Sky-blue nondoped OLEDs based on new AIEgens: ultrahigh brightness, remarkable efficiency and low efficiency roll-off†
Long
Chen‡
a,
Gengwei
Lin‡
a,
Huiren
Peng
b,
Siyang
Ding
a,
Wenwen
Luo
a,
Rongrong
Hu
a,
Shuming
Chen
*b,
Fei
Huang
a,
Anjun
Qin
a,
Zujin
Zhao
*a and
Ben Zhong
Tang
*ac
aState Key Laboratory of Luminescent Materials and Devices, South China University of Technology, Guangzhou 510640, China. E-mail: mszjzhao@scut.edu.cn
bDepartment of Electrical and Electronic Engineering, South University of Science and Technology of China, Shenzhen, Guangdong 518055, China. E-mail: chen.sm@sustc.edu.cn
cDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration and Reconstruction, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
First published on 29th September 2016
Abstract
Two novel AIEgens decorated with fluorenyl and dimesitylboryl groups are prepared. They show high thermal stability and excellent solid-state photoluminescence efficiency. Sky-blue nondoped OLEDs are achieved based on them, affording remarkable electroluminescence efficiencies (12.2 cd A−1 and 5.3%), ultrahigh brightness (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 cd m−2) and low efficiency roll-off (11.0 cd A−1 at 1000 cd m−2).
810 cd m−2) and low efficiency roll-off (11.0 cd A−1 at 1000 cd m−2).
Organic light-emitting diodes (OLEDs), invented by Tang and co-workers in 1987, have drawn intensive attention because of their promising applications in flat-panel display and solid-state lighting.1–3 To achieve commercially viable OLED products with high electroluminescence (EL) efficiency and reliable working stability, robust luminescent materials with excellent solid-state photoluminescence (PL) efficiency is of vital importance. Unfortunately, most conventional organic chromophores are only emissive in good solvents, but suffer detrimental aggregation-caused quenching (ACQ) of their fluorescence due to the formation of excimers or exciplexes in the solid state.4,5 In order to mitigate the ACQ problem, scientists have proposed various methods to avoid the aggregation of the chromophores, typically, e.g. chemical modification with branched groups or physical doping in transparent organic semiconductors. But all of them inevitably encounter some adverse side-effects such as lowered carrier mobility, voltage-dependent EL spectra, phase separation, complicated procedures, and so on.6,7 Aggregation-induced emission (AIE) is a unique photophysical phenomenon that fights against the ACQ effect. The luminogens with an AIE nature (AIEgens) are almost nonfluorescent in dilute solutions, but become highly fluorescent once they form aggregates, such as nanoparticles or solid films.8 By adopting an AIE motif in the molecular design, highly efficient solid-state luminescent materials are readily achievable.9–16 For instance, just by combining the AIE units with the ACQ functional groups at the molecular level, new AIEgens with excellent PL efficiencies are attainable, allowing nondoped OLEDs utilizing them as light-emitting layers to perform efficiently, with EL efficiencies reaching or even exceeding the theoretical limit.17–22
Tetraphenylethene (TPE) is a marvelous AIEgen that has attracted considerable interest due to its facile synthesis and outstanding AIE nature.23–26 To date, a library of TPE-based functional materials have been developed for application in OLEDs, but most of them possess a better hole-transporting ability than an electron-transporting one.27–29 Whereas light emitters with a high hole mobility are very important for achieving high-performance OLEDs with simplified structures, virtually, those with an enhanced electron-transporting ability, which are currently very rare, hold equal importance.21 Decorating TPE with electron-withdrawing units was verified as a valid method to prepare efficient solid-state emitters with an enhanced electron-transporting ability.30–32 For example, three coordinate organoboranes, such as dimesitylboryl groups, are intrinsically electron-deficient, owing to the empty pz orbital on the boron atom. TPE derivatives functionalized with dimesitylboryl groups can exhibit good electron-transporting ability, and thus, can serve as light-emitting and electron-transporting materials in OLEDs at the same time.31,33 However, these materials reach a limit where the thermal stability of the 4-(dimesitylboryl)phenyl group therein is not so high, which may undermine the working stability of the OLEDs. Therefore, to simultaneously improve the thermal stability and device performance, a fluorenyl unit was introduced into the dimesitylboryl group in this work, for this bulky group can effectively prevent organoboranes from decomposition.34–36 Furthermore, two novel AIEgens with excellent solid-state emission efficiency and high thermal stability are obtained. The nondoped OLED devices based on these AIEgens show remarkable EL efficiency and excellent stability.
