch-clamp electrophysiology in HEK293T cells, we subsequently evaluated the activity of VLOGO towards different GIRK channel subtypes using the thallium flux assay technique (Fig. 3).17 We found that VLOGO activates GIRK channels containing the GIRK1 subunit, whilst not affecting homodimeric GIRK2 channels. VLOGO exhibits similar potency at GIRK channels (GIRK1/2: EC50 = 2.0 ± 0.11 μM; GIRK1/4: EC50 = 1.9 ± 0.06 μM) as the non-substituted photoswitchable GIRK channel agonist LOGO.9 However, the efficacy of VLOGO is significantly reduced (GIRK1/2: %Emax = 49.4 ± 4.7; GIRK1/4: %Emax = 30.9 ± 5.2).
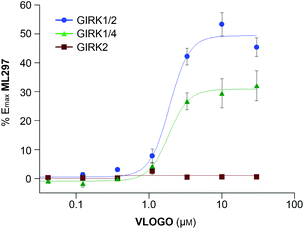 | ||
| Fig. 3 Potency, efficacy and selectivity profile of VLOGO. Shown are fits to representative data obtained from testing multiple concentrations of VLOGO on cell lines stably expressing GIRK1/2 (blue circles), GIRK1/4 (green triangles) and GIRK2 (red squares). The measured potencies for GIRK1/2 and GIRK1/4 were 2.0 ± 0.11 μM and 1.9 ± 0.06 μM, respectively. Efficacy values were normalised to the maximum activity observed using ML297 (10 μM) on GIRK1/2-expressing cells. Error bars represent mean ± SEM obtained from triplicate wells. Reported potency values represent the mean ± SEM obtained from three independent experiments.18 | ||
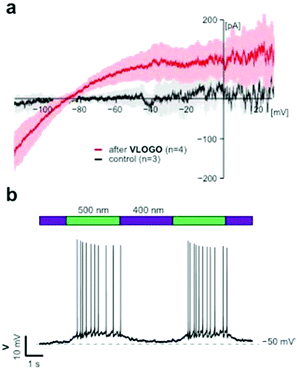
After demonstrating that VLOGO can optically control cellular currents in HEK293T cells heterologously expressing GIRK1/2 channels, we next investigated if it could be used to control GIRK channels in excitable cells.19 For this endeavour we conducted electrophysiological experiments on CA1 hippocampal neurons found in acute brain slice preparations from wild type mice. Firstly, we recorded current–voltage (I–V) relationships comparing the control traces with those obtained in the presence of VLOGO (Fig. 4a). Due to the inwardly rectifying nature of GIRK channels, we would expect to see increasing amounts of inward current as the voltage becomes more negative. After the application of VLOGO (100 μM) and subsequent wash out, the hippocampal neuron exhibited increasing inward current at voltages lower than −90 mV during a −120 mV to +30 mV voltage ramp. This is indicative of GIRK channel opening.7 Most importantly VLOGO was able to reversibly silence spontaneous action potential firing under violet light (400 nm) illumination when the hippocampal neurons were held at depolarised potentials (Fig. 4b). Changing the illumination wavelength to 500 nm then efficiently restored the action potential firing.
