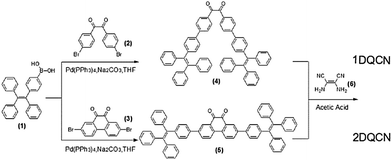
Dicyanopyrazine capped with tetraphenylethylene: polymorphs with high contrast luminescence as organic volatile sensors†
Chao
Ge
 ,
Yang
Liu
*,
Xin
Ye
,
Xiaoxin
Zheng
,
Quanxiang
Han
,
Jie
Liu
and
Xutang
Tao
,
Yang
Liu
*,
Xin
Ye
,
Xiaoxin
Zheng
,
Quanxiang
Han
,
Jie
Liu
and
Xutang
Tao
State Key Laboratory of Crystal Materials, Shandong University, Jinan 250100, P. R. China. E-mail: liuyangicm@sdu.edu.cn
First published on 12th September 2016
Abstract
Recently the polymorphism and phase-transition between polymorphs have attracted special attention in organic luminescent materials. In this report, we employed propeller-like tetraphenylethylenes to functionalize large conjugated and electron-withdrawing dicyanopyrazine units, constructing novel organic luminescent polymorphs with high contrast fluorescence. Through an in-depth study of the luminescence-related properties, the more planar structured 2DQCN was found to generate close intermolecular packing and demonstrated essentially different solid state luminescence behaviour compared with that of 1DQCN. It shows crystallization-induced red-shift fluorescence, in contrast to the crystallization-induced blue-shift of 1DQCN and many other AIE (aggregation-induced emission) materials. The morphology transformation of 2DQCN can be realized via solvent fuming and thermal annealing, where the transformation among its three forms was manifested by DSC measurements. Utilizing the high contrast luminescence of 2DQCN polymorphs, we demonstrated their real applications as selective visual sensors for detecting volatile organic compounds. The results, revealing insights into the structure–property relationship of luminescent polymorphs, should provide useful directions for the understanding, design and utilization of luminescent colour-switching materials.
Introduction
Efficient organic luminescent materials have been widely used in many light-emitting fields, such as organic light-emitting diodes (OLEDs), biological fluorescent probing and labelling.1–6 Among them, solid-state organic emitters are universally regarded to be more desirable because of their convenient fabrication and use.7–9 During the massive investigations on the emission behaviours of organic materials in the solid state, researchers have noted that the polymorphism in many organic luminescent materials has attracted special interest.10,11 Being known as polymorphs, the multiple forms or crystal structures of a solid luminescent material may exhibit totally distinct emissive properties.12–14 This means the solid-state emission is controlled by the molecular structures as well as the molecular packing styles.15,16 Polymorphs can switch fluorescence in response to external stimuli, and thus have potential applications in sensors, security materials, and information display/storage.17–20 Additionally, the definite molecular conformation and packing style in the polymorphs of organic emitters make them ideal models for disclosing structure–property relationships. So, the exploration of polymorphism characteristics is of particular significance and has attracted tremendous attention in these years.21–24 In addition to modifying the emission intensity and colour of organic chromophores via chemical structure modification, so far increasing attention has been paid to intermolecular interactions, such as hydrogen bonds and π–π interactions which can profoundly influence the packing motifs and cause polymorph transformation, to achieve emission changes.25,26 It is worth noting that organic polymorphism is a simple and effective method to modify material emission, which can avoid the lengthy process of the synthesis of traditional organic materials.27 Accordingly, design of new organic polymorphs and comprehending the relationship among the molecular configuration, the molecular arrangement and the optical properties have proved to be important for both academic research and advancement in real applications.28–30 In this field, Fraser and co-workers reported polymorphism of difluoroboron avobenzone and found that different polymorphs exhibited green, cyan, and blue emission by changing the intermolecular interaction and the mode of the molecular packing.31 Park, Yamaguchi and Wang et al. emphasized the importance of the intermolecular interactions that were responsible for the specific molecular stacking architecture, thus leading to different optical properties in different environments.32–34 Araki, Xu and Nakamura et al. analyzed the fluorescence changes in different morphologies realized by changing the modes of the molecular packing under various external stimuli.35–37 Our group has studied fluorenyl-containing tetrasubstituted ethylene derivatives that exhibited reversible solid-state