The molecular structures of the two new AIEgens (DPE-BFDB and TPE-BFDB) are illustrated in Scheme 1. They were successfully synthesized according to the synthetic routes outlined in Scheme S1 (ESI†). The detailed steps for the synthesis and characterization data are given in the ESI.† Both DPE-BFDB and TPE-BFDB show good solubility in common organic solvents such as chloroform and THF but poor solubility in water and methanol. Their thermal stabilities were tested using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). DPE-BFDB and TPE-BFDB show high decomposition temperatures (Td) of 321 and 343 °C (Table 1), respectively, according to a 5% loss of initial weight. A high glass-transition temperature (Tg) of 183 °C was recorded for TPE-BFDB from the DSC curve (Fig. S1, ESI†). The thermal stabilities of both new AIEgens are enhanced apparently compared to those of previously reported similar TPE derivatives with 4-(dimesitylboryl)phenyl groups,18,33,37 indicating that the introduction of the fluorenyl group to the dimesitylboryl one can ameliorate molecular thermal stability.
| Compounds | λ abs (nm) | λ em (nm) | Φ F (%) | α AIE | T g/Td (°C) | HOMO/LUMOe (eV) | E g (eV) | ||
|---|---|---|---|---|---|---|---|---|---|
| THF | Filmb | THF | Filmb | ||||||
| a Measured in THF solutions (10 μM). b Spin-coated amorphous film. c Absolute fluorescence quantum yield determined using a calibrated integrating sphere. d The AIE effect calculated by ΦF (Film)/ΦF (THF). e Determined by cyclic voltammetry. f Optical energy band gap. | |||||||||
| DPE-BFDB | 381 | 521 | 519 | 5.5 | 82 | 15 | −/321 | −5.48/−2.78 | 2.70 |
| TPE-BFDB | 364 | 490 | 486 | 1.9 | 80 | 42 | 183/343 | −5.52/−2.70 | 2.82 |
The photophysical properties of the two new AIEgens were characterized using UV-visible absorption and PL spectroscopies. In Fig. 1A, DPE-BFDB is found to have a maximum absorption peak at 381 nm, while TPE-BFDB shows an absorption maximum at 364 nm. The blue-shifted absorption of TPE-BFDB compared to that of DPE-BFDB may result from its twist molecular structure, which impairs electronic delocalization and electronic donor–acceptor interaction. DPE-BFDB exhibits weak emission at 521 nm in THF solution with a low fluorescence quantum yield (ΦF) of 5.5%. TPE-BFDB, however, shows a blue-shifted PL peak at 490 nm, with an even lower ΦF of 1.9%. The larger ΦF of DPE-BFDB in THF solution compared to that of TPE-BFDB further evidence that DPE-BFDB possesses a better conjugation, which could be positive to the restriction of intramolecular rotation (RIR) and thus suppress the nonradiative relaxation.38 Both DPE-BFDB and TPE-BFDB become highly emissive in spin-coated films with PL peaks at 519 and 486 nm, respectively. The ΦF values, measured using a calibrated integrating sphere, are as high as 82 and 80% for DPE-BFDB and TPE-BFDB, respectively, denoting an obvious AIE effect with αAIE39 values of 15 and 42, respectively. These results indicate that they are indeed AIE-active and highly efficient in the solid state.