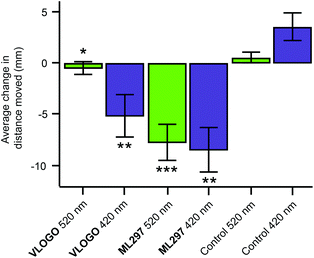
After accomplishing the control of native GIRK channels in mouse hippocampal neurons using VLOGO, we investigated if it could be used to optically control the movement of living animals. For these experiments we selected zebrafish larvae (Danio rerio) as light can easily be delivered to them due to their transparency.20 Accordingly, zebrafish larvae 5–7 days post fertilisation were exposed to 2 minute intervals of alternating violet light (420 nm) and green light (520 nm) (Fig. S5†). After the first cycle of violet and green light, the distance (mm) that the zebrafish larvae moved in a 10 second time period within a representative section of each 2 minute interval was measured to give the background swimming behaviour for each condition. The zebrafish larvae were then incubated with VLOGO (100 μM) for 1 hour and the same protocol was used to determine its effect by calculating the change in swimming distance. Pleasingly, the zebrafish larvae showed significantly different swimming distances in the presence of VLOGO, which could be modulated by changing the illumination from violet to green light (Fig. 5). The zebrafish larvae exhibited reduced swimming distances when illuminated with violet light compared to the control zebrafish. After illuminating with green light for a total of 2 minutes, the swimming distance of the zebrafish was not significantly different from the control experiment. We then performed additional experiments using the non-photoswitchable GIRK activator ML297 in an effort to dissect the effect of VLOGO from the native responses to violet and green light. These experiments confirmed that there is almost no change in the swimming distances of zebrafish larvae when illumination is changed between violet and green light. This is significantly different from the results obtained using VLOGO in conjunction with violet and green light.
Conclusion
In summary, we have developed a photochromic compound that enables the optical control of GIRK channels using visible light. The photoswitch, VLOGO, is transiently active in the dark and when illuminated with violet light. Green light illumination then rapidly converts it to its significantly less active cis-isomer. We have demonstrated that VLOGO opens GIRK channels bearing the GIRK1 subunit and that it can be used to optically reduce neuronal excitability in dissociated hippocampal neurons. VLOGO also exhibits in vivo activity, controlling the motility of zebrafish larvae in a light dependent manner. Future work is concentrated on the development of VLOGO as a tool for the optical control of GIRK channels in higher animals.Conflict of interest statement
C. D. W. receives royalties from the sale of the thallium-sensitive dye, Thallos, through a licensing agreement between Vanderbilt University and TEFlabs. C. D. W. is an owner of WaveFront Biosciences, the maker of the Panoptic instrument used to perform the thallium flux assays.Live subject statement
Animal procedures were in accord with EU and national law and were licensed by the Regierung Oberbayern.Acknowledgements
J. B. T. thanks the Danish National Research Foundation Center for DNA Nanotechnology (DNRF81) and Aarhus University, Faculty of Science and Technology for financial support. D. B. K. thanks the Friedrich-Ebert-Stiftung for a PhD scholarship. D. T. was supported by the European Research Council (Advanced Grant 268795). D. T. and H. B. thank the Centre for Integrated Protein Science Munich (CIPSM). D. M. B. thanks the European Commission for a Marie Skłodowska-Curie Intra-European Fellowship (PIEF-GA-2013-627990). We thank Achim Keidel for assistance with the chemical synthesis and Dr. Martin Sumser for helpful discussions during the preparation of this manuscript.References
- (a) H. Hibino, A. Inanobe, K. Furutani, S. Murakami, I. Findlay and Y. Kurachi, Physiol. Rev., 2010, 90, 291–366 CrossRef CAS PubMed; (b) C. Lüscher and P. A. Slesinger, Nat. Rev. Neurosci., 2010, 11, 301–315 CrossRef PubMed; (c) N. Dascal and U. Kahanovitch, Int. Rev. Neurobiol., 2015, 123, 27–85 CrossRef PubMed.
- R. Luján, E. Marron Fernandez de Velasco, C. Aguado and K. Wickman, Trends Neurosci., 2014, 37, 20–29 CrossRef PubMed.
- K. B. Walsh, Front. Pharmacol., 2011, 2, 64 CAS.
- (a) F. Lesage, F. Duprat, M. Fink, E. Guillemare, T. Coppola, M. Lazdunski and J. P. Hugnot, FEBS Lett., 1994, 353, 37–42 CrossRef CAS PubMed; (b) G. Krapivinsky, E. A. Gordon, K. Wickman, B. Velimirović, L. Krapivinsky and D. E. Clapham, Nature, 1995, 374, 135–141 CrossRef CAS PubMed.
- Y. J. Liao, Y. N. Jan and L. Y. Jan, J. Neurosci., 1996, 16, 7137–7150 CAS.