luminescence switching caused by the transformation of molecular geometries and packing motifs.38 These reports clearly demonstrated the tuning effect of the molecular arrangements on the fluorescence changes. Besides, the compounds with tetrasubstituted ethylenes are found to be particularly applicable to construct luminescent polymorphs, because of their loose packing and ease of phase transformation.39 However, most of the organic luminescent polymorphs display a low contrast ratio in luminescence and colour, making the contrast not obvious to distinguish by the naked eye.40 So in this work, we employed tetraphenylethylene (TPE) to functionalize large conjugated units bearing strong electron-withdrawing groups, aiming to construct luminescent molecules with special intermolecular interactions. The coplanar and electron-deficient centres of the molecules hopefully strengthen the intermolecular stacking, adding an opposite effect for the peripheral propeller-like TPEs. In order to design and achieve novel organic luminescent polymorphs with high contrast fluorescence, we will conduct an in-depth analysis of the relationship between the structures and luminescence performances, and develop new sensing applications.Results and discussion
Here we report two compounds, 5,6-bis(4′-(1,2,2-triphenylvinyl)-[1,1′-biphenyl]-4-yl)pyrazine-2,3-dicarbonitrile (1DQCN) and 6,11-bis(4-(1,2,2-triphenylvinyl)phenyl)dibenzoquinoxaline-2,3-dicarbonitrile (2DQCN), whose structures are shown in Scheme 1. The compounds are composed of multiple functional groups, where the dicyanopyrazine as the centre is capped with bilateral TPEs. TPE as an AIE (aggregation-induced emission)-active unit has proved to significantly enhance the solid-state luminescence efficiency with its non-planar and bulky structure. Dicyanopyrazine as an electron withdrawing unit can not only increase the molecular electron affinity, but may also effectively result in extra intermolecular interactions via π–π and C–H⋯N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C hydrogen bonds. Such structure characteristics affected by both a twisted conformation and multiple intermolecular interactions ultimately make the polymorphs possible.
C hydrogen bonds. Such structure characteristics affected by both a twisted conformation and multiple intermolecular interactions ultimately make the polymorphs possible.
1DQCN and 2DQCN were prepared by a facile two-step approach and the synthetic routes to the two compounds are depicted in Scheme 2. The first key synthesis step to obtain the intermediate products 1,2-bis(4′-(1,2,2-triphenylvinyl)-[1,1′-biphenyl]-4-yl)ethane-1,2-dione (4) and 2,7-bis(4-(1,2,2-triphenylvinyl)phenyl)phenanthrene-9,10-dione (5) is the palladium-catalyzed Suzuki-coupling reaction, which involves the reaction of 4-(1,2,2-triphenylvinyl)phenylboronic acid (1) coupled with 1,2-bis(4-bromophenyl)ethane-1,2-dione (2) and 2,7-dibromophenanthrene-9,10-dione (3) in high yields, respectively. Then a subsequent acetic acid mediated coupling reaction produced the target compounds 1DQCN and 2DQCN, by treating the intermediate compounds (4) and (5) with 2,3-diaminofumaronitrile (6), respectively. We successfully isolated and obtained the pure solid 1DQCN and 2DQCN by using column chromatography. Detailed synthesis procedures and characterization of the key intermediates and final products are presented in the ESI.† The identity and purity of the products were verified by 1H NMR, 13C NMR, and high-resolution mass spectral data with satisfactory results.
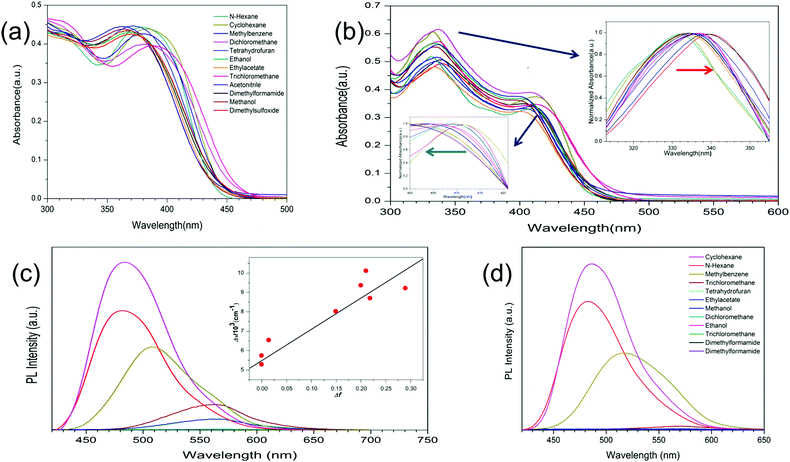
Both 1DQCN and 2DQCN have good solubility in common organic solvents, such as tetrahydrofuran (THF), dichloromethane and acetone. The UV-visible absorption and fluorescence spectra of 1DQCN and 2DQCN in different solvents are shown in Fig. 1. The two compounds exhibit intense absorption in the UV-visible region, both characterized by two absorption bands. Taking 2DQCN as an example, the absorption at λmax = 330–340 nm should correspond to the π → π* transitions of the large polycyclic π-conjugated system; the other absorption band at λmax = 400–412 nm should be attributed to a charge-transfer transition, due to the electron-withdrawing effect of the dicyanopyrazine group. Since the more coplanar centre part of 2DQCN will generate an enlarged π-conjugated system compared with that of 1DQCN, the absorption band of 2DQCN exhibits a small bathochromic shift. In addition, it is worth noting that the two compounds' absorption shows obvious dependence on the polarity of the solvents. As shown in the insets of Fig. 1(b), the π → π* transition bands (330–340 nm) red-shift, while the charge-transfer transition bands (400–412 nm) blue-shift being accompanied by an increase of the solvent polarity.