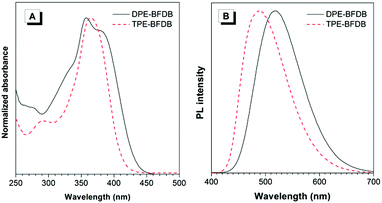 | ||
| Fig. 1 (A) Absorption (in THF, 10 μM) and (B) PL spectra (in films) of DPE-BFDB and TPE-BFDB. Excitation wavelength: 360 nm. | ||
The AIE properties of DPE-BFDB and TPE-BFDB could be further verified by their PL behaviours in THF/water mixtures. In Fig. 2, it can be found that both AIEgens emit weakly when the water content is lower than 60%. However, they turn out to be highly emissive when the water content becomes higher than 60%. Since both AIEgens are soluble in THF but insoluble in water, they must have existed as aggregates at high water contents. According to the RIR mechanism of the AIE phenomenon,8 the intramolecular rotation of both AIEgens that is active in good solvents is then restricted due to steric hindrance in the aggregate state, which will depress the nonradiative relaxation and promote the radiative decay of the excited state, rendering the AIEgen aggregates highly fluorescent.
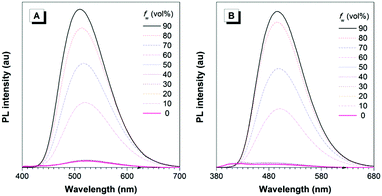 | ||
| Fig. 2 PL spectra of (A) DPE-BFDB and (B) TPE-BFDB in THF/water mixtures with different water fractions (fw). Excitation wavelength: 360 nm. | ||
Theoretical calculations were performed to gain deeper insight into the electronic structures of the two new AIEgens. Calculation results demonstrate that DPE-BFDB and TPE-BFDB have low LUMO energy levels of −1.88 and −1.86 eV, respectively (Fig. S2, ESI†). These values are much lower than that of the parent TPE (−1.22 eV), indicating that the introduction of the dimesitylboryl groups has effectively lowered their LUMO energy levels. In order to evaluate their energy levels experimentally, we investigated their electrochemical properties using cyclic voltammetry (CV). DPE-BFDB and TPE-BFDB exhibit a similar quasi-reversible oxidation process, with onset potentials at 1.08 and 1.12 V, respectively (Fig. S3, ESI†). The HOMO energy levels of DPE-BFDB and TPE-BFDB are calculated to be −5.48 and −5.52 eV, and the LUMO energy levels are −2.78 and −2.70 eV, respectively. The measured LUMO energy levels are close to or even lower than that of widely used electron-transporting materials, such as 1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene (TPBi, −2.7 eV),40 demonstrating their good potential of electron injection.
