Hard–soft chemistry of Sr1−xCaxCrO3–δ solid solutions†‡
A. M.
Arevalo-Lopez
,
A.
Srinath
and
J. P.
Attfield
 *
*
Centre for Science at Extreme Conditions (CSEC) and School of Chemistry, University of Edinburgh, Edinburgh, EH9 3FD, UK. E-mail: j.p.attfield@ed.ac.uk
First published on 30th September 2016
Abstract
Sr1−xCaxCrO3−δ (x = 0.25, 0.50, 0.75) solid solutions have been synthesised using the ‘hard–soft’ technique and structurally characterised. Sr1−xCaxCrO3 phases were prepared under ‘hard’ high pressure and high temperature synthesis and then reduced at ‘soft’ low temperature conditions. Analysis by powder X-ray diffraction and Rietveld refinements reveals that the reduction of each composition gives two crystalline products, with 6H and 15R structure types previously reported in SrCrO3−δ. analogues. These results demonstrate that the 6H and 15R structures are stable for very high calcium contents up to x = 0.75 in Sr1−xCaxCrO3−δ, although different structural motifs are observed for CaCrO3−δ. The Sr1−xCaxCrO3−δ phase diagram shows that the lower temperature for superstructure formation has a maximum near x = 0.5, evidencing an influence of Ca/Sr cation disorder on oxygen vacancy order.
Introduction
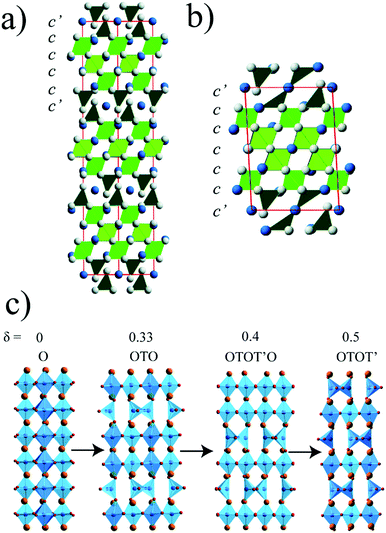
Transition metal oxide perovskites show a plethora of physical phenomena such as superconductivity, colossal magnetoresistance, ferroelectricity, charge and spin dependent transport, leading to useful applications. The orders associated with these properties may be of vacancies, atoms, charges, orbital or spin states. Many chemical techniques are used to control these orders and so tune the materials properties. For instance, high pressure synthesis helps to stabilise metal oxides in unusual oxidation states and coordination environments.1 Topotactic control over the anion lattices in transition metal oxides has also provided access to new structures and unusual oxidation states.2Combination of high pressure and temperature synthesis (‘hard’ chemistry) with topotactic control (‘soft’ chemistry) over the oxygen content at low temperatures has recently been demonstrated as a route to obtain new materials, using chromium perovskites as exemplars. Two new phases were discovered when the ‘hard’ high pressure phase SrCrO3 was reduced under low temperature conditions; 15R–SrCrO2.8 and 6H-SrCrO2.75.3 They show a long range oxide vacancy ordering along the (111) direction of the cubic perovskite defining long periodicity 15-layer rhombohedral (ccc′cc)3 and 6-layer hexagonal (c′ccccc) stacking sequences with mixed cubic close packed (c) and oxygen-deficient (c′) layers. The coordination of Cr4+ in the c′ layer has changed from octahedral to tetrahedral, which is generally more stable at ambient pressure, see Fig. 1a and b. They also show a charge modulation, accompanied by a spin density wave below an antiferromagnetic transition at 272 K in the 15R phase. The SrCrO3 and 15R–SrCrO2.8 phases were subsequently stabilised epitaxially as thin films with rapid oxygen uptake or loss on cycling between the two phases.4 Moreover, the 15R superstructure was also obtained via chemical substitution without requiring ‘hard–soft’ synthesis in SrCr1−xFexO3−y perovskites (0.4 ≤ x ≤ 0.6). These are ferromagnetic materials with ordering temperatures of 225–340 K.5 ‘Hard–soft’ chemistry was also applied to CaCrO3 and three new reduced CaCrO3−δ phases (δ = 0.33, 0.4 and 0.5) were obtained.6 Contrary to the SrCrO3−δ phases, CaCrO3−δ show vacancy ordering along the (100) direction of the cubic perovskite cell, and are related to the brownmillerite structure, see Fig. 1c. The brownmillerite CaCrO2.5 has Cr3+ in an unusual tetrahedral environment and shows antiferromagnetic ordering at 220 K.7
In view of the very different vacancy superstructures of SrCrO3−δ and CaCrO3−δ phases, it is of interest to discover how these structures evolve across the Sr1−xCaxCrO3−δ series, and whether any new intermediate structures might be formed. Here we report the ‘hard–soft’ synthesis of Sr1−xCaxCrO3−δ phases for x = 0.25, 0.5 and 0.75, and the vacancy superstructures adopted.