Because of the two TPE units in the molecules, 1DQCN and 2DQCN demonstrate typical AIE characteristics. While attributed to the large polycyclic π-conjugated dicyanopyrazine parts, their solutions still release a faint light under UV irradiation. We measured their fluorescence spectra according to the different polarities of the solvent. As shown in Fig. 1(c) and (d), 1DQCN and 2DQCN distinctly show almost the same emission wavelength in the same solvent (e.g., 485 nm of 1DQCNvs. 486 nm of 2DQCN, in cyclohexane). The fluorescence intensities of the two compounds decrease and the emission peaks show an apparent red-shift as the solvent polarity increases. We believe that the solvatochromism is caused by photoinduced intramolecular charge transfer (ICT) in the excited state, owing to the electron-withdrawing effect of the dicyanopyrazine group. To further understand the solvent effects, the Stokes shifts of 1DQCN in different solvents were measured and the Lippert–Mataga equation was used to quantitatively describe its solvatochromic behaviour.41–43 The linear dependence of ΔV (the Stokes shift of 1DQCN) on Δf (the solvent polarity parameters, related to the dielectric constant and refractive index of the solvent) together with the large slope of the Δf–ΔV plot (the inset of Fig. 1(c)) suggests that the ICT excited-state has a larger dipolar moment than the ground state due to substantial charge redistribution, which is probably derived from the relaxation of the initially formed Franck–Condon excited state, instead of a direct transition from the ground state.
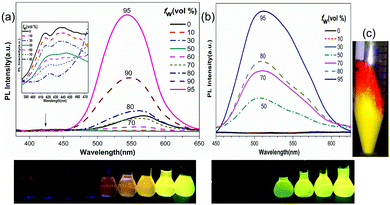
Considering the AIE characteristics of the two compounds, we also measured their photoluminescence (PL) spectra in THF solutions upon adding water. As shown in Fig. 2, the PL of the two compounds in pure THF is quite weak or even with a flat line parallel to the abscissa of the spectra. Upon adding water to the solutions to a water fraction (fw) of more than 50%, where the solubility of the mixture is worsened so much that the molecules form aggregates, the PL intensities start to increase dramatically. With a final water fraction of fw = 95%, the PL intensity increases several hundred times. It is interesting to mention that although 1DQCN bears a less coplanar centre than 2DQCN, the fluorescence of 1DQCN is not totally quenched under the condition of low water content. Its THF solution exhibits a weak orange emission when irradiated under the UV lamp, with two weak emission bands at approximately 420 nm and 550 nm, respectively. Upon adding water, the emission band at the shorter wavelength decreases persistently. In contrast, the one at a longer wavelength of 550 nm first decreases at small water fractions, then increases rapidly accompanied by aggregate formation. Like many other TPE or silole derivatives, the restriction of intramolecular rotation in the aggregated state is believed to account for the AIE characteristics in the two compounds.44 We consider that the difference in their AIE behaviours originates from the rotational steric hindrance. As shown in the geometry-optimized molecular structure plots (optimized by using density functional theory calculations at the DFT/B3LYP/6-31G(d) level, shown in Fig. S1, ESI†), the V-shaped 1DQCN should have more severe rotational steric hindrance than the linear 2DQCN molecule. In any case, the high conjugation and twisted structures of bulky aromatic substituents are efficiently beneficial to facilitate the aggregation luminescence of the materials.