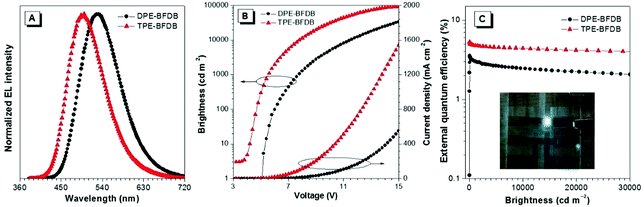
DPE-BFDB and TPE-BFDB were subjected to an EL property study in nondoped OLEDs, in view of their good thermal stabilities and remarkable solid-state PL efficiencies. We fabricated multilayer nondoped OLED devices with a configuration of ITO/HATCN (20 nm)/NPB (40 nm)/emitters (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al (100 nm), in which DPE-BFDB and TPE-BFDB act as light-emitting layers, dipyrazinoquinoxaline-2,3,6,7,10,11-hexacarbonitrile (HATCN) functions as a hole-injecting layer, and 4,4′-bis[(1-naphthyl) (phenyl)amino]biphenyl (NPB) and TPBi serve as hole/electron-transporting layers. The current density–voltage–brightness characteristics and external quantum efficiency curves are depicted in Fig. 3, and the key performance data are summarized in Table 2. Whereas the device based on DPE-BFDB radiates green light with a low turn-on voltage at 5.0 V, the device of TPE-BFDB shows sky-blue light with a lower turn-on voltage at 3.2 V. The lower turn-on voltage could be ascribed to the higher electron mobility of TPE-BFDB than DPE-BFDB (Fig. S4, ESI†), measured using the space-charge-limited current (SCLC) method. The maximum brightness (Lmax) of DPE-BFDB is moderate (33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 cd m−2), but that of TPE-BFDB is as high as 92
610 cd m−2), but that of TPE-BFDB is as high as 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 cd m−2, which is among the highest brightness of OLED devices emitting similar light.17,18,41–43 With the functionalization by electron-transporting dimesitylboryl groups, the LUMO energy levels are lowered, allowing for easy injection and transport of electrons. Hence, the ultrahigh brightness should result from the good injection and balance of charge carriers in TPE-BFDB. The maximum current (ηc) and external quantum (ηext) efficiency achieved by the device based on DPE-BFDB are 10.9 cd A−1 and 3.6%, respectively. The device of TPE-BFDB affords even better ηc and ηext values of 12.2 cd A−1 and 5.3%, respectively. The theoretical limit-exceeded ηext value should be attributed to the enhancement of the light out-coupling efficiency by horizontal orientation for TPE-BFDB.44–46 Significantly, it is noteworthy that both devices exhibit ultralow efficiency roll-off as the brightness increases. Taking TPE-BFDB as an example, the device based on it shows a current efficiency of 11.0 cd A−1 at 1000 cd m−2, which only reduces by less than 10% of the peak value. When the brightness increases to 10
810 cd m−2, which is among the highest brightness of OLED devices emitting similar light.17,18,41–43 With the functionalization by electron-transporting dimesitylboryl groups, the LUMO energy levels are lowered, allowing for easy injection and transport of electrons. Hence, the ultrahigh brightness should result from the good injection and balance of charge carriers in TPE-BFDB. The maximum current (ηc) and external quantum (ηext) efficiency achieved by the device based on DPE-BFDB are 10.9 cd A−1 and 3.6%, respectively. The device of TPE-BFDB affords even better ηc and ηext values of 12.2 cd A−1 and 5.3%, respectively. The theoretical limit-exceeded ηext value should be attributed to the enhancement of the light out-coupling efficiency by horizontal orientation for TPE-BFDB.44–46 Significantly, it is noteworthy that both devices exhibit ultralow efficiency roll-off as the brightness increases. Taking TPE-BFDB as an example, the device based on it shows a current efficiency of 11.0 cd A−1 at 1000 cd m−2, which only reduces by less than 10% of the peak value. When the brightness increases to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, the current efficiency can still retain a high value of 10.1 cd A−1 (Fig. S5, ESI†), clearly demonstrating the good stability of the devices.
000 cd m−2, the current efficiency can still retain a high value of 10.1 cd A−1 (Fig. S5, ESI†), clearly demonstrating the good stability of the devices.
| Emitters | λ EL (nm) | V on (V) | L max (cd m−2) | η ext (%) | η p (lm W−1) | η c (cd A−1) | η c,1000 (cd A−1) |
η
c,10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (cd A−1) 000 (cd A−1) |
CIE (x,y) |
|---|---|---|---|---|---|---|---|---|---|
a Abbreviations: λEL = electroluminescence maximum, Von = turn-on voltage at 1 cd m−2, Lmax = maximum brightness, ηext = maximum external quantum efficiency, ηp = maximum power efficiency, ηc = maximum current efficiency, ηc,1000 = current efficiency at 1000 cd m−2, ηc,10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 = current efficiency at 10 000 = current efficiency at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, CIE = Commission Internationale de I'Eclairage coordinates. Device configuration: ITO/HATCN (20 nm)/NPB (40 nm)/emitters (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al (100 nm). 000 cd m−2, CIE = Commission Internationale de I'Eclairage coordinates. Device configuration: ITO/HATCN (20 nm)/NPB (40 nm)/emitters (20 nm)/TPBi (40 nm)/LiF (1 nm)/Al (100 nm).