Experimental
The “hard” high pressure syntheses of Sr1−xCaxCrO3 perovskite precursors used a similar approach to previous preparations of SrCrO38,9 and CaCrO3.10 Stoichiometric amounts of Ca3Cr2O8, Sr3Cr2O8 and Cr2O3 were combined in the appropriate ratio. Sr3Cr2O8 (Ca3Cr2O8) was prepared by heating pellets of SrCO3 (CaCO3) and Cr2O3 in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 1100 °C in a tube furnace under flowing Ar gas, followed by quenching into liquid nitrogen. High pressure syntheses were carried out using a two-stage Walker-type press. Samples were heated at temperatures of 900–1100 °C and pressures 8–9 GPa with reaction times of 30 min. The Sr1−xCaxCrO3 perovskite phases were reduced at “soft” conditions in a tube furnace at 400–525 °C under a flowing 10% H2/90% Ar gas mixture at ambient pressure for 12 hours. Laboratory powder X-ray diffraction (PXRD) profiles from flat-plate samples were recorded on a Bruker D2 instrument using Cu-Kα radiation.
1 ratio at 1100 °C in a tube furnace under flowing Ar gas, followed by quenching into liquid nitrogen. High pressure syntheses were carried out using a two-stage Walker-type press. Samples were heated at temperatures of 900–1100 °C and pressures 8–9 GPa with reaction times of 30 min. The Sr1−xCaxCrO3 perovskite phases were reduced at “soft” conditions in a tube furnace at 400–525 °C under a flowing 10% H2/90% Ar gas mixture at ambient pressure for 12 hours. Laboratory powder X-ray diffraction (PXRD) profiles from flat-plate samples were recorded on a Bruker D2 instrument using Cu-Kα radiation.
Results and discussion
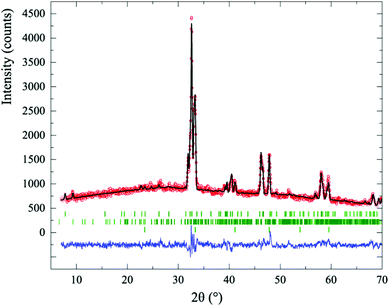
The Sr1−xCaxCrO3 (x = 0.25, 0.5 and 0.75) solid solutions made by “hard” high pressure syntheses were found to have the perovskite structure with lattice parameters similar to those reported previously.11 These precursor materials were initially reduced at 400 °C for 12 hours. PXRD was performed after each 12 hour reduction to determine if any new phases had been formed, and the reaction was repeated at a temperature 25 °C higher until decomposition occurred.Powder X-ray diffraction patterns for all of the Sr1−xCaxCrO3−δ (x = 0.25, 0.50, 0.75) compositions showed that two new crystalline products were formed at temperatures between 400 and 525 °C. Both phases had complex diffraction patterns related to that of their perovskite precursor. It was difficult to acquire single-phase samples of the two products because of their similar formation conditions, so their oxygen contents could not be measured directly. However the two phases were identified as being isostructural with 15R-SrCrO2.8 and 6H–SrCrO2.75 on the basis of their low angle X-ray diffraction peaks, and oxygen contents were assigned on this basis. These mixed-phase products showed a superstructure peak at 2θ ≈ 7.8°, similar to that for 15R–SrCrO2.8, and at 2θ ≃ 9.3° as observed for 6H–SrCrO2.75 (Fig. 2).
Powder X-ray diffraction peaks from the δ = 0.2 Sr1−xCaxCrO2.8 phases were indexed on a rhombohedral (R) unit cell (space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) with a long (≈35 Å) c-axis. This structure has a repeat sequence of 15 close-packed SrO3 layers stacked perpendicular to the (111) (body-diagonal) direction of the cubic perovskite structure. This 15R polymorph was the predominant reaction product during initial, lower temperature reductions. X-ray diffraction peaks from the δ = 0.25 phase were indexed on a primitive monoclinic cell, showing a distorted stacking of six hexagonal cubic close-packed layers perpendicular to the cubic(111) direction. This 6H polymorph was seen to emerge later in the reduction sequence, consistent with a smaller oxygen content.
m) with a long (≈35 Å) c-axis. This structure has a repeat sequence of 15 close-packed SrO3 layers stacked perpendicular to the (111) (body-diagonal) direction of the cubic perovskite structure. This 15R polymorph was the predominant reaction product during initial, lower temperature reductions. X-ray diffraction peaks from the δ = 0.25 phase were indexed on a primitive monoclinic cell, showing a distorted stacking of six hexagonal cubic close-packed layers perpendicular to the cubic(111) direction. This 6H polymorph was seen to emerge later in the reduction sequence, consistent with a smaller oxygen content.
