Fluorescent C-dot nanocomposites as efficient photothermal agents and multi-modal imaging tracers†
Zhe
Liu
*ab,
Qien
Xu
ab,
Yihong
Li
ab and
Waner
Chen
ab
aWenzhou Institute of Biomaterials and Engineering, Wenzhou Medical University, Wenzhou 325011, Zhejiang, China. E-mail: liuzhe@wibe.ac.cn
bWenzhou Institute of Biomaterials and Engineering, Chinese Academy of Sciences, Wenzhou 325001, Zhejiang, China
First published on 12th September 2016
Abstract
Fluorescent C-dot nanocomposites (MB-CDs) have been developed as efficient photothermal agents for potential thermal ablation of cancerous cells. They are also promising multi-modal imaging tracers for both dual-channel cell tracking and photoacoustic imaging of ocular blood vessels. These features endow them with competency to serve in biomarker-targeted molecular imaging as well as in photothermal therapy against cancers.
Fluorescence (FL) imaging has witnessed a wide range of biomedical applications in the past few decades due to its outstanding advantages in terms of high sensitivity and satisfactory resolution.1–3 Various fluorescence imaging tracers, such as organic dyes, semiconductor quantum dots, rare-earth up-regulation materials and fluorophore-included nanoparticles, have been used to track biological or pathological processes in vitro and in vivo. In particular by incorporation of photo-induced ultrasonic wave reception, photoacoustic (PA) imaging is capable of combining high optical sensitivity and acoustic deep tissue penetration for medical diagnosis with enhanced precision.4–6 For instance, photoacoustic endoscopy has been successfully applied to detect superficial lesions (e.g. breast cancers and melanoma).7 By developing novel multi-modal tracers for both fluorescence and photoacoustic imaging, real-time tracking of multiple biomarkers with one single probe may be achieved.
Furthermore, it has attracted emerging attention to design multifunctional agents for not only diagnostic imaging but also for efficacious therapy.8,9 Upon activation of photo-sensitive agents by pulsed laser irradiation, a moderate temperature increase can be generated from physiological 37.5 °C to 40–45 °C, and thus photothermal therapy (PTT) or hyperthermia may be implemented for cancer treatments.10,11 Since cancerous cells are more sensitive to heating compared to normal cells, the specific cancer damage can be realized. Moreover, by means of PTT, the side-effects of radiotherapy or chemotherapy may be avoided or minimized for the sake of the patient's recovery.
Carbon dots (C-dots) have received much attention in the aspect of bio-imaging materials and drug delivery systems (DDSs) due to their superior properties such as chemical inertness, ready functionalization, colloidal stability and excellent biocompatibility.12–14 Numerous materials such as milk, honey, citric acid and glucose can be used as carbon sources to synthesize C-dots via bottom-up or top-down strategies.15–17 Although various C-dots have been developed using different techniques, the utilization of C-dot nanocomposites for cell tracking, multi-modal imaging and photothermal agents has been rarely studied so far.18 Hence, we developed C-dot nanocomposites with the embedding of methylene blue (MB), a clinical PA agent and fluorescent dye, and investigated their availability for efficient photothermal agents, dual-channel cell tracking in vitro and photoacoustic imaging of ocular blood vessels in vivo. Therefore, it has provided an unprecedented opportunity to utilize these nanocomposites for potential biomarker-targeted imaging along with photothermal ablation on cancer lesions.