The polymorphism of the compounds was discovered in the process of purification, where a precipitation method was used by slowly adding a 2DQCN solution in dichloromethane dropwise into a vigorously stirred methanol solvent. After centrifugation and removal of the solvent, the yellow powders were kept in the centrifuge tube. Interestingly we found that the yellow materials turned red gradually from the upper part to the bottom part (Fig. 2(c)). After about three days, nearly all the materials became red. Fig. S2 (ESI†) depicts the whole process. We have checked the chemical identity and purity of the compound by 1H and 13C NMR, excluding the possibility of any chemical changes. The experience of our former studies on fluorenyl-substituted ethylenes reminded us that the colour change of the compound may have arisen from polymorphism.38 Thus we examined the powder X-ray diffraction (PXRD) patterns of the materials with different colours. The results reveal their different morphologies, being amorphous for the yellow powder while crystalline for the red ones. This suggests that the colour change from yellow to red is actually a crystallization process. We repeated the crystallization of 1DQCN and 2DQCN from their solutions in methanol/dichloromethane by using slow volatilization and cooling methods. Because of the large dimensions of the molecules, we still didn't grow crystals with large enough size for single-crystal XRD structure determination. Nevertheless, as shown in Fig. 3(c) and Fig. S4 (ESI†), from the two isolated phases we confirmed the morphology-dependent luminescence of 2DQCN in the red crystalline state and the yellow amorphous state. The contrasts in the emission colour of the two different morphologies are extremely high and easy to identify by the naked eye. Another noteworthy phenomenon in the emission of 2DQCN is the crystallization-induced red-shift fluorescence, which is apparently different from that of many other AIE chromophores. For AIE materials, especially those bearing tetrasubstituted ethylenes, they normally possess another crystallization-induced blue-shift characteristic, which is attributed to a more twisty non-planar conformation and weak intermolecular interactions in crystals compared with the more planarized conformation in the amorphous state.45 This reveals that the introduction of the coplanar and electron-deficient dicyanopyrazine into the molecular structures does affect the stacking mode of the molecules, where a more coplanar conformation and close packing are anticipated in the 2DQCN crystals.
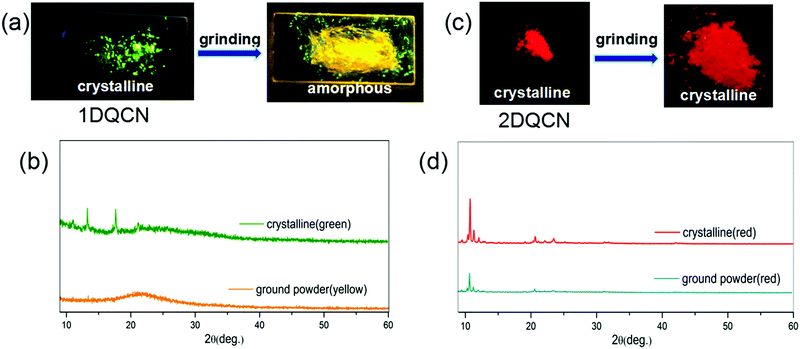 | ||
| Fig. 3 Fluorescence images of (a) 1DQCN and (c) 2DQCN before and after grinding under UV illumination. The corresponding PXRD patterns of (b) 1DQCN and (d) 2DQCN powder in different forms. | ||
According to previous studies, a great number of fluorescent polymorphs bearing propeller-shaped tetrasubstituted ethylene groups and AIE characteristics are usually inclined to be affected by external stimuli because of the loose packing and mutable conformations.46 Mechanochromic luminescence is one of the most typical responses.47 Thus we checked the stimuli-responsive luminescence behaviour of the two compounds by grinding their crystals. The crystals of 1DQCN grown from a mixture of solvents exhibit green emission, as shown in Fig. 3(a). When we ground the 1DQCN crystals with a pestle, the fluorescence of 1DQCN switched simultaneously during the process of grinding, from green-emissive to yellow-emissive fluorescence, which corresponds to the emission in the amorphous state. The emission spectra of both 1DQCN and 2DQCN before and after being ground are shown in Fig. S3 (ESI†). Fig. 3(b) presents the PXRD patterns of the initial crystals and the ground powders, from which we can see that the original crystals have sharp diffraction peaks while the diffraction pattern of the ground powders is nearly a flat line with inconspicuous broad peaks. This indicates that the crystallinity of 1DQCN is obviously reduced by grinding, generating a disordered molecular packing in the ground powders. However, what's really interesting is the phenomenon occurring in 2DQCN. Although it exhibits high contrast in both colour and luminescence between the two morphologies, no matter how long we ground its crystals, the colour and luminescence showed no changes (Fig. 3(c)). The PXRD pattern in Fig. 3(d) shows the diffraction peaks of the ground powders being coincident with those of the initial crystals, confirming the fact that the red samples before and after grinding belong to the same crystalline morphology. Grinding of the yellow amorphous samples was also conducted, which showed no changes either (Fig. S4, ESI†). The results indicate that the more planar molecular conformation of 2DQCN generates strong intermolecular forces and close packing. Thus compared to 1DQCN, the crystals of 2DQCN possess higher lattice energy and better structural stability. The force of mechanical grinding cannot destroy the unit cell structure of 2DQCN crystals.