Rietveld fits using 15R and 6H starting models from previously reported SrCrO3−δ phases3 gave good fits to the PXRD data. The Rietveld refinement for x = 0.25 is shown in Fig. 2 and illustrates the coexistence of the three phases δ = 0, 0.2 and 0.25 seen in many samples. Phase fractions from Rietveld refinements demonstrate that the proportion of the 6H (δ = 0.25) phase increases relative to the 15R (δ = 0.2) fraction as the level of Ca doping x increases. For example, in samples reduced at 500 °C, Sr0.75Ca0.25CrO3−δ had respective phase fractions of 26/24/50% (±1%) for the δ = 0 (precursor), 0.2 (15R) and 0.25 (6H) phases while Sr0.5Ca0.5CrO3−δ had corresponding fractions of 0/21/79%, and Sr0.25Ca0.75CrO3−δ had 0/17/83%.
Variations of the lattice parameters in the 15R and 6H Sr1−xCaxCrO3−δ phases are plotted in Fig. 3. All cell lengths generally decrease as Ca is smaller than Sr. The 15R-Sr1−xCaxCrO2.8 phases show a steady decline in both a and c lattice parameters with x, from a = 5.51430(4) Å and c = 34.4645(3) Å for SrCrO2.8 to a = 5.406(2) Å and c = 33.50(2) Å for Sr0.25Ca0.75CrO2.8. The a and b lattice parameters for the 6H phases also decrease with x, but there appears to a correlation between c and the β angle leading to anomalous changes between x = 0.50 and x = 0.75 in Fig. 3. Cell volume decreases by similar amounts, 6.6 and 4.0% for 15R and 6H respectively, in the two series between x = 0 and 0.75.
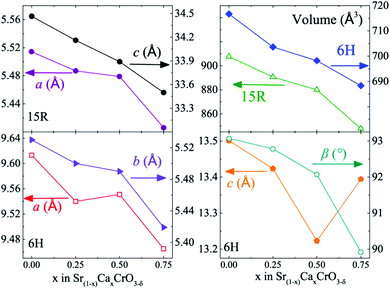 | ||
| Fig. 3 Plots of cell parameters and volume against calcium content (x) for 15R (δ = 0.2) and 6H (δ = 0.25) Sr1−xCaxCrO3−δ phases. Data for x = 0 are taken from ref. 3. | ||
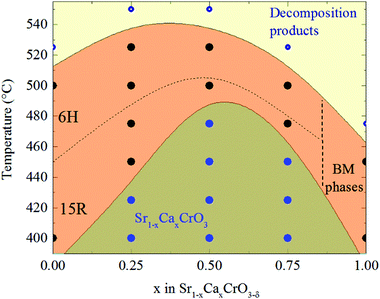
The surprising result of this study is that the reduced Sr1−xCaxCrO3−δ phases have the same 15R and 6H structures as the previously reported SrCrO3−δ phases,3 rather than the CaCrO3−δ type structures,6 even when the calcium content is as large as x = 0.75 (Fig. 4). None of the brownmillerite type superstructures observed for CaCrO3−δ were seen in the PXRD pattern of Sr0.25Ca0.75CrO3−δ. Vacancies in Sr1−xCaxCrO3−δ phases are formed in (111)-type c′ planes, like SrCrO3−δ, but unlike CaCrO3−δ in which the deficient layers are parallel to cubic (100) planes. The reconstructed c′ layers in both Sr1−xCaxCrO3−δ phases show direct relaxation of octahedral to tetrahedral coordination of the adjacent chromium cations without the formation of a five coordinate square pyramidal intermediate, most probably resulting from the novel “hard–soft” approach as discussed elsewhere.3,6 The Sr1−xCaxCrO3−δ structures, like SrCrO3−δ, distribute their oxide vacancies into widely (five- or six-layer) spaced (111) planes between layers of the parent perovskite in-between.