By using soybean milk as the carbon source, C-dot nanocomposites were synthesized via a green and one-pot microwaving protocol (Scheme 1). MB was added to the soybean milk as a cocktail formulation followed by microwaving to fabricate MB-embedded nanocomposites (MB-CDs). Different conditions with varying microwaving times and temperatures have been screened, and real-time photoluminescence (PL) was investigated to detect the formation of C-dot nanocomposites. It was found that MB-CDs reacted for 5 h exhibited prominent PL compared to those with a shorter microwaving time (Fig. S1d vs. a–c, ESI†). Temperature selection concluded that at 120 or 180 °C, MB-CDs were either pre-mature or over-consumed (Fig. S2b vs. a and c, ESI†). Thus, 5 hour microwaving at 160 °C has been taken as the optimized synthetic condition. These as-prepared MB-CDs were then centrifuged and dialyzed to afford the pure and stable C-dot nanocomposites (Fig. 1a). Considering that MB as a small fluorescent molecule is prone to be eliminated by enzymes in the blood circulation, the MB embedded into the C-dots helps protect them from rapid body clearance and further enhances their bio-compatibility.19
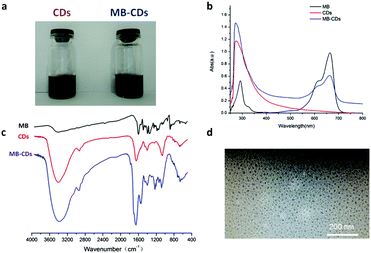 | ||
| Fig. 1 Characterization of C-dot nanocomposites, MB-CDs (a) using UV-Vis absorption spectra (b), FT-IR (c) and the morphological image by transmission electron microscopy (d). | ||
To characterize these nanocomposites and verify the embedding structure, optical UV-Vis absorbance and infrared spectra have been acquired. The nanocomposites of MB-CDs displayed characteristic absorbance peaks of C-dots without MB inclusion at 280 nm due to the π → π* electron transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C to n → π* of C
C to n → π* of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.20 The existence of identical characteristic peaks of free MB at 665 nm further confirmed their hybrid composition (Fig. 1b). Besides that, FT-IR spectra further consolidated the successful embedding of MB into C-dots in that the strong bands at 3402, 1652, 1402 and 1062 cm−1 proved the presence of hydroxyl and carbonyl groups which render energy traps and endow C-dots with high fluorescence (Fig. 1c).21,22 The size and the morphology of MB-CDs could be visualized by transmission electron microscopy (TEM). It showed that they had a uniform spherical shape and the size was 10.9 ± 3.5 nm in diameter (Fig. 1d), which was in the optimal size range of 5–30 nm for C-dots as theranostic agents.22,23 The zeta potential was measured to be −12.4 ± 0.6 mV which implied acceptable stability from aggregation for them to serve biomedical applications.24
O groups.20 The existence of identical characteristic peaks of free MB at 665 nm further confirmed their hybrid composition (Fig. 1b). Besides that, FT-IR spectra further consolidated the successful embedding of MB into C-dots in that the strong bands at 3402, 1652, 1402 and 1062 cm−1 proved the presence of hydroxyl and carbonyl groups which render energy traps and endow C-dots with high fluorescence (Fig. 1c).21,22 The size and the morphology of MB-CDs could be visualized by transmission electron microscopy (TEM). It showed that they had a uniform spherical shape and the size was 10.9 ± 3.5 nm in diameter (Fig. 1d), which was in the optimal size range of 5–30 nm for C-dots as theranostic agents.22,23 The zeta potential was measured to be −12.4 ± 0.6 mV which implied acceptable stability from aggregation for them to serve biomedical applications.24
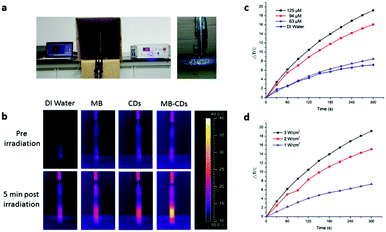
In order to use MB-CDs as photothermal agents, their photothermal effect was then tested by applying a pulsed laser to irradiate MB-CDs at 808 nm for 5 min, and the thermal images and the temperature increase were recorded (Fig. 2a). As shown in Fig. 2b, MB-CDs exhibited a significant photothermal effect compared to deionized water, free MB and C-dots without MB embedding. The temperature increase of MB-CDs was 19.2 °C, while only a minor increase of 4.8 °C, 5.6 °C and 8.5 °C was observed for deionized water, free MB and CD solution, respectively. To further elucidate the relationship between the temperature increase and the photothermal parameters, MB-CDs with varying embedded MB concentrations were synthesized and applied to the photothermal measurement. It is displayed in the temperature increase curves in Fig. 2c that the photothermal effect had a close relationship with the MB-CD concentration, and the synergy of MB and C-dots might contribute to the enhanced photothermal effect for MB-CDs. In addition, the energy density of laser irradiation was considered a critical factor. A higher energy density of 3 W cm−2 led to nearly 3 times more temperature increase compared to 1 W cm−2 (Fig. 2d). The irradiation distance between the laser point and MB-CD solution was also optimized which shows that 1–3 cm was satisfactory to afford an apparent photothermal effect, and a distance beyond 4–5 cm could not induce a notable temperature increase (Fig. S3, ESI†).