|
|||||||||
| DPE-BFDB | 530 | 5.0 | 33610 | 3.6 | 5.9 | 10.9 | 9.2 | 7.5 | 0.29, 0.53 |
| TPE-BFDB | 497 | 3.2 | 92810 | 5.3 | 8.7 | 12.2 | 11.0 | 10.1 | 0.20, 0.37 |
In summary, two novel TPE derivatives (DPE-BFDB and TPE-BFDB) have been facially synthesized and their photophysical and EL properties have been investigated. Both molecules are demonstrated to feature AIE characteristics with excellent solid-state PL efficiencies. The introduction of a fluorenyl group to the dimesitylboryl one significantly enhances molecular thermal stability, while the presence of dimesitylboryl groups effectively lowers the LUMO energy levels. The OLED devices fabricated with both luminogens exhibit outstanding EL efficiencies, ultrahigh brightness and very low efficiency roll-off, which are deemed to be of vital significance to the commercial application of OLEDs. It should be noted that the devices have not yet been optimized thoroughly. Therefore, devices with better performances could be expected through device engineering, owing to the good optoelectronic properties of both AIEgens.
We acknowledge the financial support from the National Natural Science Foundation of China (51273053 and 21673082), the National Key Basic Research and Development Program of China (973 program, 2015CB655004 and 2013CB834702) Founded by MOST, the Guangdong Natural Science Funds for Distinguished Young Scholar (2014A030306035), the Guangdong Innovative Research Team Program of China (201101C0105067115), Science and Technology Project of Guangdong Province (2016B090907001), the Natural Science Foundation of Guangdong Province (2016A030312002), the Innovation and Technology Commission of Hong Kong (ITC-CNERC14SC01) and the Fundamental Research Funds for the Central Universities (2015PT020 and 2015ZY013).
References
- C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS.
- C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897 CrossRef PubMed.
- J. Kido, M. Kimura and K. Nagai, Science, 1995, 267, 1332 CrossRef CAS PubMed.
- U. Lemmer, S. Heun, R. F. Mahrt, U. Scherf, M. Hopmeier, U. Siegner, E. O. Göbel, K. Müllen and H. Bässler, Chem. Phys. Lett., 1995, 240, 373 CrossRef CAS.
- R. Jakubiak, C. J. Collison, W. C. Wan, L. J. Rothberg and B. R. Hsieh, J. Phys. Chem. A, 1999, 103, 2394 CrossRef CAS.
- S. Hecht and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2001, 40, 74 CrossRef CAS.
- L. Chen, S. Xu, D. McBranch and D. Whitten, J. Am. Chem. Soc., 2000, 122, 9302 CrossRef CAS.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- Z. Zhao, S. Chen, C. Y. K. Chan, J. W. Y. Lam, C. K. W. Jim, P. Lu, Z. Chang, M. Xue, H. S. Kwok, H. Qiu and B. Z. Tang, Chem. – Asian J., 2012, 7, 484 CrossRef CAS PubMed.
- J. Huang, N. Sun, Y. Dong, R. Tang, P. Lu, P. Cai, Q. Li, D. Ma, J. Qin and Z. Li, Adv. Funct. Mater., 2013, 23, 2329 CrossRef CAS.
- X. Zhan, N. Sun, Z. Wu, J. Tu, L. Yuan, X. Tang, Y. Xie, Q. Peng, Y. Dong, Q. Li, D. Ma and Z. Li, Chem. Mater., 2015, 27, 1847 CrossRef CAS.