- (a) J. M. Lewohl, W. R. Wilson, R. D. Mayfield, S. J. Brozowski, R. A. Morrisett and R. A. Harris, Nat. Neurosci., 1999, 2, 1084–1090 CrossRef CAS PubMed; (b) G. Weigl and W. Schreibmayer, Mol. Pharmacol., 2001, 60, 282–289 Search PubMed; (c) T. T. Yow, E. Pera, N. Absalom, M. Heblinski, G. A. Johnston, J. R. Hanrahan and M. Chebib, Br. J. Pharmacol., 2001, 163, 1017–1033 CrossRef PubMed; (d) P. Aryal, H. Dvir, S. Choe and P. A. Slesinger, Nat. Neurosci., 2009, 12, 988–995 CrossRef CAS PubMed.
- (a) K. Kaufmann, I. Romaine, E. Days, C. Pascual, A. Malik, L. Yang, B. Zou, Y. Du, G. Sliwoski, R. D. Morrison, J. Denton, C. M. Niswender, J. S. Daniels, G. A. Sulikowski, X. Xie, C. W. Lindsley and C. D. Weaver, ACS Chem. Neurosci., 2013, 4, 1278–1286 CrossRef CAS PubMed; (b) N. Wydeven, E. Marron Fernandez de Velasco, Y. Du, M. A. Benneyworth, M. C. Hearing, R. A. Fischer, M. J. Thomas, C. D. Weaver and K. Wickman, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10755–10760 CrossRef CAS PubMed; (c) S. J. Ramos-Hunter, D. W. Engers, K. Kaufmann, Y. Du, C. W. Lindsley, C. D. Weaver and G. A. Sulikowski, Bioorg. Med. Chem. Lett., 2013, 23, 5195–5198 CrossRef CAS PubMed.
- (a) T. Fehrentz, M. Schönberger and D. Trauner, Angew. Chem., Int. Ed., 2011, 50, 12156–12182 CrossRef CAS PubMed; (b) A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC; (c) C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446–8476 CrossRef CAS PubMed; (d) W. Szymański, J. M. Beierle, H. A. V. Kistemaker, W. A. Velema and B. L. Feringa, Chem. Rev., 2013, 113, 6114–6178 CrossRef PubMed; (e) W. A. Velema, W. Szymanski and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 2178–2191 CrossRef CAS PubMed; (f) J. Broichhagen, J. A. Frank and D. Trauner, Acc. Chem. Res., 2015, 48, 1947–1960 CrossRef CAS PubMed; (g) A. Damijonaitis, D. M. Barber and D. Trauner, Neurotransmitter, 2016, 3, e1292 Search PubMed; (h) M. M. Lerch, M. J. Hansen, G. M. van Dam, W. Szymanski and B. L. Feringa, Angew. Chem., Int. Ed., 2016, 55, 10978–10999 CrossRef CAS PubMed.
- D. M. Barber, M. Schönberger, J. Burgstaller, J. Levitz, C. D. Weaver, E. Y. Isacoff, H. Baier and D. Trauner, Chem. Sci., 2016, 7, 2347–2352 RSC.
- (a) M. Protic-Sabljic, N. Tuteja, P. Munson, J. Hauser, K. Kraemer and K. Dixon, Mol. Cell. Biol., 1986, 6, 3349–3356 CrossRef CAS PubMed; (b) D. Brash, J. Rudolph, J. Simon, A. Lin, G. Mckenna, H. Baden, A. Halperin and J. Ponten, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 10124–10128 CrossRef CAS PubMed; (c) G. Banerjee, N. Gupta, A. Kapoor and G. Raman, Cancer Lett., 2005, 223, 275–284 CrossRef CAS PubMed.
- K. Kalka, H. Merk and H. Mukhtar, J. Am. Acad. Dermatol., 2000, 42, 389–413 CrossRef CAS PubMed.
- C. C. Frazier, Int. J. Dermatol., 1996, 35, 312–316 CrossRef CAS PubMed.
- (a) J. García-Amorós and D. Velasco, Beilstein J. Org. Chem., 2012, 8, 1003–1017 CrossRef PubMed; (b) H. M. Dhammika Bandarab and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC; (c) Y. Yang, R. P. Hughes and I. Aprahamian, J. Am. Chem. Soc., 2012, 134, 15221–15224 CrossRef CAS PubMed; (d) Y. Yang, R. P. Hughes and I. Aprahamian, J. Am. Chem. Soc., 2014, 136, 13190–13193 CrossRef CAS PubMed; (e) M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry and G. A. Woolley, Acc. Chem. Res., 2015, 48, 2662–2670 CrossRef CAS PubMed; (f) D. Bléger and S. Hecht, Angew. Chem., Int. Ed., 2015, 54, 11338–11349 CrossRef PubMed.
- (a) J. Broichhagen, M. Schönberger, S. C. Cork, J. A. Frank, P. Marchetti, M. Bugliani, A. M. J. Shapiro, S. Trapp, G. A. Rutter, D. J. Hodson and D. Trauner, Nat. Commun., 2014, 5, 5116 CrossRef CAS PubMed; (b) J. Broichhagen, J. A. Frank, N. R. Johnston, R. K. Mitchell, K. Šmid, P. Marchetti, M. Bugliani, G. A. Rutter, D. Trauner and D. J. Hodson, Chem. Commun., 2015, 51, 6018–6021 RSC.
- (a) D. Bléger, J. Schwarz, A. M. Brouwer and S. Hecht, J. Am. Chem. Soc., 2012, 134, 20597–20600 CrossRef PubMed; (b) S. Samanta, A. A. Beharry, O. Sadovski, T. M. McCormick, A. Babalhavaeji, V. Tropepe and G. A. Woolley, J. Am. Chem. Soc., 2013, 135, 9777–9784 CrossRef CAS PubMed; (c) C. Knie, M. Utecht, F. Zhao, H. Kulla, S. Kovalenko, A. M. Brouwer, P. Saalfrank, S. Hecht and D. Bléger, Chem. – Eur. J., 2014, 20, 16492–16501 CrossRef CAS PubMed.
- D. B. Konrad, J. A. Frank and D. Trauner, Chem. – Eur. J., 2016, 22, 4364–4368 CrossRef CAS PubMed.
- C. D. Weaver, D. Harden, S. I. Dworetzky, B. Robertson and R. J. Knox, J. Biomol. Screening, 2004, 9, 671–677 CrossRef CAS PubMed.
- The data were acquired using 494 ± 10 nm excitation (20 ms). The excitation wavelength of 494 nm could result in trans/cis isomerisation of VLOGO compromising the measured values. Therefore, we added a second light pulse at 405 ± 10 nm to ensure that VLOGO remained as its trans-isomer [494 ± 10 nm excitation (20 ms) then 405 ± 10 nm (200 ms)].
- H. M. D. Bandara and S. C. Burdette, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- (a) S. Szobota, P. Gorostiza, F. Del Bene, C. Wyart, D. L. Fortin, K. D. Kolstad, O. Tulyathan, M. Volgraf, R. Numano, H. L. Aaron, E. K. Scott, R. H. Kramer, J. Flannery, H. Baier, D. Trauner and E. Y. Isacoff, Neuron, 2007, 54, 535–545 CrossRef CAS PubMed; (b) C. Wyart, F. Del Bene, E. Warp, E. K. Scott, D. Trauner, H. Baier and E. Y. Isacoff, Nature, 2009, 461, 407–411 CrossRef CAS PubMed; (c) H. Janovjak, S. Szobota, C. Wyart, D. Trauner and E. Y. Isacoff, Nat. Neurosci., 2010, 13, 1027–1032 CrossRef CAS PubMed; (d) J. Levitz, C. Pantoja, B. Gaub, H. Janovjak, A. Reiner, A. Hoagland, D. Schoppik, B. Kane, P. Stawski, A. F. Schier, D. Trauner and E. Y. Isacoff, Nat. Neurosci., 2013, 16, 507–516 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterisation data. See DOI: 10.1039/c6ob02153k |
| This journal is © The Royal Society of Chemistry 2017 |