Now we can conclusively compare the luminescence-related properties between 1DQCN and 2DQCN. With a more planar molecular structure, 2DQCN has almost the same emission wavelength as 1DQCN in solutions, but essentially different luminescence behaviours in the solid state. 1DQCN exhibits crystallization-induced blue-shift fluorescence like many other AIE materials; in contrast 2DQCN shows crystallization-induced red-shift fluorescence. What's more, 1DQCN is capable of mechanochromic luminescence, while 2DQCN is not. The results reveal the fact that tiny chemical structure modifications, which have hardly any effect on the optical properties of the isolated molecules, may substantially change the luminescence behaviours in the aggregate state.
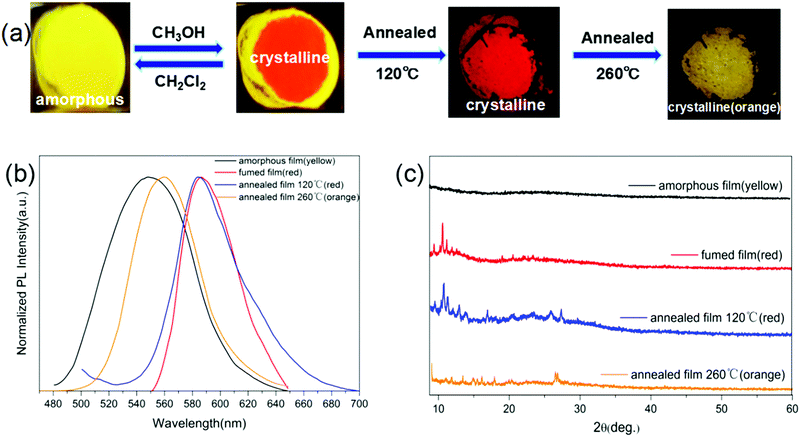
Although without mechanochromic luminescence ability, the morphology conversion of 2DQCN can be realized via solvent fuming and thermal annealing. As shown in Fig. 4(a), a spin-coated 2DQCN film on a quartz substrate emits yellow light, indicating an amorphous morphology. Upon fuming the centre parts by using methanol vapour, the fumed parts became red-emissive in about ten seconds, indicating a crystallization process. The conversion can be reversed by fuming with dichloromethane vapour. We can monitor the process and distinguish the morphology easily by the red or yellow luminescence colour. The yellow-to-red crystallization is also able to proceed under thermal annealing (Fig. 4(a)). A yellow amorphous film turned red in several minutes under 120 °C annealing. What's more, if we continued to anneal the red crystalline film at 260 °C for 10 min, it converted into a new phase with orange emission. The TGA of 2DQCN (Fig. S5, ESI†) and the consistent NMR spectra after annealing (Fig. S6, ESI†) further excluded any chemical changes of the sample. Fig. 4(b) depicts the fluorescence spectra of all the samples in the morphology conversion process, from which we can see that the spectral profiles of the two red crystalline films obtained via methanol fuming and thermal annealing coincide with each other, and the orange phase obtained through 260 °C annealing differs from both the yellow amorphous and red crystalline phases. Fig. 4(c) presents the PXRD patterns of all the samples, confirming the same crystalline structure of the two red films, and the orange phase of 2DQCN formed by 260 °C annealing belonging to a new crystalline phase.
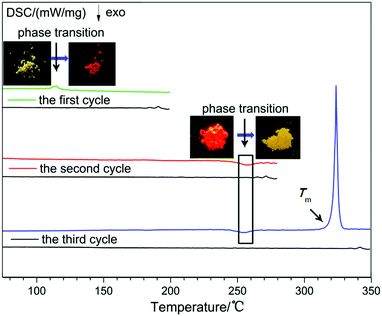
To unearth the thermal annealing process of 2DQCN, differential scanning calorimetric (DSC) curves were recorded for three cycles of heating and cooling. As shown in Fig. 5, in the first cycle for the yellow amorphous sample, the DSC curves showed an endothermic peak at around 112 °C; no additional peak was observed in the cooling process. After the first cycle we checked the samples remaining in the aluminium crucible, finding that the yellow samples had turned into red ones (the inset of Fig. 5). This suggests that the endothermic peak at 112 °C corresponds to the crystallization point from an amorphous to a crystalline state, and it is a monotropic conversion, so we obtained red crystals upon cooling to room temperature. The red crystals were sequentially heated in the second cycle. A weak exothermic peak was observed at 250 °C, which after checking the samples in the crucible was confirmed to be corresponding to the phase transition from the red crystals to the orange crystals. The monotropic transformation suggests that the thermodynamic stability of the orange crystal should be higher than that of the red crystal, that is, the red crystal is a kinetically stable polymorph and the orange crystal is a thermodynamically stable one. In the third cycle with the red crystals as starting materials, an endothermic peak at 320 °C appeared, corresponding to the melting of the orange crystal. In the cooling process no crystallization peak was observed, indicating that the crystallization capacity of 2DQCN is quite low. After cooling of the melted sample, it transformed into a glassy state.