Combining results from the present study with those from the earlier investigations of the SrCrO3−δ and CaCrO3−δ systems enables an approximate phase diagram for the hard–soft chemistry of the Sr1−xCaxCrO3−δ system to be constructed as shown in Fig. 4. This displays several striking features. The lower temperature for formation of Sr1−xCaxCrO3−δ superstructure phases under reduction in 10% H2/90% Ar shows an approximately symmetric variation with a maximum near x = 0.5. Both SrCrO3 and CaCrO3 form reduced superstructure phases at 400 °C, whereas the onset for Sr0.5Ca0.5CrO3 under the same reduction conditions is between 475 °C, where no reduction product was observed, and 500 °C. This effect is unlikely to be due to changes in the average crystal structures, as these are expected to vary monotonically with x. Instead this observation evidences a disorder effect, as Ca/Sr cation disorder is maximised at x = 0.5. A possible explanation is that this cation disorder traps oxygen vacancies, so higher temperatures are needed to enable the vacancies to overcome kinetic barriers to migrate and self-organise into the well-separated c′ defect layers in the 6H and 15R structures for x = 0.5. This effect of cation disorder on vacancy migration might be relevant to understanding of oxide ion migration in chromium perovskite mixed conductors used in fuel cell anodes such as (La1−xSrx)(Cr1−yMy)O3−δ (M = Mn, Fe, Co, Ni).12 The upper stability limit for the latter Sr1−xCaxCrO3−δ superstructure phases also shows a maximum at intermediate x, being 525 °C for x = 0.25 and 0.50, but 500 °C for x = 0 and 0.75, so cation disorder again appears to play a role. The brownmillerite-related superstructures observed for CaCrO3−δ decompose above 450 °C.6 The region of the phase diagram between x = 0.75 and 1 clearly merits further investigation to discover how structure evolves between the SrCrO3−δ and CaCrO3−δ types, and whether any new intermediate structures are present.
Conclusions
In summary, reduced phases in the Sr1−xCaxCrO3 system prepared by hard–soft chemistry for x = 0.25, 0.50, and 0.75 compositions form the same 15R and 6H superstructure phases as observed in SrCrO3−δ. The alternative brownmillerite related superstructures reported for CaCrO3−δ are not observed even in the Ca-rich x = 0.75 composition. The Sr1−xCaxCrO3−δ phase diagram shows that the lower temperature for superstructure formation has a maximum near x = 0.5, evidencing an influence of Ca/Sr cation disorder on oxygen vacancy order. The region of the phase diagram between x = 0.75 and 1 requires further investigation to discover how structure evolves between the reduced SrCrO3−δ and CaCrO3−δ types, and whether any further intermediate superstructures remain to be discovered.Acknowledgements
We acknowledge support from EPSRC and the Royal Society.References
- J. A. Rodgers, A. J. Williams and J. P. Attfield, Z. Naturforsch., B: J. Chem. Sci., 2006, 61, 1515 CAS.
- M. A. Hayward, Semicond. Sci. Technol., 2014, 29, 064010 CrossRef.
- A. M. Arevalo-Lopez, J. A. Rodgers, M. S. Senn, F. Sher, J. Farnham, W. Gibbs and J. P. Attfield, Angew. Chem., Int. Ed., 2012, 51, 10791 CrossRef CAS PubMed.
- K. H. L. Zhang, P. V. Sushko, R. Colby, Y. Du, M. E. Bowden and S. A. Chambers, Nat. Commun., 2014, 5, 4669 CAS.
- A. M. Arevalo-Lopez, F. Sher, J. Farnham, A. J. Watson and J. P. Attfield, Chem. Mater., 2013, 25, 2346 CrossRef CAS.
- A. M. Arevalo-Lopez, B. Liang, M. S. Senn, C. Murray, C. Tang and J. P. Attfield, J. Mater. Chem. C, 2014, 2, 9364 RSC.
- A. M. Arevalo-Lopez and J. P. Attfield, Dalton Trans., 2015, 44, 10661 RSC.
- A. J. Williams, A. Gillies, J. P. Attfield, G. Heymann, H. Huppertz, M. J. Martínez-Lopez and J. A. Alonso, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 104409 CrossRef.
- L. Ortega-San-Martin, A. J. Williams, J. Rodgers, J. P. Attfield, G. Heymann and J. Huppertz, Phys. Rev. Lett., 2007, 99, 255701 CrossRef PubMed.
- M. A. Alario-Franco, E. Castillo-Martinez and A. M. Arevalo-Lopez, High Pressure Res., 2009, 29, 254 CrossRef CAS.
- E. Castillo-Martinez, A. Duran and M. A. Alario-Franco, J. Solid State Chem., 2008, 181, 895 CrossRef CAS.
- P. I. Cowin, C. T. G. Petit, R. Lan, J. T. S. Irvine and S. Tao, Adv. Energy Mater., 2011, 1, 314 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Rietveld refinement fits and results. See DOI: 10.1039/c6qm00136j |
| ‡ Open data for this article are at http://datashare.is.ed.ac.uk/handle/10283/838 |
| This journal is © the Partner Organisations 2017 |