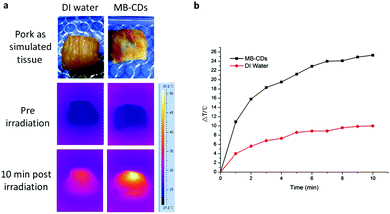
Encouraged by these findings, we performed a phantom photothermal imaging with a piece of pork as simulated tissue and injected MB-CDs to further confirm their availability as photothermal agents (Fig. 3). In comparison to the injection with deionized water as a control, the injection with MB-CDs gave rise to a prominent temperature increase of 25.3 °C after laser irradiation for 10 min. This distinct effect is sufficient enough for the photothermal therapy against cancer lesions when cancerous cells are differentiated and targeted.25,26
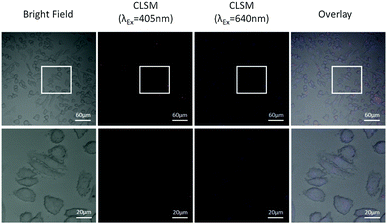
Afterwards, the nanocomposites were used as multi-modal tracers for cancer cell dual-channel imaging. After co-incubation with breast cancer cells MDA-MB231 for 30 min, MB-CDs were internalized via the passive cell membrane penetration and accumulated into the cells. By confocal laser scanning microscopy at dual-channel wavelengths of 405 nm and 640 nm, these nanocomposites inside the cells could be clearly visualized (Fig. 4). Due to the different excitation wavelengths of MB and C-dots, the overlay of images at 405 nm and 640 nm for enhanced precision on cell labelling and tracking has been achieved, which provides unprecedented convenience to targeted bio-imaging of multiple biomarkers with one single agent at one time.
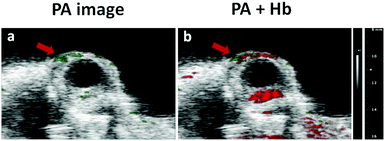
Considering that MB is a clinical PA agent, these nanocomposites with embedded MB should be able to image superficial tissues such as ocular blood vessels. Although ocular blood vessels are generally imaged by optical coherence tomography (OCT) or ultrasound, photoacoustic imaging has become a promising modality for providing much functional and structural information of pathological lesions in ocular vessels or capillaries.27–29 Thus in a proof-of-principle in vivo experiment, MB-CDs were injected into a nude mouse via the tail vein, and blood circulation pumped them into the eyes where the PA images were captured (Fig. 5a). By combining the PA images resulting from endogenous hemoglobin (Hb) and exogenous MB-CDs, it was more clearly concluded that MB-CDs afforded obvious signals in the blood vessels (Fig. 5b). This demonstrated that the as-developed MB-CDs as novel photoacoustic imaging tracers might be applied for the diagnosis and treatment of ocular vasculature-related diseases. To the best of our knowledge, this is the first report to utilize C-dot nanocomposites for the in vivo ocular photoacoustic imaging so far.
In conclusion, C-dot nanocomposite MB-CDs have been synthesized and well characterized. They can be used as efficient photothermal agents with a prominent temperature increase for potential thermal ablation of cancerous cells.
Besides, they are promising multi-modal imaging tracers for both dual-channel cell tracking and photoacoustic imaging of ocular blood vessels. These excellent features endow them with competency to serve in biomarker-targeted molecular imaging as well as simultaneous photothermal therapy against cancers upon completion of specific bio-conjugation on MB-CDs.
This work was financially supported by the National Natural Science Foundation of China (21575106), the Scientific Research Foundation for Returned Scholars, Ministry of Education of China, Zhejiang Qianjiang Talents Program and Wenzhou Government's Start-up Fund.
Notes and references
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC.
- M. Garland, J. J. Yim and M. Bogyo, Cell Chem. Biol., 2016, 23, 122–136 CrossRef CAS PubMed.
- O. Kocaoglu and E. E. Carlson, Nat. Chem. Biol., 2016, 12, 472–478 CrossRef CAS PubMed.
- S. Zackrisson, S. M. van de Ven and S. S. Gambhir, Cancer Res., 2014, 74, 979–1004 CrossRef CAS PubMed.
- J. Yao and L. V. Wang, Contrast Media Mol. Imaging, 2011, 6, 332–345 CrossRef CAS PubMed.