Considering the high contrast and rapid switching ability of 2DQCN polymorphs, we studied their potential applications as chemical sensors for detecting volatile organic compounds (VOCs). As shown in Fig. 6, the visual and luminescent sensor patterns for a large family of VOCs were established based on the vapochromism luminescence of the polymorphs. The red crystals of 2DQCN compacted onto a quartz plate act as the starting panels; then each panel is exposed to different VOC vapours. The red 2DQCN film gradually changed its colour and luminescence to yellow upon exposure to the VOC vapors. By comparison of the extent of change we can distinguish among different VOCs. Fig. 6 recorded the extent of colour and fluorescence changes of a series of panels after exposure to VOCs for 1 min, 5 min and 10 min, respectively. We can see that exposure to n-hexane, cyclohexane, methanol, and dimethyl sulfoxide (DMSO) vapors did not alter the colour and luminescence of the panels; but when the red 2DQCN film was exposed to chloroalkane (CH2Cl2, CHCl3, CCl4, CH2ClCH2Cl), toluene, ethyl acetate (EtOAc), tetrahydrofuran (THF), acetone and dimethyl formamide (DMF) vapors, the red panels underwent an obvious colour and luminescence change from red to yellow. In addition, the speed of the colour change for different VOCs is different. We regard the vapochromism of 2DQCN to have resulted from direct interactions between the crystalline lattice and the adsorbed VOC molecules. VOCs that are able to disturb the packing in 2DQCN crystals induce amorphization accompanied by a colour change. So the solvent vapors with moderate polarities, such as CH2Cl2 and THF, can significantly increase the response, while the solvents with either low polarities or high polarities, such as cyclohexane and DMSO, can hardly affect the crystal lattice and trigger no response. Fig. S7 (ESI†) shows the XRD patterns of 2DQCN crystals before and after being fumed with different solvents. Therefore, by ingenious design 2DQCN can hopefully be used as a visual colour and luminescence response sensor for detecting VOCs with high sensitivity and selectivity, as a convenient auxiliary to the complicated chromatography (GC)-mass spectrometry-based VOC sensing systems.48,49
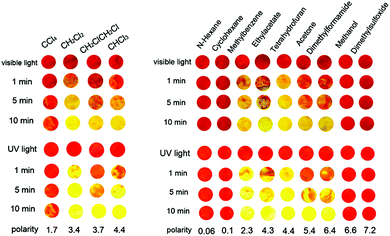 | ||
| Fig. 6 Responses of vapochromic and vapoluminescent sensor patterns of 2DQCN crystalline films after exposure to different VOCs for 1 min, 5 min and 10 min, respectively. | ||
Conclusions
In summary, we have designed and synthesized two luminescent switching materials 1DQCN and 2DQCN by decorating the electron-withdrawing dicyanopyrazine with propeller-like TPEs. This kind of architecture on one side endows the compounds with remarkable intramolecular charge transfer characteristics, and on the other hand leads to polymorphism with high contrast colour and luminescence. Owing to a more coplanar molecular conformation, 2DQCN shows crystallization-induced red-shift fluorescence, while the more twisted 1DQCN exhibits crystallization-induced blue-shift fluorescence. And moreover, grinding of 1DQCN crystals leads to amorphization and mechanochromic luminescence, while grinding of 2DQCN crystals doesn't affect their crystalline lattice, due to a stronger intermolecular force and closer packing. 2DQCN demonstrates three distinguishable polymorphs with yellow, red, and orange colour and luminescence. Switching between the polymorphs can be achieved by solvent fuming and thermal annealing. Taking advantage of the high contrast and rapid switching morphology-dependent fluorescence, 2DQCN can be used as a visual sensor for detecting VOCs. The present study discloses a particular structure–property relationship between the luminescent polymorphs of organic emitters, and also provides a perspective of the potential applications of these luminescence-switching polymorphs in the fields of optical displays and visual sensors for multiple external stimuli.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 51321091, 51227002, 51303095, and 51272129), the Program of Introducing Talents of Disciplines to Universities in China (111 program no. b06015), and the Fundamental Research Funds of Shandong University.Notes and references
- Y. Watanabe, H. Sasabe, D. Yokoyama, T. Beppu, H. Katagiri and J. Kido, J. Mater. Chem. C, 2016, 4, 3699–3704 RSC.
- H. Yanagi, T. Morikawa and S. Hotta, Appl. Phys. Lett., 2002, 81, 1512–1514 CrossRef CAS.
- K. P. Divya, S. Savithri and A. Ajayaghosh, Chem. Commun., 2014, 50, 6020–6022 RSC.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS PubMed.
- U. Resch-Genger, M. Grabolle, S. Cavaliere-Jaricot, R. Nitschke and T. Nann, Nat. Methods, 2008, 5, 763–775 CrossRef CAS PubMed.
- W. Qin, K. Li, G. Feng, M. Li, Z. Yang, B. Liu and B. Z. Tang, Adv. Funct. Mater., 2014, 24, 635–643 CrossRef CAS.
- M. Shimizu and T. Hiyama, Chem. – Asian J., 2010, 5, 1516–1531 CrossRef CAS PubMed.
- F. G. Gao and A. J. Bard, J. Am. Chem. Soc., 2000, 122, 7426–7427 CrossRef CAS.
- Y. Liu, X. Chen, Y. Lv, S. Chen, J. WY Lam, F. Mahtab, H. S. Kwok, X. Tao and B. Z. Tang, Chem. – Eur. J., 2012, 18, 9929–9938 CrossRef CAS PubMed.
- N. Sanz, P. L. Baldeck, J.-F. Nicoud, Y. Le Fur and A. Ibanez, Solid State Sci., 2001, 3, 867–875 CrossRef CAS.
- S. P. Anthony and S. M. Draper, J. Phys. Chem. C, 2010, 114, 11708–11716 CAS.
- P. Galer, R. C. Korošec, M. Vidmar and B. Šket, J. Am. Chem. Soc., 2014, 136, 7383–7394 CrossRef CAS PubMed.
- Z. Zhang, Y. Zhang, D. Yao, H. Bi, I. Javed, Y. Fan, H. Zhang and Y. Wang, Cryst. Growth Des., 2009, 9, 5069–5076 CAS.
- X. Ye, Y. Liu, Y. Lv, G. Liu, X. Zheng, Q. Han, K. A. Jackson and X. Tao, Angew. Chem., Int. Ed., 2015, 127, 8087–8091 CrossRef.
- Y. Li, G. Vamvounis and S. Holdcroft, Macromolecules, 2002, 35, 6900–6906 CrossRef CAS.
- B.-K. An, J. Gierschner and S. Y. Park, Acc. Chem. Res., 2011, 45, 544–554 CrossRef PubMed.
- Q. Qi, J. Zhang, B. Xu, B. Li, S. X.-A. Zhang and W. Tian, J. Phys. Chem. C, 2013, 117, 24997–25003 CAS.
- S. P. Anthony, Chem. – Asian J., 2012, 7, 374–379 CrossRef CAS PubMed.
- S. M. Jeong, S. Song, S. K. Lee and N. Y. Ha, Adv. Mater., 2013, 25, 6194–6200 CrossRef CAS PubMed.
- Z. Zhang, B. Xu, J. Su, L. Shen, Y. Xie and H. Tian, Angew. Chem., Int. Ed., 2011, 50, 11654–11657 CrossRef CAS PubMed.
- Z. Zhang, X. Song, S. Wang, F. Li, H. Zhang, K. Ye and Y. Wang, J. Phys. Chem. Lett., 2016, 7, 1697–1702 CrossRef CAS PubMed.
- M.-S. Yuan, D.-E. Wang, P. Xue, W. Wang, J.-C. Wang, Q. Tu, Z. Liu, Y. Liu, Y. Zhang and J. Wang, Chem. Mater., 2014, 26, 2467–2477 CrossRef CAS.
- K. Wang, H. Zhang, S. Chen, G. Yang, J. Zhang, W. Tian, Z. Su and Y. Wang, Adv. Mater., 2014, 26, 6168–6173 CrossRef CAS PubMed.
- S. Varghese and S. Das, J. Phys. Chem. Lett., 2011, 2, 863–873 CrossRef CAS PubMed.
- S.-J. Yoon and S. Park, J. Mater. Chem., 2011, 21, 8338–8346 RSC.
- Y. Li, F. Li, H. Zhang, Z. Xie, W. Xie, H. Xu, B. Li, F. Shen, L. Ye and M. Hanif, Chem. Commun., 2007, 231–233 RSC.
- Z. He, L. Zhang, J. Mei, T. Zhang, J. W. Lam, Z. Shuai, Y. Q. Dong and B. Z. Tang, Chem. Mater., 2015, 27, 6601–6607 CrossRef CAS.