- L. Chen, Y. Jiang, H. Nie, P. Lu, H. H. Y. Sung, I. D. Williams, H. S. Kwok, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, Adv. Funct. Mater., 2014, 24, 3621 CrossRef CAS.
- W.-L. Gong, B. Wang, M. P. Aldred, C. Li, G.-F. Zhang, T. Chen, L. Wang and M.-Q. Zhu, J. Mater. Chem. C, 2014, 2, 7001 RSC.
- M.-M. Xue, Y.-M. Xie, L.-S. Cui, X.-Y. Liu, X.-D. Yuan, Y.-X. Li, Z.-Q. Jiang and L.-S. Liao, Chem. – Eur. J., 2016, 22, 916 CrossRef CAS PubMed.
- Y. Liu, Q. Bai, J. Li, S. Zhang, C. Zhang, F. Lu, B. Yang and P. Lu, RSC Adv., 2016, 6, 17239 RSC.
- X. Tang, L. Yao, H. Liu, F. Shen, S. Zhang, H. Zhang, P. Lu and Y. Ma, Chem. – Eur. J., 2014, 20, 7589 CrossRef CAS PubMed.
- J. Yang, J. Huang, Q. Li and Z. Li, J. Mater. Chem. C, 2016, 4, 2663 RSC.
- Z. Zhao, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem., 2012, 22, 23726 RSC.
- Z. Zhao, S. Chen, J. W. Y. Lam, P. Lu, Y. Zhong, K. S. Wong, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 2221 RSC.
- L. Chen, Y. Jiang, H. Nie, R. Hu, H. S. Kwok, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 17215 CAS.
- G. Lin, H. Peng, L. Chen, H. Nie, W. Luo, Y. Li, S. Chen, R. Hu, A. Qin, Z. Zhao and B. Z. Tang, ACS Appl. Mater. Interfaces, 2016, 8, 16799 CAS.
- B. Chen, Y. Jiang, L. Chen, H. Nie, B. He, P. Lu, H. H. Y. Sung, I. D. Williams, H. S. Kwok, Z. Zhao and B. Z. Tang, Chem. – Eur. J., 2014, 20, 1931 CrossRef CAS PubMed.
- J. Huang, N. Sun, J. Yang, R. Tang, Q. Li, D. Ma and Z. Li, Adv. Funct. Mater., 2014, 24, 7645 CrossRef CAS.
- H. Li, Z. Chi, X. Zhang, B. Xu, S. Liu, Y. Zhang and J. Xu, Chem. Commun., 2011, 47, 11273 RSC.
- C. Y. K. Chan, Z. Zhao, J. W. Y. Lam, J. Liu, S. Chen, P. Lu, F. Mahtab, X. Chen, H. H. Y. Sung, H. S. Kwok, Y. Ma, I. D. Williams, K. S. Wong and B. Z. Tang, Adv. Funct. Mater., 2012, 22, 378 CrossRef CAS.
- H. Shi, J. Yang, X. Dong, X. Wu, P. Zhou, F. Cheng and M. M. F. Choi, RSC Adv., 2014, 4, 19418 RSC.
- Z. Zhao, Z. Li, J. W. Y. Lam, J.-L. Maldonado, G. Ramos-Ortiz, Y. Liu, W. Yuan, J. Xu, Q. Miao and B. Z. Tang, Chem. Commun., 2011, 47, 6924 RSC.
- S. Jin, Y. Tian, F. Liu, J. Chen, S. Deng and N. Xu, J. Mater. Chem. C, 2015, 3, 8066 RSC.
- Z. Zhao, P. Lu, J. W. Y. Lam, Z. Wang, C. Y. K. Chan, H. H. Y. Sung, I. D. Williams, Y. Ma and B. Z. Tang, Chem. Sci., 2011, 2, 672 RSC.