- G. P. Luke, D. Yeager and S. Y. Emelianov, Ann. Biomed. Eng., 2012, 40, 422–437 CrossRef PubMed.
- T. J. Yoon and Y. S. Cho, World J. Gastrointest. Endosc., 2013, 5, 534–539 CrossRef PubMed.
- J. Bartelmess, S. J. Quinn and S. Giordani, Chem. Soc. Rev., 2015, 44, 4672–4698 RSC.
- S. D. Jo, S. H. Ku, Y. Y. Won, S. H. Kim and I. C. Kwon, Theranostics, 2016, 6, 1362–1377 CrossRef PubMed.
- P. Wust, B. Hildebrandt, G. Sreenivasa, B. Rau, J. Gellermann, H. Riess, R. Felix and P. M. Schlag, Lancet Oncol., 2002, 3, 487–497 CrossRef CAS PubMed.
- R. Cavaliere, E. C. Ciocatto, B. C. Giovanella, C. Heidelberger, R. O. Johnson, M. Margottini, B. Mondovi, G. Moricca and A. Rossi-Fanelli, Cancer, 1967, 20, 1351–1381 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- R. L. Liu, D. Q. Wu, S. H. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., Int. Ed., 2009, 48, 4598–4601 CrossRef CAS PubMed.
- Y. M. Long, C. H. Zhou, Z. L. Zhang, Z. Q. Tian, L. Bao, Y. Lin and D. W. Pang, J. Mater. Chem., 2012, 22, 5917–5920 RSC.
- W. Kwon, S. Do, J. H. Kim, M. S. Jeong and S. W. Rhee, Sci. Rep., 2015, 5, 12604 CrossRef CAS PubMed.
- C. A. Salinas, A. M. Ariza, C. Pritz, R. M. Camprubí, B. Fernandez, R. M. J. Ruedas, F. A. Megia, F. A. Lapresta, G. F. Santoyo, F. A. Schrott and V. L. F. Capitan, Chem. Commun., 2013, 49, 1103–1105 RSC.
- H. Li, Z. Kang, Y. Li and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- W. Wang, L. Cheng and W. G. Liu, Sci. China: Chem., 2014, 57, 522–539 CrossRef CAS.
- E. Morgounova, Q. Shao, B. J. Hackel, D. D. Thomas and S. Ashkenazi, J. Biomed. Opt., 2013, 18, 56004 CrossRef PubMed.
- C. Liu, P. Zhang, F. Tian, W. Li, F. Li and W. Liu, J. Mater. Chem., 2011, 21, 13163–13167 RSC.
- L. Cao, X. Wang, J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S. Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318–11319 CrossRef CAS PubMed.
- A. Talib, S. Pandey, M. Thakur and H. F. Wu, Mater. Sci. Eng., C, 2015, 48, 700–703 CrossRef CAS PubMed.
- A. Mewada, S. Pandey, M. Thakur, D. Jadhav and M. Sharon, J. Mater. Chem. B, 2014, 2, 698–705 RSC.
- V. Gallardo, M. E. Morales, M. A. Ruiz and A. V. Delgado, Eur. J. Pharm. Sci., 2005, 26, 170–175 CrossRef CAS PubMed.
- Z. Lin, Y. Liu, X. Ma, S. Hu, J. Zhang, Q. Wu, W. Ye, S. Zhu, D. Yang, D. Qu and J. Jiang, Sci. Rep., 2015, 5, 11709 CrossRef PubMed.
- M. P. Melancon, A. M. Elliott, A. Shetty, Q. Huang, R. J. Stafford and C. Li, J. Controlled Release, 2011, 156, 265–272 CrossRef CAS PubMed.
- P. A. Keane and S. R. Sadda, Ophthalmology, 2014, 121, 2489–2500 CrossRef PubMed.
- C. L. Shields, M. Pellegrini, S. R. Ferenczy and J. A. Shields, Retina, 2014, 34, 1495–1512 CrossRef PubMed.
- N. S. Aburn and R. C. Sergott, Curr. Opin. Ophthalmol., 1993, 4, 3–6 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, including synthesis and characterization of C-dot nanocomposites (MB-CDs), and the protocols for photothermal imaging, cell tracking and PA imaging of ocular blood vessels. See DOI: 10.1039/c6qm00160b |
| This journal is © the Partner Organisations 2017 |