- J. Bernstein, Polymorphism in molecular crystals, International Union of Crystallography, Oxford University Press, New York, 2002 Search PubMed.
- X. Cheng, Y. Zhang, S. Han, F. Li, H. Zhang and Y. Wang, Chem. – Eur. J., 2016, 22, 4899–4903 CrossRef CAS PubMed.
- T. Nicolini, A. Famulari, T. Gatti, J. Martí-Rujas, F. Villafiorita-Monteleone, E. V. Canesi, F. Meinardi, C. Botta, E. Parisini and S. V. Meille, J. Phys. Chem. Lett., 2014, 5, 2171–2176 CrossRef CAS PubMed.
- G. Zhang, J. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160–2162 CrossRef CAS PubMed.
- S.-J. Yoon, J. W. Chung, J. Gierschner, K. S. Kim, M.-G. Choi, D. Kim and S. Y. Park, J. Am. Chem. Soc., 2010, 132, 13675–13683 CrossRef CAS PubMed.
- K. Nagura, S. Saito, H. Yusa, H. Yamawaki, H. Fujihisa, H. Sato, Y. Shimoikeda and S. Yamaguchi, J. Am. Chem. Soc., 2013, 135, 10322–10325 CrossRef CAS PubMed.
- H. Zhang, Z. Zhang, K. Ye, J. Zhang and Y. Wang, Adv. Mater., 2006, 18, 2369–2372 CrossRef CAS.
- T. Mutai, H. Satou and K. Araki, Nat. Mater., 2005, 4, 685–687 CrossRef CAS PubMed.
- B. Xu, Z. Chi, X. Zhang, H. Li, C. Chen, S. Liu, Y. Zhang and J. Xu, Chem. Commun., 2011, 47, 11080–11082 RSC.
- H. Tsuji, G. M. O. Favier, C. Mitsui, S. Lee, D. Hashizume and E. Nakamura, Chem. Lett., 2011, 40, 576–578 CrossRef CAS.
- Y. Lv, Y. Liu, D. Guo, X. Ye, G. Liu and X. Tao, Chem. – Asian J., 2014, 9, 2885–2890 CrossRef CAS PubMed.
- X. Luo, W. Zhao, J. Shi, C. Li, Z. Liu, Z. Bo, Y. Q. Dong and B. Z. Tang, J. Phys. Chem. C, 2012, 116, 21967–21972 CAS.
- W. Z. Yuan, Y. Tan, Y. Gong, P. Lu, J. W. Lam, X. Y. Shen, C. Feng, H. H. Y. Sung, Y. Lu and I. D. Williams, Adv. Mater., 2013, 25, 2837–2843 CrossRef CAS PubMed.
- A. G. Gilani, S. Hosseini, M. Moghadam and E. Alizadeh, Spectrochim. Acta, Part A, 2012, 89, 231–237 CrossRef PubMed.
- R. Wang and R. Zenobi, J. Am. Soc. Mass Spectrom., 2010, 21, 378–385 CrossRef CAS PubMed.
- D. Rácz, M. Nagy, A. Mándi, M. Zsuga and S. Kéki, J. Photochem. Photobiol., A, 2013, 270, 19–27 CrossRef.
- J. Chen, C. C. Law, J. W. Lam, Y. Dong, S. M. Lo, I. D. Williams, D. Zhu and B. Z. Tang, Chem. Mater., 2003, 15, 1535–1546 CrossRef CAS.
- J. Wang, J. Mei, R. Hu, J. Z. Sun, A. Qin and B. Z. Tang, J. Am. Chem. Soc., 2012, 134, 9956–9966 CrossRef CAS PubMed.
- Y. Lv, Y. Liu, X. Ye, G. Liu and X. Tao, CrystEngComm, 2015, 17, 526–531 RSC.
- Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC.
- C.-H. Wu, C.-T. Feng, Y.-S. Lo, T.-Y. Lin and J.-G. Lo, Chemosphere, 2004, 56, 71–80 CrossRef CAS PubMed.
- F. Dincer, M. Odabasi and A. Muezzinoglu, J. Chromatogr. A, 2006, 1122, 222–229 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The geometry-optimized molecular structure plots, the whole transformation process of 2DQCN, the PL spectra of 1DQCN and 2DQCN after grinding, the fluorescence images and PXRD patterns of the 2DQCN amorphous state after grinding, the TGA of 2DQCN, the XRD patterns of 2DQCN crystalline films before and after exposure to VOCs, details of synthesis, elemental analysis, HRMS, and NMR spectra. See DOI: 10.1039/c6qm00146g |
| This journal is © the Partner Organisations 2017 |