- W. Z. Yuan, S. Chen, J. W. Y. Lam, C. Deng, P. Lu, H. H. Y. Sung, I. D. Williams, H. S. Kwok, Y. Zhang and B. Z. Tang, Chem. Commun., 2011, 47, 11216 RSC.
- L. Chen, C. Zhang, G. Lin, H. Nie, W. Luo, Z. Zhuang, S. Ding, R. Hu, S.-J. Su, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 2775 RSC.
- G. Mu, W. Zhang, P. Xu, H. Wang, Y. Wang, L. Wang, S. Zhuang and X. Zhu, J. Phys. Chem. C, 2014, 118, 8610 CAS.
- L. Chen, G. Lin, H. Peng, H. Nie, Z. Zhuang, P. Shen, S. Ding, D. Huang, R. Hu, S. Chen, F. Huang, A. Qin, Z. Zhao and B. Z. Tang, J. Mater. Chem. C, 2016, 4, 5241 RSC.
- Y. Liu, Y. Lv, H. Xi, X. Zhang, S. Chen, J. W. Y. Lam, R. T. K. Kwok, F. Mahtab, H. S. Kwok, X. Tao and B. Z. Tang, Chem. Commun., 2013, 49, 7216 RSC.
- X. Xu, S. Ye, B. He, B. Chen, J. Xiang, J. Zhou, P. Lu, Z. Zhao and H. Qiu, Dyes Pigm., 2014, 101, 136 CrossRef CAS.
- Z. Chang, Y. Jiang, B. He, J. Chen, Z. Yang, P. Lu, H. S. Kwok, Z. Zhao, H. Qiu and B. Z. Tang, Chem. Commun., 2013, 49, 594 RSC.
- H. Shi, D. Xin, S. Bai, L. Fang, X. Duan, J. Roose, H. Peng, S. Chen and B. Z. Tang, Org. Electron., 2016, 33, 78 CrossRef CAS.
- H. Nie, B. Chen, C. Quan, J. Zhou, H. Qiu, R. Hu, S.-J. Su, A. Qin, Z. Zhao and B. Z. Tang, Chem. – Eur. J., 2015, 21, 8137 CrossRef CAS PubMed.
- Z. Zhao, S. Chen, X. Shen, F. Mahtab, Y. Yu, P. Lu, J. W. Y. Lam, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 46, 686 RSC.
- Z. Gao, C. S. Lee, I. Bello, S. T. Lee, R.-M. Chen, T.-Y. Luh, J. Shi and C. W. Tang, Appl. Phys. Lett., 1999, 74, 865 CrossRef CAS.
- W. L. Jia, X. D. Feng, D. R. Bai, Z. H. Lu, S. Wang and G. Vamvounis, Chem. Mater., 2004, 17, 164 CrossRef.
- C.-T. Chen, W.-S. Chao, H.-W. Liu, Y. Wei, J.-H. Jou and S. Kumar, RSC Adv., 2013, 3, 9381 RSC.
- T. H. Huang, J. T. Lin, L. Y. Chen, Y. T. Lin and C. C. Wu, Adv. Mater., 2006, 18, 602 CrossRef CAS.
- J. Y. Kim, T. Yasuda, Y. S. Yang and C. Adachi, Adv. Mater., 2013, 25, 2666 CrossRef CAS PubMed.
- J. Frischeisen, D. Yokoyama, A. Endo, C. Adachi and W. Brütting, Org. Electron., 2011, 12, 809 CrossRef CAS.
- D. Yokoyama, A. Sakaguchi, M. Suzuki and C. Adachi, Org. Electron., 2009, 10, 127 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, thermal properties, electrochemical properties, electronic structures, electron mobility and EL characteristic curves of DPE-BFDB and TPE-BFDB. See DOI: 10.1039/c6qm00075d |
| ‡ L. Chen and G. Lin contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |